Abstract
Agouti-related peptide (AGRP)-expressing neurons are activated by fasting—this causes hunger1,2,3,4, an aversive state that motivates the seeking and consumption of food5,6. Eating returns AGRP neuron activity towards baseline on three distinct timescales: rapidly and transiently following sensory detection of food cues6,7,8, slowly and longer-lasting in response to nutrients in the gut9,10, and even more slowly and permanently with restoration of energy balance9,11. The rapid regulation by food cues is of particular interest as its neurobiological basis and purpose are unknown. Given that AGRP neuron activity is aversive6, the sensory cue-linked reductions in activity could function to guide behaviour. To evaluate this, we first identified the circuit mediating sensory cue inhibition and then selectively perturbed it to determine function. Here, we show that a lateral hypothalamic glutamatergic → dorsomedial hypothalamic GABAergic (γ-aminobutyric acid-producing)12 → AGRP neuron circuit mediates this regulation. Interference with this circuit impairs food cue inhibition of AGRP neurons and, notably, greatly impairs learning of a sensory cue-initiated food-acquisition task. This is specific for food, as learning of an identical water-acquisition task is unaffected. We propose that decreases in aversive AGRP neuron activity6 mediated by this food-specific circuit increases the incentive salience13 of food cues, and thus facilitates the learning of food-acquisition tasks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
MATLAB scripts to run and analyse 2AFC behaviour are freely available on Github (https://github.com/jbwallace123/2AFC_BPOD_ANALYSIS). For more details on installation please visit: https://sites.google.com/site/bpoddocumentation/installing-bpod.
References
Aponte, Y., Atasoy, D. & Sternson, S. M. AgRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 8, 1289–1291 (2005).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Hull, C. L. Principles of Behavior (D. Appleton-Century, 1943).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Mandelblat-Cerf, Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015).
Beutler, L. R. et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475 (2017).
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016).
Berridge, K. C. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur. J. Neurosci. 35, 1124–1143 (2012).
Shimp, C. P. Probabilistically reinforced choice behavior in pigeons. J. Exp. Anal. Behav. 9, 443–455 (1966).
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M. & Callaway, E. M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA 107, 21848–21853 (2010).
Wickersham, I. R., Finke, S., Conzelmann, K.-K. & Callaway, E. M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49 (2007).
de Jong, J. W. et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101, 133–151 (2019).
Petrovich, G. D. Lateral hypothalamus as a motivation–cognition interface in the control of feeding behavior. Front. Syst. Neurosci. 12, 14 (2018).
Sternson, S. M. & Eiselt, A.-K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 79, 401–423 (2017).
Stamatakis, A. M. et al. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. 36, 302–311 (2016).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
Li, Y. et al. Hypothalamic circuits for predation and evasion. Neuron 97, 911–924.e5 (2018).
Bonnavion, P., Mickelsen, L. E., Fujita, A., de Lecea, L. & Jackson, A. C. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 594, 6443–6462 (2016).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Krashes, M. J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Balleine, B. W. The meaning of behavior: discriminating reflex and volition in the brain. Neuron 104, 47–62 (2019).
Allen, W. E. et al. Thirst-associated preoptic neurons encode an aversive motivational drive Science 357, 1149–1155 (2017).
Leib, D. E. et al. The forebrain thirst circuit drives drinking through negative reinforcement. Neuron 96, 1272–1281.e4 (2017).
Zimmerman, C. A., Leib, D. E. & Knight, Z. A. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18, 459–469 (2017).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Tong, Q., Ye, C.-P., Jones, J. E., Elmquist, J. K. & Lowell, B. B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Leshan, R. L., Björnholm, M., Münzberg, H. & Myers, M. G. Jr. Leptin receptor signaling and action in the central nervous system. Obesity 14 (Suppl 5), 208S–212S (2006).
Paxinos, G. & Franklin, K. The Mouse Brain In Stereotaxic Coordinates (Academic, 1997).
Han, W. et al. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 23, 103–112 (2016).
Acknowledgements
We thank M. Andermann, Y. Livneh and J. Resch for advice regarding photometry, behavioural experiments and analyses; the Lowell laboratory for helpful discussions; H. Kucukdereli and Y. Livneh for coding assistance; Z. Yang for technical assistance; C. Wu for assistance with creating viral plasmids; and the Boston Children’s Viral Core for packaging/preparation of viral plasmids. This research was funded by the following NIH grants to B.B.L.: NIH P30 DK046200, NIH P30 DK057521, R01 DK075632, R01 DK089044, R01 DK096010; to J.B.: F32DK118807; and to M.J.K.: 1ZIADK075087-07.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.B. and B.B.L.; methodology: J.B., C.L., J.C.M., A.S.G., J.S.S., M.J.K. and B.B.L.; validation: J.B., C.L. and J.C.M.; formal analysis: J.B. and C.L.; resources: B.B.L. and M.J.K.; writing, original draft: J.B., C.L. and B.B.L.; writing, review and editing: J.B., C.L., J.C.M., AS.G., J.S.S., M.J.K. and B.B.L.; funding acquisition: J.B. and B.B.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 DMHLEPR neuron photometry responses are greater with pellet drops than with gut-infusions of caloric substances and inhibiting DMHLEPR neurons does not affect food intake following an overnight fast.
a, Heat maps of additional well-trained mice included in Fig. 1d of DMHLEPR neuron fibre photometry responses to cue (light) onset. Each heat map represents a single behavioural session. Single trials have been sorted by response time. Green line indicates light onset (time = 0), black line indicates time when the mouse poked within the correct port. b, Heat maps of all naive mice included in Fig. 1d of DMHLEPR neuron fibre photometry responses to cue (light) onset. Each heat map represents a single behavioural session. Single trials have been sorted by response time. Green line indicates light onset (time = 0), black line indicates time when the mouse poked within the correct port. It should be noted that naive mice perform significantly fewer trials than experienced mice. c, Gastric infusion experimental design. d–f, Plot of fibre photometry responses from DMHLEPR neurons aligned to gut infusions of Ensure (d, e), saline (d, e), or caloric and non-caloric sweeteners (f). These were also aligned to food pellet drops post-infusion. Mice were either food deprived or fed ad libitum for sated Ensure infusions. Light colour tones indicate S.E.M. g, Quantification of fluorescence changes ten minutes post-gut infusions of saline, Ensure, and Ensure when animals were fed ad libitum (sated Ensure). n = 9 (mice); Repeated measures one-way ANOVA, ***P = 0.0007. h, Comparison between peak fibre photometry responses during the last 30 s of Ensure infusion (red) and first 30 s of pellet drop response (beige) when animals were given saline infusions. Peak response is significantly larger with pellet drops vs. Ensure infusions. n = 9 (animals); Two-tailed, paired t-test, *P = 0.0241. i, hM4Di-mediated inhibition of DMHLEPR neurons does not affect food intake in a post-fast refeeding assay. n = 12 (mice). Two-way ANOVA, P = 0.9992. All data represents the mean ± S.E.M.
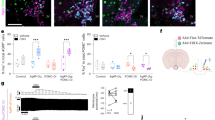
Extended Data Fig. 2 LHVGLTU2→DMH neurons preferentially synapse onto DMHPDYN-GFP neurons.
a, Sample image of rabies labelling of afferents to DMHLEPR neurons. (Top left) Sample of injection site of AAV-DIO-TVA, AAV-DIO-RG, and EnvA-GFP within the DMH. (Right and bottom row) Sample images of rabies labelled afferents. Scale bar = 200 μM. A/P levels are inferred based on histological landmarks. n = 3 (animals). b, Monosynaptic connection probability of optically evoked IPSCs and EPSCs to vDMHPDYN-GFP neurons. X-axis = afferent source. (c, d) Quantification of oIPSC (a) and oEPSC (b) amplitude from all candidate afferents to DMHLEPR neurons in the presence and absence of TTX and 4AP. Two-tailed, paired t-test, *P ≤ 0.05. (b) P = 1.664 (NAc), P = 0.0285 (BNST), P = 0.6413 (LH), P = 0.0165 (VTA) (c) P = 0.9366 (LH). e, Experimental design schematic. AAV-DIO-ChR2 injected in LHVGLUT2 neurons and oEPSCs were measured in vDMHPDYN-GFP neurons. Bottom: Example current-clamp recordings in vDMHPDYN-GFP neuron during optogenetic activation of LHVGLUT2 neurons. f, LHVGLUT2 neurons preferentially connect to about 80% of DMHPDYN-GFP neurons and have sparse connectivity to DMHPDYN-GFP negative neurons. n = 15 (cells) GFP+, 17 (cells) GFP-. g, oEPSC amplitude is unchanged when TTX and 4AP are added. n = 9 (ACSF) and 11 (TTX+4AP). Two-tailed unpaired t-test, P = 0.1666. h, AAV-DIO-ChR2 was injected into the LH of Vglut2-IRES-Cre mice. Whole-cell recordings were performed in vDMHPDYN-GFP negative neurons while stimulating LHVGLUT2 terminals. Bottom: representative ChR2-assisted circuit mapping traces from vDMHPDYN-GFP negative neurons in the presence and absence of TTX and 4AP. i, Summarized postsynaptic current amplitude in DMHPDYN-GFP negative neurons in the presence and absence of TTX and 4AP. n = 9 (ACSF) and 8 (TTX, 4AP). Two-tailed, unpaired t-test, P = 0.26. j, Summarized input resistance in both PDYN-GFP positive and negative neurons. n = 20 (GFP+) and 17 (GFP–). Two-tailed, unpaired t-test, P = 0.14. k, Summarized series resistance in both PDYN-GFP positive and negative neurons. n = 20 (GFP+) and 17 (GFP–). Two-tailed, unpaired t-test, P = 0.24. l, m, Averaged current onset latency (l) and jitter (m) in PDYN-GFP positive neurons in the absence of TTX and 4AP. n = 8 (cells). All data represents the mean ± S.E.M.
Extended Data Fig. 3 LHVGLUT2→DMH neurons are appetitive, do not send collaterals to the LHb or PAG, have negligible responses to non-edible object presentations, and respond differently to water when compared to food.
a, Representative wire plots of an XFP and ChR2 expressing animals throughout the entire 20-min RTPP session across three different conditions: lights-on ad libitum, lights-off ad libitum, and lights-on fasted. Blue line represents stimulation side of the RTPP chamber and black represents the non-stimulation side. b–d, Total distance travelled is unchanged between XFP and ChR2 expressing animals throughout the RTPP session across all three conditions (lights-on ad libitum, lights-off ad libitum, and lights-on fasted). n = 7 (animals) for both XFP and ChR2-expressing groups. Unpaired t-test, P = 0.7498 (b), P = 0.5277 (c), P = 0.3482 (d). e, DIO-TVA-mCherry was injected into the LH of Vglut2-IRES-Cre mice and pseudotyped EnvA-GFP was injected into the DMH to map collaterals of LHVGLUT2 → DMH neurons. f, Injection sites within the LH (left) and DMH (right). Scale bar = 500 μM (LH) and 200 μM (DMH). g, Collaterals were found within the BNST, MPO/LPO, and AHA and were absent in LH targets known to promote aversion such as the LHb and PAG. n = 3 (animals). Scale bar = 500 μM. h, Averaged axonal fibre-photometry response in LHVGLUT2→vDMH terminals across all animals when presented with a non-edible object. Vertical line represents object presentation. n = 7 (animals). i, Summarized Z-score of LHVGLUT2→vDMH axonal fibre photometry response to pellet (n = 9) and object (n = 7) drops in fasted mice. One-way ANOVA; Kruskal–Wallis, ****P < 0.0001. j, Summarized mean peak response in LHVGLUT2→vDMH axonal fibre photometry response to pellet drops when animals were fed ad libitum. n = 9; Two-tailed, unpaired t-test, *P = 0.0129. k, Averaged axonal fibre-photometry response in LHVGLUT2→vDMH terminals across all animals when given a 500 mg food pellet when food restricted (green) or, a bowl of water when water restricted (blue). Vertical line represents food/water presentation. l, m, Quantification of mean peak response of food (in food-deprived mice) and water presentations (in water-deprived mice) (l, P = 0.0206). n = 9 (food) and 8 (water). Peak water response occurred at a much later time point than food responses suggesting that the neurons respond differently to water cues when compared to food (m, P = 0.0391). n = 9 (food) and 8 (water); Two-tailed, unpaired t-test, *P < 0.05. A/P levels are inferred based on histological landmarks from the referenced mouse atlas. All data represents the mean ± S.E.M.
Extended Data Fig. 4 AAV-fDIO-hM4Di efficiently inhibits LHVGLUT2 neurons, CNO does not affect DMHLEPR activation, and hM4Di inhibition does not affect sustained AGRP inhibition.
a, Validation of AAV-fDIO-hM4Di construct using whole-cell patch clamp electrophysiology. Upon CNO wash-on (red), the rheobase increase is indicative of a successfully hyperpolarized LHVGLUT2 neuron given that a higher injection of current is needed to fire an action potential during CNO-wash on. b, CNO wash-on also eliminated spontaneous action potential firing in LHVGLUT2 neurons in fDIO-hM4Di expressing neurons. c, d, XFP controls were used to determine if CNO would affect DMHLEPR activation in the absence of hM4Di. CNO did not affect DMHLEPR neuron photometry responses (c, P = 0.7616) or behavioural performance (d). n = 3 (mice). e, Inhibiting LH→DMH afferents does not affect behavioural response time in well-trained mice. n = 4 (mice). Relates to Fig. 3a–c. Two-tailed t-test, P = 0.7068. f, CNO treatment does not affect the later consummatory response in AGRP neurons when mice are given a large (250 mg) food pellet. n = 4 (mice); Two-tailed t-test, P = 0.61. g, Inhibiting LH→DMH afferents in well-trained mice does not affect behavioural performance. n = 4 (mice), Two-tailed t-test, P = 0.3819. Relates to Fig. 3a–c. h, Inhibition of LHVGLUT2 neurons does not affect behavioural performance. n = 4 (mice), Two-tailed t-test P = 0.3661. Relates to Fig. 3d–f. i, j, Inhibition of LHVGLUT2 neurons does not affect behavioural accuracy (i) or response time (j). n = 4 (mice), Two-tailed t-test, P = 0.7107. Relates to Fig. 3g–i. All data represents the mean ± S.E.M.
Extended Data Fig. 5 TeNT-mediated silencing of DMHLEPR, DMHPDYN, and LH→DMH neurons significantly attenuates learning a cue-initiated food acquisition task by increasing mistakes made during the task but TeNT-mediated silencing of DMHLEPR neurons does not affect learning a cue-initiated water acquisition task.
a, b, DMHLEPR neuron silencing leads to an increase in response errors in the 2AFC task. TeNT-expressing mice largely miss the response window (a) in the initial phases of training and have an increase in false alarms (b). n = 5 (GFP), 5 (TeNT). a, Red dotted line indicates line of best fit (***P < 0.0001, plateau = 5.735 (GFP), -2.910 (TeNT); tau = 2.923 (GFP), 12.64 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = 0.05. b, Red dotted line indicates line of best fit (***P < 0.0001, plateau = 0.1324 (GFP), -162.8 (TeNT); tau = 6.679 (GFP), 117.6 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = 0.6. c, TeNT-expressing Lepr-IRES-Cre mice take significantly longer to nose poke following cue-light presentation. n = 5 (GFP), 5 (TeNT).). Red dotted line indicates line of best fit (***P < 0.0001, plateau = 1.319 (GFP), 0.633 (TeNT); tau = 4.272 (GFP), 9.860 (TeNT)). Two-way repeated measures ANOVA, main effect of days = **P < 0.01, main effect of group = 0.08. d, Body weight is unaffected upon DMHLEPR silencing with TeNT (7 weeks post-surgery). n = 5 (GFP), 5 (TeNT). Two-tailed, unpaired t-test, P = 0.7043. e, Food-intake does not significantly differ with DMHLEPR silencing following an overnight fast. n = 5 (GFP), 6 (TeNT). Two-tailed, unpaired t-test, P = 0.9495. f, AAV-DIO-TeNT injection into DMHPDYN neurons. Bottom: Example images of TeNT expression. Scale bar = 500 μM. n = 5 (mice). g, h, Similar to (Fig. 2b) in DMHPDYN neurons. n = 7 (GFP), 5 (TeNT). Red line indicates = line of best fit (***P < 0.0001, plateau = 82.83 (GFP), 65.52 (TeNT); tau = 5.619 (GFP), 7.337 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = **P < 0.01. h, Similar to (Fig. 2c) in DMHPDYN neurons (learning criterion >70% correct across three consecutive days). >20 on the y-axis is to indicate that by day 20, mice had not learned. 7/7 GFP and 1/6 TeNT mice learned. n = 7 (GFP), 5 (TeNT). Two-tailed, unpaired t-test, **P = 0.0094. i, j, DMHPDYN neuron silencing leads to an increase in response errors in the 2AFC task. TeNT-expressing mice largely miss the response window (i) in the initial phases of training and do not have a difference in false alarm rate (j). n = 7 (GFP), 5 (TeNT). (i) Red dotted line indicates line of best fit (***P < 0.0001, plateau = 7.142 (GFP), 20.89 (TeNT); tau = 0.8597 (GFP), 1.96 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ***P < 0.001, main effect of group = *P < 0.05. j, Line of best fit could not be calculated. Two-way repeated measures ANOVA, main effect of days = ***P < 0.001, main effect of group = 0.8267. k, TeNT-expressing Pdyn-Cre mice take significantly longer to nose poke following cue-light presentation. n = 7 (GFP), 5 (TeNT).). Red dotted line indicates line of best fit (***P < 0.0001, plateau = 1.185 (GFP), 2.748 (TeNT); tau = 2.122 (GFP), 3.939 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = **** P < 0.0001. l, Body weight increases upon long term DMHPDYN silencing with TeNT (20 weeks post-surgery). n = 7 (GFP), 5 (TeNT). Two-tailed, unpaired t-test, **P = 0.0086. m, Food-intake does not significantly differ with DMHPDYN silencing following an overnight fast. n = 7 (GFP), 5 (TeNT). Two-tailed, unpaired t-test, P = 0.2668. n, AAV-DIO-TeNT injection into the LH and rAAV-Cre injection into the DMH in wild-type mice. Bottom: Example images of TeNT expression. Scale bar = 500 μM. n = 6 (mice). o, p, Similar to (Fig. 2b) in LH→DMH neurons. n = 5 (GFP), 6 (TeNT). Red line = line of best fit (***P < 0.0001, slope = 2.033 (GFP), 1.488 (TeNT); Y-intercept = 44.50 (GFP), 30.02 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = P = 0.0548. p, Similar to (Fig. 2c) in LH→DMH neurons (learning criterion >70% correct across three consecutive days). 5/5 GFP and 2/6 TeNT-expressing mice learned. n = 5 (GFP), 6 (TeNT). Two-tailed, unpaired t-test, P = 0.0789. q, r, LH→DMH neuron silencing leads to an increase in response errors in the 2AFC task. TeNT-expressing mice largely miss the response window (q) in the initial phases of training and do not have a difference in false alarm rate (r). n = 5 (GFP), 6 (TeNT). q, Red dotted line indicates line of best fit (***P < 0.0001, slope = -0.299 (GFP), -0.4169 (TeNT); Y-intercept = 16.75 (GFP), 39.91 (TeNT)). Two-way repeated measures ANOVA, main effect of days = *P = 0.0416, main effect of group = P = 0.1896. r, Line of best fit is not significantly different for TeNT and GFP-expressing animals (P = 0.1724, plateau = -22.34 (GFP), -74.42 (TeNT); Tau = 23.91 (GFP), 83.89 (TeNT)). Two-way repeated measures ANOVA, main effect of days = *P = 0.0101, main effect of group = 0.9094. s, TeNT-expression in LH→DMH neurons significantly increases nose poke response following cue-light presentation. n = 5 (GFP), 6 (TeNT).). Red dotted line indicates line of best fit (***P < 0.0001, plateau = 1.790 (GFP), 3.314 (TeNT); tau = 1.818 (GFP), 8.042 (TeNT)). Two-way repeated measures ANOVA, main effect of days = ***P = 0.0004, main effect of group = ****P < 0.0001. t, Body weight is unaffected upon LH→DMH silencing with TeNT (8 weeks post-surgery). n = 5 (GFP), 6 (TeNT). Two-tailed, unpaired t-test, P = 0.2820. u, Food-intake does not significantly differ with LH→DMH silencing following an overnight fast. n = 5 (GFP), 6 (TeNT). Two-tailed, unpaired t-test, P = 0.8666. v–x, DMHLEPR neuron silencing does not affect response errors or response times in the 2AFC task when dehydrated mice receive water rewards. n = 4 (GFP), 6 (TeNT). v, Red dotted line indicates line of best fit (plateau = 57.74, tau = 4.477). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = 0.942. w, Red dotted line indicates line of best fit (plateau = -15.32, tau = 11.67). Two-way repeated measures ANOVA, main effect of days = *P < 0.05, main effect of group = 0.13. x, Red dotted line indicates line of best fit (plateau = 0.7882, tau = 2.929). Two-way repeated measures ANOVA, main effect of days = ****P < 0.0001, main effect of group = 0.82. All data represents the mean ± S.E.M.
Extended Data Fig. 6 PDYN-expressing DMH neurons exclusively project to the ARC.
AAV-DIO-ChR2-mCherry was injected into the DMH of Pdyn-Cre mice to search for long range projections of DMHPDYN neurons. The ARC was the only area of the brain that received DMHPDYN innervation. A portion of this data was previously published in Garfield et al. 201612.
Extended Data Fig. 7 Schematic representation of viral spread and fibre placement.
a–c, Schematic representing the extent of viral spread (transparent shaded regions) and fibre placement (solid ovals) for every animal related to studies presented in Fig. 1b-d, Extended Data Fig. 1a (a), Fig. 2a-e, Extended Data Fig. 3a-d (b) and Fig. 2f–k, Extended Data Fig. 3h–m (c). Please note that different animals are represented by different colours and that “hits” and “misses” are represented on different hemispheres (miss is on the left). GENERAL CRITERION FOR HITS AND MISSES: Animals were deemed a “hit” if viral expression was within the area of interest and fibre placement was either within or immediately dorsal to the area of interest (that is, the DMH or the LH). Excluded animals are specified by the “miss” label. Reason each animal was considered a “miss”: Animal 5; fibre placement dorso-lateral to DMH (a); Animal 10; unilateral viral expression and fibre placement lateral to midline/DMH (b); Animal 10; viral expression ventro-medial to LH, fibre placement lateral to the DMH (c).
Extended Data Fig. 8 Schematic representation of viral spread and fibre placement within Lepr-Cre animals.
Schematic representing the extent of viral spread (transparent shaded regions) and fibre placement (solid ovals) for every animal related to studies presented in Fig. 3a–c and Extended Data Fig. 4e–g. Please note that individual animals are represented by different colours. Bilateral hM4Di injections were targeted to the LH and bilateral AAV6-FlpO injections were targeted to the DMH. GCaMP6s injections into the DMH were unilateral. CRITERION FOR HITS AND MISSES: Animals were deemed a “hit” if viral expression was within the area of interest (that is, hM4Di bilateral in the LH, GCaMP unilateral in the DMH) and fibre placement was either within or immediately dorsal to the area of interest (that is, the DMH). For clarity, hits are shown on the top and misses on the bottom. Reason each animal was considered a “miss”: Animal 1; viral expression (hM4Di) was medial to DMH, fibre placement ventral to DMH. Animal 2; viral expression (hM4Di) was unilateral, fibre placement ventral to DMH. Animal 3; viral expression (hM4Di) was unilateral, fibre placement dorsal to DMH. Animal 4; hM4Di expression undetected, fibre placement ventral to DMH.
Extended Data Fig. 9 Schematic representation of viral spread and fibre placement within Vglut2-FlpO::Lepr-Cre animals.
Schematic representing the extent of viral spread (transparent shaded regions) and fibre placement (solid ovals) for every animal related to studies presented in Fig. 3d–f and Extended Data Fig. 4h. Please note that individual animals are represented by different colours. Bilateral hM4Di injections were targeted to the LH and GCaMP6s injections were unilateral into the DMH. GENERAL CRITERION FOR HITS AND MISSES: Animals were deemed a “hit” if viral expression was within the area of interest (that is, hM4Di bilateral in the LH, GCaMP unilateral in the DMH) and fibre placement was either within or immediately dorsal to the area of interest (that is, the DMH). For clarity, hits are shown on the top and misses on the bottom. Reason each animal was considered a “miss”: Animal 1; viral expression of hM4Di was dorsal to LH. Animal 2; hM4Di viral expression was unilateral and dorsal to LH and minimal GCaMP6s expression was detected lateral to DMH.
Extended Data Fig. 10 Schematic representation of viral spread and fibre placement within Vglut2-FlpO::AGRP-Cre animals.
Schematic representing the extent of viral spread (transparent shaded regions) and fibre placement (solid ovals) for every animal related to studies presented in Fig. 3g–i and Extended Data Fig. 4i, j. Please note that different animals are represented by different colours. Bilateral hM4Di injections were targeted to the LH and GCaMP6s injections were unilateral into the ARC. Purple regions indicate areas with no viral expression. GENERAL CRITERION FOR HITS AND MISSES: Animals were deemed a “hit” if viral expression was within the area of interest (that is, hM4Di bilateral in the LH, GCaMP unilateral in the ARC) and fibre placement was either within or immediately dorsal to the area of interest (that is, the ARC). For clarity, hits are shown on the top and misses on the bottom. Reason each animal was considered a “miss”: Animal 1; viral expression of hM4Di was unilateral and dorsal to LH and GCaMP6s expression was undetectable. Animal 2; viral expression of hM4Di was unilateral and dorsal to LH and GCaMP6s expression was undetectable.
Extended Data Fig. 11 Schematic representation of viral spread.
a–d, Schematic representing the extent of viral spread (transparent shaded regions) for every animal related to studies presented in Fig. 4a–c, Extended Data Fig. 5a–e (a), Extended Data Fig. 5f–m (b), Extended Data Fig. 5n–u (c) and Fig. 4d–f and Extended Data Fig. 5v–x (d). Please note that different animals are represented by different colours. GENERAL CRITERION FOR HITS AND MISSES: Animals were deemed a “hit” if viral expression was within the area of interest (that is, the DMH or the LH). Excluded animals are specified by the “miss” label. Reason each animal was considered a “miss”: Animal 7, viral expression had spread to the DMH and VMH (a); Animal 6, viral expression was dorsal to DMH (b); Animal 7, viral expression was barely detected (b); Animal 7, viral expression was dorsal to LH (c); Animal 8, diffuse, widespread viral expression dorsal to LH (c); Animal 9, viral expression was dorsal to LH and within zona incerta (c); Animal 10, viral expression was dorsal to LH and within zona incerta (c); Animal 7; viral expression was dorsal to DMH (d); Animal 8, viral expression was barely detectable in a small region within the anterior DMH (d).
Extended Data Fig. 12 Example of method used to draw anatomical boundaries in histological images.
DAPI-stained images were taken and contrast was enhanced (left). Images were matched with the appropriate A/P coordinate within the histological atlas (middle) then, boundaries were drawn using the axis as a template (right). All anatomical boundaries represented within the figures were drawn using this method. The atlas used for these studies was The Mouse Brain in Stereotaxic Coordinates by Paxinos and Franklin, Second Edition.
Supplementary information
Supplementary Information
This file contains supplementary discussion.
Rights and permissions
About this article
Cite this article
Berrios, J., Li, C., Madara, J.C. et al. Food cue regulation of AGRP hunger neurons guides learning. Nature 595, 695–700 (2021). https://doi.org/10.1038/s41586-021-03729-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-021-03729-3
This article is cited by
-
Prefrontal cortex-to-hypothalamic outputs orchestrate cue-potentiated palatable food consumption via AMPKβ2 signaling
Cell Discovery (2026)
-
Lateral hypothalamus and eating: cell types, molecular identity, anatomy, temporal dynamics and functional roles
Experimental & Molecular Medicine (2025)
-
Fat sensory cues in early life program central response to food and obesity
Nature Metabolism (2025)
-
A food-sensitive olfactory circuit drives anticipatory satiety
Nature Metabolism (2025)
-
Hypothalamic-hindbrain circuit for consumption-induced fear regulation
Nature Communications (2024)