Abstract
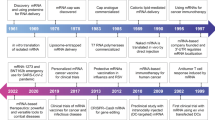
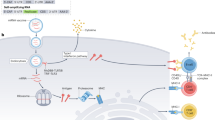
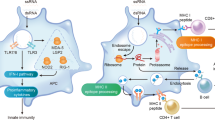
Messenger RNA (mRNA) is an emerging class of therapeutic agent for the prevention and treatment of a wide range of diseases. The recent success of the two highly efficacious mRNA vaccines produced by Moderna and Pfizer–BioNTech to protect against COVID-19 highlights the huge potential of mRNA technology for revolutionizing life science and medical research. Challenges related to mRNA stability and immunogenicity, as well as in vivo delivery and the ability to cross multiple biological barriers, have been largely addressed by recent progress in mRNA engineering and delivery. In this Review, we present the latest advances and innovations in the growing field of mRNA nanomedicine, in the context of ongoing clinical translation and future directions to improve clinical efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sharp, P. A. The centrality of RNA. Cell 136, 577–580 (2009).
Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Xiao, Y. et al. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 51, 3828–3845 (2022).
Emiliano, B. et al. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 14, 4448–4455 (2022).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976).
Langer, R. Controlling the movement of molecules. Q. Rev. Biophys. 52, e5 (2019).
Ostro, M. J., Giacomoni, D., Lavelle, D. O. N., Paxton, W. & Dray, S. Evidence for translation of rabbit globin mRNA after liposomemediated insertion into a human cell line. Nature 274, 921–923 (1978).
Dimitriadis, G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 274, 923–924 (1978).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2, 29 (2017).
Thess, A. et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 23, 1456–1464 (2015).
Karikó, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D.mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Gupta, A., Andresen, J. L., Manan, R. S. & Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 178, 113834 (2021).
Granot, Y. & Peer, D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—an innate immune system standpoint. Semin. Immunol. 34, 68–77 (2017).
Patel, A. K. et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 31, 1805116 (2019).
Moffett, H. F. et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 8, 389 (2017).
Kong, N. et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 11, eaaw1565 (2019).
Islam, M. A. et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2, 850–864 (2018).
Kong, N. et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc. Natl Acad. Sci. USA 119, e2112696119 (2022).
Kowalski, P. S., Rudra, A., Miao, L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Huang, X. et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. https://doi.org/10.1038/s41565-022-01174-5 (2022).
Tang, Z. et al. Insights from nanotechnology in COVID-19 treatment. Nano Today 36, 101019 (2021).
Tang, Z. et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 5, 847–860 (2020).
Pardi, N., Muramatsu, H., Weissman, D. & Karikó, K. in Synthetic Messenger RNA and Cell Metabolism Modulation: Methods and Protocols (ed. Rabinovich, P. M.) 29–42 (Humana Press, 2013).
Rosa, S. S., Prazeres, D. M. F., Azevedo, A. M. & Marques, M. P. C. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine 39, 2190–2200 (2021).
Tsui, N. B., Ng, E. K. & Lo, Y. D. J. C. C. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 48, 1647–1653 (2002).
McKinlay, C. J. et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl Acad. Sci. USA 114, E448–E456 (2017).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Lee, B. L. & Barton, G. M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 24, 360–369 (2014).
Devoldere, J., Dewitte, H., De Smedt, S. C. & Remaut, K. Evading innate immunity in nonviral mRNA delivery: don’t shoot the messenger. Drug Discov. Today 21, 11–25 (2016).
García, M. A., Meurs, E. F. & Esteban, M. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89, 799–811 (2007).
Anderson, B. R. et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 38, 5884–5892 (2010).
Silverman, R. H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNAse l during the interferon antiviral response. J. Virol. 81, 12720–12729 (2007).
George, C. X., John, Lijo & Samuel, C. E. An RNA editor, adenosine deaminase acting on double-stranded RNA (ADAR1). J. Interferon Cytokine Res. 34, 437–446 (2014).
Ross, J. & Sullivan, T. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood 66, 1149–1154 (1985).
Cao, J. et al. High-throughput 5′ UTR engineering for enhanced protein production in non-viral gene therapies. Nat. Commun. 12, 4138 (2021).
Orlandini von Niessen, A. G. et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol. Ther. 27, 824–836 (2019).
Sample, P. J. et al. Human 5′ UTR design and variant effect prediction from a massively parallel translation assay. Nat. Biotechnol. 37, 803–809 (2019).
Zeng, C. et al. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Adv. Mater. 32, 2004452 (2020).
Linares-Fernández, S., Lacroix, C., Exposito, J.-Y. & Verrier, B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 26, 311–323 (2020).
Holtkamp, S. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108, 4009–4017 (2006).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Karikó, K., Muramatsu, H., Keller, J. M. & Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 20, 948–953 (2012).
Anderson, B. R. et al. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 39, 9329–9338 (2011).
Kormann, M. S. D. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 29, 154–157 (2011).
Andries, O. et al. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 217, 337–344 (2015).
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Baiersdörfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Ramanathan, A., Robb, G. B. & Chan, S.-H. mRNA capping: biological functions and applications. Nucleic Acids Res. 44, 7511–7526 (2016).
Schlake, T., Thess, A., Thran, M. & Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 76, 301–328 (2019).
Leppek, K. et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 13, 1536 (2022).
Van Dülmen, M., Muthmann, N. & Rentmeister, A. Chemo-enzymatic modification of the 5′ cap maintains translation and increases immunogenic properties of mRNA. Angew. Chem. Int. Ed. 60, 13280–13286 (2021).
Bollu, A., Peters, A. & Rentmeister, A. Chemo-enzymatic modification of the 5′ cap to study mRNAs. Acc. Chem. Res. 55, 1249–1261 (2022).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Zhang, Y., Sun, C., Wang, C., Jankovic, K. E. & Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 121, 12181–12277 (2021).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989).
Rejman, J. et al. mRNA transfection of cervical carcinoma and mesenchymal stem cells mediated by cationic carriers. J. Control. Release 147, 385–391 (2010).
Kauffman, K. J., Webber, M. J. & Anderson, D. G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 240, 227–234 (2016).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Cui, S. et al. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 7, 473–479 (2018).
Xia, Y., Tian, J. & Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 79, 56–68 (2016).
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Gref, R. et al. Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994).
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 124, 8657–8661 (2012).
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
Zhang, X., Goel, V. & Robbie, G. J. Pharmacokinetics of patisiran, the first approved RNA interference therapy in patients with hereditary transthyretin-mediated amyloidosis. J. Clin. Pharmacol. 60, 573–585 (2020).
Richner, J. M. et al. Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170, 273–283.e12 (2017).
Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell 168, 1114–1125.e10 (2017).
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251 (2017).
Pardi, N. et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 8, 14630 (2017).
Sajid, A. et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 13, eabj9827 (2021).
Mukherjee, A. et al. Engineered mutant α-ENaC subunit mRNA delivered by lipid nanoparticles reduces amiloride currents in cystic fibrosis-based cell and mice models. Sci. Adv. 6, eabc5911 (2020).
Szőke, D. et al. Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema. Nat. Commun. 12, 3460 (2021).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Jiang, L. et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 24, 1899–1909 (2018).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 11, eaat9143 (2019).
Yin, H. et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 35, 1179–1187 (2017).
Song, C.-Q. et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat. Biomed. Eng. 4, 125–130 (2020).
Riley, R. S. et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7, eaba1028 (2021).
Miao, L. et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11, 2424 (2020).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Moderna. Protocol mRNA-1273-P301. Moderna https://covid19crc.org/wp-content/uploads/2020/09/mRNA-1273-P301-Protocol-2020.pdf (2020).
Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine EUA letter of authorization. https://www.fda.gov/media/144412/download (2020).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Cheng, X. & Lee, R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 99, 129–137 (2016).
Huang, X. et al. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 17, 748–780 (2022).
Tao, W. et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 12, eaay1063 (2020).
Zhu, X. et al. Long-circulating siRNA nanoparticles for validating prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA 112, 7779–7784 (2015).
Kaczmarek, J. C. et al. Systemic delivery of mRNA and DNA to the lung using polymer–lipid nanoparticles. Biomaterials 275, 120966 (2021).
Lin, Y.-X. et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 13, eaba9772 (2021).
Xiao, Y. et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 13, 758 (2022).
Kaczmarek, J. C. et al. Optimization of a degradable polymer–lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 18, 6449–6454 (2018).
Lv, H., Zhang, S., Wang, B., Cui, S. & Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 114, 100–109 (2006).
Akinc, A., Lynn, D. M., Anderson, D. G. & Langer, R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J. Am. Chem. Soc. 125, 5316–5323 (2003).
Green, J. J., Langer, R. & Anderson, D. G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 41, 749–759 (2008).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020).
Rui, Y. et al. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci. Adv. 8, eabk2855 (2022).
Benner, N. L. et al. Oligo(serine ester) charge-altering releasable transporters: organocatalytic ring-opening polymerization and their use for in vitro and in vivo mRNA delivery. J. Am. Chem. Soc. 141, 8416–8421 (2019).
McKinlay, C. J., Benner, N. L., Haabeth, O. A., Waymouth, R. M. & Wender, P. A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl Acad. Sci. USA 115, E5859–E5866 (2018).
Haabeth, O. A. W. et al. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc. Natl Acad. Sci. USA 115, E9153–E9161 (2018).
Haabeth, O. A. et al. An mRNA SARS-CoV-2 vaccine employing charge-altering releasable transporters with a TLR-9 agonist induces neutralizing antibodies and T cell memory. ACS Cent. Sci. 7, 1191–1204 (2021).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012).
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28, 117–129 (2021).
Maruggi, G., Zhang, C., Li, J., Ulmer, J. B. & Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 27, 757–772 (2019).
Buschmann, M. D. et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines 9, 65 (2021).
McKay, P. F. et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 11, 3523 (2020).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Li, Z. et al. Exon–intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015).
Pamudurti, N. R. et al. Translation of circRNAs. Mol. Cell 66, 9–21.e27 (2017).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e9 (2017).
Koch, L. Translated circular RNAs. Nat. Rev. Genet. 18, 272–273 (2017).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2015).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Kauffman, K. et al. Improved immune cell expression with circular RNA (oRNA) in vivo. American Society of Gene & Cell Therapy (ASGCT) Conference 2022 https://www.ornatx.com/wp-content/uploads/2022/05/ASGCT-Poster-221.pdf (2022).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell 74, 508–520.e4 (2019).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022).
Chen, R. et al. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01393-0 (2022).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Entos Pharmaceuticals. The challenge: effective nucleic acid delivery. Entos Pharmaceuticals https://www.entospharma.com/fusogenix (2022).
Shmulevitz, M. & Duncan, R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 19, 902–912 (2000).
Sun, Y. et al. Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics. Nat. Chem. 14, 274–283 (2022).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Lee, S. M. et al. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection in vivo. Angew. Chem. Int. Ed. 60, 5848–5853 (2021).
Xue, L. et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 144, 9926–9937 (2022).
Zhang, D. et al. Targeted delivery of mRNA with one-component ionizable amphiphilic Janus dendrimers. J. Am. Chem. Soc. 143, 17975–17982 (2021).
Zhang, D. et al. One-component multifunctional sequence-defined ionizable amphiphilic Janus dendrimer delivery systems for mRNA. J. Am. Chem. Soc. 143, 12315–12327 (2021).
Park, J. H. et al. Virus‐mimicking cell membrane‐coated nanoparticles for cytosolic delivery of mRNA. Angew. Chem. Int. Ed. 134, e202113671 (2022).
Li, Y. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 34, 2109984 (2022).
Zhang, S. et al. Selective encapsulation of therapeutic mRNA in engineered extracellular vesicles by DNA aptamer. Nano Lett. 21, 8563–8570 (2021).
Yang, Z. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4, 69–83 (2020).
Keener, A. B. J. N. How extracellular vesicles can enhance drug delivery. Nature 582, S14–S15 (2020).
Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 20, 6–7 (2021).
Popowski, K. D. et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter 5, 2960–2974 (2022).
Popowski, K. D. et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell. Vesicle 1, 100002 (2022).
Kim, M. et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 7, eabf4398 (2021).
Paunovska, K. et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 31, 1807748 (2019).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Tao, W., & Peppas, N. A. Robotic pills for gastrointestinal-tract-targeted oral mRNA delivery. Matter 5, 775–777 (2022).
Billingsley, M. M. et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 22, 533–542 (2022).
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
Li, W. et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 12, 7264 (2021).
Zhao, X. et al. Imidazole‐based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem. Int. Ed. 132, 20258–20264 (2020).
Ramishetti, S. et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv. Mater. 32, 1906128 (2020).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Kedmi, R. et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13, 214–219 (2018).
Rosenblum, D. et al. CRISPR–Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6, eabc9450 (2020).
Su, F.-Y. et al. In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles. Sci. Adv. 8, eabm7950 (2022).
Tombácz, I. et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNP. Mol. Ther. 29, 3293–3304 (2021).
Blanchard, E. L. et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat. Biotechnol. 39, 717–726 (2021).
Li, J.-Q. et al. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduct. Target. Ther. 6, 369 (2021).
Anderluzzi, G. et al. The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J. Control. Release 342, 388–399 (2022).
Langel, S. N. et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 14, eabn6868 (2022).
Abramson, A. et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 5, 975–987 (2022).
Matinas BioPharma. BioNTech and Matinas BioPharma announce exclusive research collaboration to evaluate novel delivery technology for mRNA-based vaccines. Matinas BioPharma https://www.matinasbiopharma.com/investors/news-events/press-releases/detail/419/biontech-and-matinas-biopharma-announce-exclusive-research (11 April 2022).
Matinas BioPharma. Lipid nano-crystal (LNC)—a disruptive platform for the safe and targeted delivery of therapeutics. Matinas BioPharma https://www.matinasbiopharma.com/lnc-technology/lnc-platform (2022).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Kremsner, P. G. et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 22, 329–340 (2022).
Gebre, M. S. et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 601, 410–414 (2022).
De Alwis, R. et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 29, 1970–1983 (2021).
Arcturus Therapeutics. Arcturus announces self-amplifying COVID-19 mRNA vaccine candidate ARCT-154 meets primary efficacy endpoint in phase 3 study. Arcturus Therapeutics https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-announces-self-amplifying-covid-19-mrna-vaccine (20 April 2022).
Moderna. Moderna announces first participants dosed in phase 3 study of seasonal influenza vaccine candidate (mRNA-1010). Moderna https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participants-Dosed-in-Phase-3-Study-of-Seasonal-Influenza-Vaccine-Candidate-mRNA-1010/default.aspx (7 June 2022).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Tran, E., Longo, D. L. & Urba, W. J. A milestone for CAR T cells. N. Engl. J. Med. 377, 2593–2596 (2017).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Huang, X. et al. Efficient delivery of mRNA using crosslinked nucleic acid nanogel as a carrier. ACS Mater. Lett. 2, 1509–1515 (2020).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Zhang, D. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17, 777–787 (2022).
Huang, X. et al. Intercalation-driven formation of siRNA nanogels for cancer therapy. Nano Lett. 21, 9706–9714 (2021).
Musunuru, K. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434 (2021).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Maugeri, M. et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 10, 4333 (2019).
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
Igyártó, B. Z., Jacobsen, S. & Ndeupen, S. Future considerations for the mRNA–lipid nanoparticle vaccine platform. Curr. Opin. Virol. 48, 65–72 (2021).
Zhang, L. et al. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl Acad. Sci. USA 118, e2105968118 (2021).
Aldén, M. et al. Intracellular reverse transcription of Pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr. Issues Mol. Biol. 44, 1115–1126 (2022).
Parry, R., Gifford, R. J., Lytras, S., Ray, S. C. & Coin, L. J. M. No evidence of SARS-CoV-2 reverse transcription and integration as the origin of chimeric transcripts in patient tissues. Proc. Natl Acad. Sci. USA 118, e2109066118 (2021).
Shimabukuro, T. T., Cole, M. & Su, J. R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020–January 18, 2021. J. Am. Med. Assoc. 325, 1101–1102 (2021).
Kozma, G. T., Shimizu, T., Ishida, T. & Szebeni, J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 154-155, 163–175 (2020).
Muskula, P. R. & Main, M. L. Safety with echocardiographic contrast agents. Circ. Cardiovasc. Imaging 10, e005459 (2017).
Corey, K. B. et al. A case of coronavirus disease 2019 messenger RNA vaccine tolerance and immune response despite presence of anti-polyethylene glycol antibodies. Ann. Allergy Asthma Immunol. 129, 246–248 (2022).
Pardi, N. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018).
Alameh, M.-G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl Acad. Sci. USA 118, e2005191118 (2021).
Lokugamage, M. P. et al. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 32, 1904905 (2020).
Paunovska, K. et al. Increased PIP3 activity blocks nanoparticle mRNA delivery. Sci. Adv. 6, eaba5672 (2020).
Zhang, N.-N. et al. A thermostable mRNA vaccine against COVID-19. Cell 182, 1271–1283.e16 (2020).
Chen, G.-L. et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe 3, e193–e202 (2022).
McHugh, K. J. et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 357, 1138–1142 (2017).
Lu, X. et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med. 12, eaaz6606 (2020).
Sarmadi, M. et al. Experimental and computational understanding of pulsatile release mechanism from biodegradable core-shell microparticles. Sci. Adv. 8, eabn5315 (2022).
Rouphael, N. G. et al. Immunologic mechanisms of seasonal influenza vaccination administered by microneedle patch from a randomized phase I trial. NPJ Vaccines 6, 89 (2021).
Xia, D. et al. An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proc. Natl Acad. Sci. USA 118, e2110817118 (2021).
O’Shea, J., Prausnitz, M. R. & Rouphael, N. Dissolvable microneedle patches to enable increased access to vaccines against SARS-CoV-2 and future pandemic outbreaks. Vaccines 9, 320 (2021).
Moderna. Moderna announces Omicron-containing bivalent booster candidate mRNA-1273.214 demonstrates superior antibody response against Omicron. Moderna https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Omicron-Containing-Bivalent-Booster-Candidate-mRNA-1273.214-Demonstrates-Superior-Antibody-Response-Against-Omicron/default.aspx (8 June 2022).
Sablad, M. et al. mRNA therapy for ornithine transcarbamylase deficiency. Society for Inherited Metabolic Disorders (SIMD) Conference 2019 https://secureservercdn.net/45.40.151.233/e32.83a.myftpupload.com/wp-content/uploads/2020/09/April-2019-OTC-Poster-Presented-at-SIMD.pdf?time=1654792975 (2019).
Arcturus Therapeutics. LUNAR-OTC. Arcturus Therapeutics https://arcturusrx.com/mrna-medicines-pipeline/#lunarOTC (2022).
Liu, L. et al. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Sousa, C. R. E. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Züst, R. et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 12, 137–143 (2011).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Cobb, M. Who discovered messenger RNA? Curr. Biol. 25, R526–R532 (2015).
Conry, R. M. et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55, 1397–1400 (1995).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Acknowledgements
We are supported by the US METAvivor Early Career Investigator Award (2018A020560 to W.T.), Harvard Medical School/Brigham and Women’s Hospital Anesthesia Department Basic Scientist Grant (2420 BPA075 to W.T.), Khoury Innovation Award (2020A003219 to W.T.), Gillian Reny Stepping Strong Center for Trauma Innovation Breakthrough Award (113548 to W.T.), Nanotechnology Foundation (2022A002721 to W.T.), Farokhzad Family Distinguished Chair Foundation (018129 to W.T.) and American Heart Association Collaborative Sciences Award (2018A004190 to W.T.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.L. declares the following current competing interest: Moderna. A list of all competing interests for R.L., past and present, is provided as Supplementary Table 1. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Kong, N., Zhang, X. et al. The landscape of mRNA nanomedicine. Nat Med 28, 2273–2287 (2022). https://doi.org/10.1038/s41591-022-02061-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-022-02061-1
This article is cited by
-
Lung-targeted RNA delivery systems: strategies and therapeutic applications
Journal of Nanobiotechnology (2026)
-
Peptide codes for organ-selective mRNA delivery
Nature Materials (2026)
-
Hybridization chain reaction-based DNA nanoframeworks for biosensing and therapeutic applications
Nature Protocols (2026)
-
Chemical biology of precise RNA targeting and intervention: advances and perspectives
Science China Chemistry (2026)
-
The mRNA-Based Innovative Strategy: Progress and Challenges
Nano-Micro Letters (2026)