Abstract
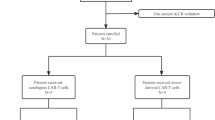
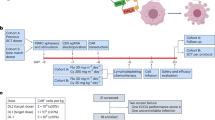
T cell acute lymphoblastic leukemia (T-ALL) is difficult to treat when it relapses after therapy or is chemoresistant; the prognosis of patients with relapsed or refractory T-ALL is generally poor. We report a case series of 17 such patients who received autologous chimeric antigen receptor (CAR) T cells expressing an anti-CD7 CAR and an anti-CD7 protein expression blocker (PEBL), which prevented CAR T cell fratricide. Despite high leukemic burden and low CAR T cell dosing, 16 of the 17 patients attained minimal residual disease-negative complete remission within 1 month. The remaining patient had CD7− T-ALL cells before infusion, which persisted after infusion. Toxicities were mild: cytokine release syndrome grade 1 in ten patients and grade 2 in three patients; immune effector cell-associated neurotoxicity syndrome grade 1 in two patients. Eleven patients remained relapse-free (median follow-up, 15 months), including all nine patients who received an allotransplant. The first patient is in remission 55 months after infusion without further chemotherapy or transplantation; circulating CAR T cells were detectable for 2 years. T cells regenerating after lymphodepletion lacked CD7 expression, were polyclonal and responded to SARS-CoV-2 vaccination; CD7+ immune cells reemerged concomitantly with CAR T cell disappearance. In conclusion, autologous anti-CD7 PEBL-CAR T cells have powerful antileukemic activity and are potentially an effective option for the treatment of T-ALL.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The CARTALL trial is currently ongoing. All requests for data related to CARTALL will be considered at the end of the trial. All other requests for data will be considered within 4 weeks, responded to in the context of obligations to patient privacy and confidentiality, and will be subject to review by the study team. Requests can be made to the corresponding authors by email at paeyej@nus.edu.sg. and/or franco.locatelli@opbg.net.
References
van Dongen, J. J. et al. T cell receptor–CD3 complex during early T cell differentiation. Analysis of immature T cell acute lymphoblastic leukemias (T-ALL) at DNA, RNA, and cell membrane level. J. Immunol. 138, 1260–1269 (1987).
Campana, D., Thompson, J. S., Amlot, P., Brown, S. & Janossy, G. The cytoplasmic expression of CD3 antigens in normal and malignant cells of the T lymphoid lineage. J. Immunol. 138, 648–655 (1987).
Teachey, D. T. & Pui, C.-H. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 20, e142–e154 (2019).
Raetz, E. A. et al. Outcome for children and young adults with T-cell ALL and induction failure in contemporary trials. J. Clin. Oncol. 41, 5025–5034 (2023).
Abaza, Y. et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am. J. Hematol. 93, 91–99 (2018).
Balduzzi, A. et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 366, 635–642 (2005).
Schrauder, A. et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J. Clin. Oncol. 24, 5742–5749 (2006).
Leung, W. et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 118, 223–230 (2011).
Bader, P. et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 3, 3393–3405 (2019).
Eckert, C. et al. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur. J. Cancer 151, 175–189 (2021).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Brentjens, R. J. et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 13, 5426–5435 (2007).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra225 (2014).
Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Hay, K. A. et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 133, 1652–1663 (2019).
Shah, N. N. et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J. Clin. Oncol. 39, 1650–1659 (2021).
Wayne, A. S. et al. Three-year results from phase I of ZUMA-4: KTE-X19 in pediatric relapsed/refractory acute lymphoblastic leukemia. Haematologica 108, 747–760 (2023).
Laetsch, T. W. et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J. Clin. Oncol. 41, 1664–1669 (2023).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Wang, T. et al. Coadministration of CD19- and CD22-directed chimeric antigen receptor T-cell therapy in childhood B-cell acute lymphoblastic leukemia: a single-arm, multicenter, phase II trial. J. Clin. Oncol. 41, 1670–1683 (2023).
Vodinelich, L. et al. A monoclonal antibody (WT1) for detecting leukemias of T-cell precursors (T-ALL). Blood 62, 1108–1113 (1983).
Link, M. et al. A single monoclonal antibody identifies T-cell lineage of childhood lymphoid malignancies. Blood 62, 722–728 (1983).
Janossy, G., Coustan-Smith, E. & Campana, D. The reliability of cytoplasmic CD3 and CD22 antigen expression in the immunodiagnosis of acute leukemia: a study of 500 cases. Leukemia 3, 170–181 (1989).
Png, Y. T. et al. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 1, 2348–2360 (2017).
Gomes-Silva, D. et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 130, 285–296 (2017).
Coustan-Smith, E. et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 10, 147–156 (2009).
Jain, N. et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 127, 1863–1869 (2016).
Wood, B. L. et al. Prognostic significance of ETP phenotype and minimal residual disease in T-ALL: a Children’s Oncology Group study. Blood 142, 2069–2078 (2023).
Pui, C.-H. et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N. Engl. J. Med. 360, 2730–2741 (2009).
Coustan-Smith, E. et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 117, 6267–6276 (2011).
Haynes, B. F., Eisenbarth, G. S. & Fauci, A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc. Natl Acad. Sci. USA 76, 5829–5833 (1979).
Sempowski, G. D., Lee, D. M., Kaufman, R. E. & Haynes, B. F. Structure and function of the CD7 molecule. Crit. Rev. Immunol. 19, 331–348 (1999).
Oh, B. L. Z. et al. Enhanced BNT162b2 vaccine-induced cellular immunity in anti-CD19 CAR T cell-treated patients. Blood 140, 156–160 (2022).
Maciocia, P. M. et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 23, 1416–1423 (2017).
Sánchez-Martínez, D. et al. Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia. Blood 133, 2291–2304 (2019).
Mamonkin, M., Rouce, R. H., Tashiro, H. & Brenner, M. K. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood 126, 983–992 (2015).
Mamonkin, M. et al. Reversible transgene expression reduces fratricide and permits 4-1BB costimulation of CAR T cells directed to T-cell malignancies. Cancer Immunol. Res. 6, 47–58 (2018).
Dai, Z. et al. The rational development of CD5-targeting biepitopic CARs with fully human heavy-chain-only antigen recognition domains. Mol. Ther. 29, 2707–2722 (2021).
Pan, J. et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J. Clin. Oncol. 39, 3340–3351 (2021).
Tan, Y. et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J. Hematol. Oncol. 16, 34 (2023).
Zhang, M. et al. Autologous nanobody-derived fratricide-resistant CD7-CAR T-cell therapy for patients with relapsed and refractory T-cell acute lymphoblastic leukemia/lymphoma. Clin. Cancer Res. 28, 2830–2843 (2022).
Locatelli, F. et al. Incidence of CD19-negative relapse after CD19-targeted immunotherapy in R/R BCP acute lymphoblastic leukemia: a review. Leuk. Lymphoma 64, 1615–1633 (2023).
Ruella, M. et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499–1503 (2018).
Li, S. et al. Eradication of T-ALL cells by CD7-targeted universal CAR-T cells and initial test of ruxolitinib-based CRS management. Clin. Cancer Res. 27, 1242–1246 (2021).
Hu, Y. et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 32, 995–1007 (2022).
Chiesa, R. et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N. Engl. J. Med. 389, 899–910 (2023).
Sadelain, M., Rivière, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Lu, P. et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood 140, 321–334 (2022).
Freiwan, A. et al. Engineering naturally occurring CD7− T cells for the immunotherapy of hematological malignancies. Blood 140, 2684–2696 (2022).
Zhang, X. et al. Analysis of 60 patients with relapsed or refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma treated with CD7-targeted chimeric antigen receptor-T cell therapy. Am. J. Hematol. 98, 1898–1908 (2023).
Shah, N. N. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 26, 1569–1575 (2020).
Del Bufalo, F. et al. Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL. Blood 142, 146–157 (2023).
Yeoh, A. E. J. et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia–Singapore acute lymphoblastic leukemia 2003 study. J. Clin. Oncol. 30, 2384–2392 (2012).
Yeoh, A. E. J. et al. Intensifying treatment of childhood B-lymphoblastic leukemia with IKZF1 deletion reduces relapse and improves overall survival: results of Malaysia–Singapore ALL 2010 study. J. Clin. Oncol. 36, 2726–2735 (2018).
Acknowledgements
This study was supported by the National Medical Research Council (NMRC) Singapore Translational Research (STaR) Award MOH-000708 (to D.C.), NMRC Research Training Fellowship NMRC/RTF/MOH/000616 (to B.L.Z.O.), NMRC Clinician Scientist Investigator Awards NMRC/CSA/0053/2008 and NMRC/CSA/0053/2013 (to A.E.J.Y.); the Cancer Science Institute of Singapore, National University of Singapore Grant NMRC/CG/NCIS/2010; and the Goh Foundation, Children’s Cancer Foundation, Singapore Totalisator Board, and VIVA Foundation for Children with Cancer. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank X. H. Tan, A. Ummul, D. A. Bte. Roslee, B. S. Lee, D. Ng and T. Y. Whoo for CAR T cell manufacturing; T. G. Soh for cell processing; N. Bte. Hussen, S. Kiong, V. Foo, H. X. Ng, V. Bertaina and C. Quintarelli for flow cytometric studies; Z. Chen, E. K. H. Chiew and G. Liew for regulatory and data management; the Malaysia–Singapore (MASPORE) leukemia study group (A. M. Tan, H. Ariffin, H. P. Lin and L. L. Chan) for providing historical data; M. Kimpo, K. L. Francisco, referring physicians and nursing staff for patient care; and patients and families for participating in the study.
Author information
Authors and Affiliations
Contributions
B.L.Z.O. directed cell manufacturing, provided clinical care and performed data analysis. N.S. established cell manufacturing technologies. E.C.-S. analyzed MRD, CAR T cells and immune reconstitution. E.C., L.P., S.H.R.L., F.Y., L.K.T. and L.Y.A.C. provided clinical care. N.L.B., N.T. and A.B. analyzed T cell responses to vaccination. S.P.C. monitored CAR T cells by ddPCR and analyzed MRD. F.D.B. and M.B. provided clinical care and performed data analysis. F.L. provided clinical care and was responsible for the clinical studies in Rome. A.E.J.Y. provided clinical care and was responsible for the clinical studies in Singapore. D.C. initiated the study, directed the translation of the technologies to the clinic and performed data analysis. B.L.Z.O., E.C.-S. and D.C. drafted the manuscript, and all authors reviewed and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.L.Z.O., N.S., E.C., L.P., S.H.R.L., F.Y., L.K.T., L.Y.A.C., N.T., S.P.C., F.D.B. and M.B. declare no competing interests. E.C.-S.’s spouse receives royalties for patents related to the development of CAR T cell technologies and is a scientific founder and stockholder of Nkarta Therapeutics and Medisix Therapeutics. N.L.B. is a co-inventor in a pending patent for a method to monitor virus-specific T cells in biological samples. A.B. is a co-inventor in a pending patent for a method to monitor virus-specific T cells in biological samples and is a cofounder of Lion TCR. F.L. has been a consultant for Amgen, Bellicum, Novimmune and Vertex and a speaker for BluebirdBio and Amgen. A.E.J.Y. has been a consultant for Amgen. D.C. receives royalties for patents related to the development of CAR T cell technologies and is a scientific founder and stockholder of Nkarta Therapeutics and Medisix Therapeutics.
Peer review
Peer review information
Nature Medicine thanks Kara Davis, Nitin Jain and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1–11.
Supplementary Data
CARTALL study protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, B.L.Z., Shimasaki, N., Coustan-Smith, E. et al. Fratricide-resistant CD7-CAR T cells in T-ALL. Nat Med 30, 3687–3696 (2024). https://doi.org/10.1038/s41591-024-03228-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03228-8
This article is cited by
-
The quintessential role for CAR T cell therapy in children, adolescents and young adults with cancer
Nature Reviews Clinical Oncology (2026)
-
Glutamine-driven metabolic reprogramming promotes CAR-T cell function through mTOR-SREBP2 mediated HMGCS1 upregulation in ovarian cancer
Journal of Translational Medicine (2025)
-
In vivo CAR-T cell engineering: concept, research progress, potential challenges and enhancement strategies
Experimental Hematology & Oncology (2025)
-
Microenvironmental regulation of solid tumour resistance to CAR T cell therapy
Nature Reviews Immunology (2025)
-
Allogeneic chimeric antigen receptor cell therapies for cancer: progress made and remaining roadblocks
Nature Reviews Clinical Oncology (2025)