Abstract
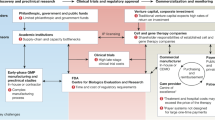
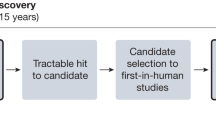
Increasing numbers of cell and gene therapies (CGTs) are emerging to treat and cure pediatric diseases. However, small market sizes limit the potential return on investment within the traditional biopharmaceutical drug development model, leading to a market failure. In this Perspective, we discuss major factors contributing to this failure, including high manufacturing costs, regulatory challenges, and licensing practices that do not incorporate pediatric development milestones, as well as potential solutions. We propose the creation of a new entity, the Pediatric Advanced Medicines Biotech, to lead late-stage development and commercialize pediatric CGTs outside the traditional biopharmaceutical model in the United States—where organized efforts to solve this problem have been lacking. The Pediatric Advanced Medicines Biotech would partner with the academic ecosystem, manufacture products in academic good manufacturing practice facilities and work closely with regulatory bodies, to ferry CGTs across the drug development ‘valley of death’ and, ultimately, increase access to lifesaving treatments for children in need.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schultz, L. M. et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J. Clin. Oncol. 40, 945–955 (2022).
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
Lorenz, B. et al. Real-world experience with Voretigene Neparvovec gene augmentation therapy in RPE65-mutation associated inherited retinal degeneration. Ophthalmology 131, 161–178 (2023).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Day, J. W. et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 20, 284–293 (2021).
Kanter, J. et al. Biologic and clinical efficacy of lentiglobin for sickle cell disease. N. Engl. J. Med. 386, 617–628 (2022).
Sheridan, C. The world’s first CRISPR therapy is approved: who will receive it? Nat. Biotechnol. 42, 3–4 (2023).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295 (2023).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216–234 (2021).
Ferrari, S. et al. Genetic engineering meets hematopoietic stem cell biology for next-generation gene therapy. Cell Stem Cell 30, 549–570 (2023).
De Wolf, D., Singh, K., Chuah, M. K. & VandenDriessche, T. Hemophilia gene therapy: the end of the beginning? Hum. Gene Ther. 34, 782–792 (2023).
Ling, Q., Herstine, J. A., Bradbury, A. & Gray, S. J. AAV-based in vivo gene therapy for neurological disorders. Nat. Rev. Drug Discov. 22, 789–806 (2023).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Urnov, F. D. Imagine CRISPR cures. Mol. Ther. 29, 3103–3106 (2021).
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Rossig, C. et al. Chimeric antigen receptor (CAR) T-cell products for pediatric cancers: why alternative development paths are needed. J. Clin. Oncol. 42, 253–257 (2023).
Aiuti, A., Pasinelli, F. & Naldini, L. Ensuring a future for gene therapy for rare diseases. Nat. Med. 28, 1985–1988 (2022).
Fox, T. et al. Access to gene therapy for rare diseases when commercialization is not fit for purpose. Nat. Med. 29, 518–519 (2023).
Castella, M. et al. Point-of-care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: experience from an academic phase I clinical trial. Front Immunol. 11, 482 (2020).
Innovative Genomics Institute. Making genetic therapies affordable and accessible. https://innovativegenomics.org/atf-report/ (2023).
Juan, M., Delgado, J., Calvo, G., Trias, E. & Urbano-Ispizua, A. Is hospital exemption an alternative or a bridge to European Medicines Agency for developing academic chimeric antigen receptor T-cell in Europe? Our experience with ARI-0001. Hum. Gene Ther. 32, 1004–1007 (2021).
Trias, E., Juan, M., Urbano-Ispizua, A. & Calvo, G. The hospital exemption pathway for the approval of advanced therapy medicinal products: an underused opportunity? The case of the CAR-T ARI-0001. Bone Marrow Transpl. 57, 156–159 (2022).
Precedence Research. Cell and gene therapy market - global industry analysis, size, share, growth, trends, regional outlook, and forecast 2023–2032. https://www.precedenceresearch.com/cell-and-gene-therapy-market (2022).
Smith, C. I. E., Bergman, P. & Hagey, D. W. Estimating the number of diseases - the concept of rare, ultra-rare, and hyper-rare. iScience 25, 104698 (2022).
Amorosi, D. Black race linked to inferior outcomes among younger patients receiving CAR-T for ALL. HemOnc Today https://www.healio.com/news/hematology-oncology/20210213/black-race-linked-to-inferior-outcomes-among-younger-patients-receiving-cart-for-all#:~:text=3%20min%20read-,Black%20race%20linked%20to%20inferior%20outcomes%20among,receiving%20CAR%2DT%20for%20ALL&text=Younger%20Black%20patients%20who%20received,at%20TCT%20Meetings%20Digital%20Experience (2021).
Sabatini, M. T. & Chalmers, M. The cost of biotech innovation: exploring research and development costs of cell and gene therapies. Pharm. Med. 37, 365–375 (2023).
Liu, A. J. & J, Legend tap Novartis to help make CAR-T drug Carvykti as they work through supply constraints. Fierce Pharma https://www.fiercepharma.com/manufacturing/jj-legend-tap-novartis-help-make-car-t-therapy-carvykti-amid-supply-constraint (2023).
Liu, A. Johnson & Johnson shelves Carvykti’s UK launch amid manufacturing shortfalls. Fierce Pharma https://www.fiercepharma.com/pharma/johnson-johnson-scraps-carvykti-launch-plan-uk-car-t-manufacturing-remains-lacking (2023).
Goodman, A. Patients with multiple myeloma may face CAR T-cell shortages. The ASCO Post https://ascopost.com/issues/september-25-2022/patients-with-multiple-myeloma-may-face-car-t-cell-shortages (2022).
Aparicio, C., Acebal, C. & Gonzalez-Vallinas, M. Current approaches to develop “off-the-shelf” chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review. Exp. Hematol. Oncol. 12, 73 (2023).
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27, 1419–1431 (2021).
Shah, N. N. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 26, 1569–1575 (2020).
Maschan, M. et al. Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients. Nat. Commun. 12, 7200 (2021).
Palani, H. K. et al. Decentralized manufacturing of anti CD19 CAR-T cells using CliniMACS Prodigy: real-world experience and cost analysis in India. Bone Marrow Transpl. 58, 160–167 (2023).
Hacein-Bey-Abina, S. et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 118, 3132–3142 (2008).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
Raper, S. E. et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 (2003).
Tucci, F., Galimberti, S., Naldini, L., Valsecchi, M. G. & Aiuti, A. A systematic review and meta-analysis of gene therapy with hematopoietic stem and progenitor cells for monogenic disorders. Nat. Commun. 13, 1315 (2022).
Scholler, J. et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4, 132ra153 (2012).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Levine, B. L. et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat. Med. 30, 338–341 (2024).
US Food and Drug Administration. FDA approves first gene therapies to treat patients with sickle cell disease. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease (8 December 2023).
Crooke, S. T. A call to arms against ultra-rare diseases. Nat. Biotechnol. 39, 671–677 (2021).
Crooke, S. T. Addressing the needs of patients with ultra-rare mutations one patient at a time: the n-lorem approach. Nucleic Acid Ther. 32, 95–100 (2022).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381, 1644–1652 (2019).
Aiuti, A., Roncarolo, M. G. & Naldini, L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 9, 737–740 (2017).
Martinez-Cibrian, N. et al. The academic point-of-care anti-CD19 chimeric antigen receptor T-cell product varnimcabtagene autoleucel (ARI-0001 cells) shows efficacy and safety in the treatment of relapsed/refractory B-cell non-Hodgkin lymphoma. Br. J. Haematol. 204, 525–533 (2023).
Elsallab, M., Bourgeois, F. & Maus, M. V. National survey of FACT-accredited cell processing facilities: assessing preparedness for local manufacturing of immune effector cells. Transplant Cell Ther. S2666-6367, 00289-6 (2024).
Elsallab, M. & Maus, M. V. Expanding access to CAR T cell therapies through local manufacturing. Nat. Biotechnol. 41, 1698–1708 (2023).
Joseph, A. In the US, scientists see barriers to the development of CAR-T cell therapies. In Spain, a hospital brews its own. STAT News https://www.statnews.com/2023/12/19/car-t-therapy-spain-hospital/ (2023).
Mast, J. Gene therapy is in crisis. For nine hours, the field’s leading minds looked for a solution. Stat News https://www.statnews.com/2023/07/31/gene-therapy-crisis-rare-disease-car-t/ (2023).
Cicalese, M. P. et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 128, 45–54 (2016).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017).
Strauss, K. A. et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the phase III SPR1NT trial. Nat. Med. 28, 1381–1389 (2022).
Strauss, K. A. et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the phase III SPR1NT trial. Nat. Med. 28, 1390–1397 (2022).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Shah, B. D. et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021).
Fumagalli, F. et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet 399, 372–383 (2022).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Thompson, A. A. et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N. Engl. J. Med. 378, 1479–1493 (2018).
Eichler, F. et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017).
Pipe, S. W. et al. Gene therapy with etranacogene dezaparvovec for hemophilia B. N. Engl. J. Med. 388, 706–718 (2023).
Tai, C. H. et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol. Ther. 30, 509–518 (2022).
Zaidman, C. M. et al. Delandistrogene moxeparvovec gene therapy in ambulatory patients (aged >/=4 to <8 years) with Duchenne muscular dystrophy: 1-year interim results from study SRP-9001-103 (ENDEAVOR). Ann. Neurol. 94, 955–968 (2023).
Ozelo, M. C. et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N. Engl. J. Med. 386, 1013–1025 (2022).
Carbonaro, D. A. et al. Preclinical demonstration of lentiviral vector-mediated correction of immunological and metabolic abnormalities in models of adenosine deaminase deficiency. Mol. Ther. 22, 607–622 (2014).
Kohn, D. B. et al. Autologous ex vivo lentiviral gene therapy for adenosine deaminase deficiency. N. Engl. J. Med. 384, 2002–2013 (2021).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
Aldoss, I. et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am. J. Hematol. 92, 858–865 (2017).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Baird, J. H. et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood 137, 2321–2325 (2021).
Frank, M. J. et al. CD22 CAR T cell therapy is safe and effective patients with large B cell lymphoma who have relapsed after CD19 CAR T cell therapy. Hemasphere 7, e3362169 (2023).
Acknowledgements
On 6 June 2023, approximately 80 CGT investigators representing more than 20 academic medical centers from the United States, Europe and Israel, alongside patient advocates, public officials representing the US FDA, the Advanced Research Projects Agency for Health and the California Institute for Regenerative Medicine and representatives from the private sector and non-profit foundations met in Washington, DC, for a think tank focused on the market failures we are witnessing for CGTs for pediatric diseases. The views discussed here germinated at this meeting54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.L.M. holds multiple patents in the arena of CAR T cell therapeutics; receives royalties from CARGO through the US National Institutes of Health and Stanford; holds equity in CARGO Therapeutics, Link Cell Therapies, Ensoma and GBM NewCo, which are developing CAR-based therapies; consults for CARGO, Link, Immatics, Ensoma and GBM NewCo; and receives research funding from Lyell Immunopharma and Tune Therapeutics. C.M.B. has filed patents in the arena of cell therapies, including CAR-modified cell therapies; is a scientific cofounder of Mana Therapeutics and Catamaran Bio; is on the Board of Directors of Cabaletta Bio; and holds stock in Repertoire Immune Medicine and Neximmune, all of which are developing cell therapies (including CAR-based therapies) for cancer or immune-mediated disorders. In addition, C.M.B. serves on the drug safety monitoring board for SOBI and on the scientific advisory board of Minovia TX. C.M.B has also served as president of FACT since 2021. N.G. is a cofounder and holds equity in CARGO Therapeutics. R.A.G. has patents related to CAR therapy and receives royalty payments related to patents from Juno Therapeutics. R.H.R. has received consulting fees from Pfizer and honoraria from Novartis. R.M.S. holds multiple patents in the area of CRISPR bioinformatics and biomanufacturing technologies; is the founder of Exthymic, a cell manufacturing instrument company; sits on the board of Indee Labs; and holds equity in Exthymic, Resilience (a Contract Development and Manufacturing Organization) and Synthego (RNA reagent manufacturer). F.D.U. is a scientific cofounder of Tune Therapeutics; holds equity in, and is a compensated advisor to, Tune Therapeutics and Cimeio Therapeutics; is a paid advisor to Ionis Pharmaceuticals; is a paid consultant to Vertex Pharmaceuticals on the exa-cel program; and receives salary support from the Danaher Corporation. A.S.W. receives institutional research funding (Children’s Hospital Los Angeles) from Kite, A Gilead Company. In addition, A.S.W. has served on advisory boards for Wugen, Kite and Institut de Recherches Internationales Servier, all for cell therapy product development. D.B.K. is an inventor for the University of California Regents for a lentiviral vector to treat ADA-SCID.
Peer review
Peer review information
Nature Medicine thanks Olaf Bodamer, Zornitza Stark and Jessica Foster for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mackall, C.L., Bollard, C.M., Goodman, N. et al. Enhancing pediatric access to cell and gene therapies. Nat Med 30, 1836–1846 (2024). https://doi.org/10.1038/s41591-024-03035-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03035-1
This article is cited by
-
The quintessential role for CAR T cell therapy in children, adolescents and young adults with cancer
Nature Reviews Clinical Oncology (2026)
-
Perspectives on the FDA platform technology designation program for the approval of gene therapies: a Swiss multi-stakeholder exploratory interview study
Orphanet Journal of Rare Diseases (2025)
-
Unlocking patient access to gene therapy: five key practices
Gene Therapy (2025)
-
A clinical roadmap for base and prime editing
Nature Reviews Bioengineering (2025)
-
Platform solutions for commercial challenges to expanding patient access and making gene editing sustainable
Nature Biotechnology (2025)