Abstract
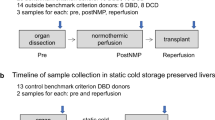
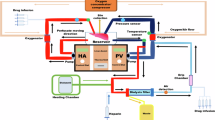
The shortage of suitable donor organs has resulted in the use of suboptimal, high-risk, extended-criteria donor (ECD) livers, which are at an increased risk of failure after transplantation. Compared with traditional static cold storage, dynamic preservation by ex situ machine perfusion reduces the risks associated with the transplantation of ECD organs. Ex situ machine perfusion strategies differ in timing (that is, speed of procurement and transport), perfusion duration and perfusion temperature. For ‘back-to-base’ protocols, the donor liver is statically cold stored during transportation to the recipient hospital (the ‘base’) and then perfused, instead of transporting the liver using a portable perfusion system. While dual hypothermic (8–12 °C) oxygenated machine perfusion (DHOPE) allows safe prolongation of preservation duration and reduces ischemia–reperfusion injury-related complications, including post-transplant cholangiopathy, normothermic machine perfusion (NMP) at 35–37 °C facilitates ex situ viability testing of both liver parenchyma and bile ducts. Here, we describe a clinical protocol for ‘back-to-base’ combined DHOPE and NMP, linked by a period of controlled oxygenated rewarming (COR), which we call the DHOPE–COR–NMP protocol. This protocol enables restoration of mitochondrial function after static ischemic preservation and minimizes both ischemia–reperfusion and temperature-shift-induced injury during the start of NMP. The NMP phase allows viability assessment before final donor liver acceptance for transplantation. Sequential DHOPE and COR–NMP may reduce the risks associated with transplantation of ECD livers and facilitate enhanced utilization, thereby helping to alleviate the organ shortage.
Key points
-
Here, the authors describe a clinical protocol for ‘back-to-base’ combined dual hypothermic oxygenated machine preservation and normothermic machine perfusion, linked by a period of controlled oxygenated rewarming.
-
This protocol minimizes both ischemia–reperfusion and temperature-shift-induced injury during the start of normothermic machine perfusion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 March 2025
In the version of this article initially published, "(ml/min" was missing after "Target flow" in Fig. 2, while in the bottom row of Table 3, the order of values was shown incorrectly, from "–0.3 to –0.5." The figure and table are amended in the HTML and PDF versions of the article.
References
Tullius, S. G. & Rabb, H. Improving the supply and quality of deceased-donor organs for transplantation. N. Engl. J. Med. 379, 693–694 (2018).
Orman, E. S. et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 21, 1040–1050 (2015).
den Dulk, A. C. et al. High peak alanine aminotransferase determines extra risk for nonanastomotic biliary strictures after liver transplantation with donation after circulatory death. Transpl. Int. 28, 492–501 (2015).
Dubbeld, J. et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 97, 744–753 (2010).
O’Neill, S., Roebuck, A., Khoo, E., Wigmore, S. J. & Harrison, E. M. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl. Int. 27, 1159–1174 (2014).
Blok, J. J. et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. 22, 1107–1114 (2016).
Callaghan, C. J. et al. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open 3, e003287 (2013).
Guarrera, J. V. et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am. J. Transpl. 10, 372–381 (2010).
Dutkowski, P. et al. HOPE for human liver grafts obtained from donors after cardiac death. J. Hepatol. 60, 765–772 (2014).
van Rijn, R. et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 104, 907–917 (2017).
Patrono, D. et al. Hypothermic oxygenated machine perfusion for liver transplantation: an initial experience. Exp. Clin. Transplant. 16, 172–176 (2018).
Schlegel, A. et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 60, 103014 (2020).
van Rijn, R. et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N. Engl. J. Med. 384, 1391–1401 (2021).
van Leeuwen, O. B. et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- and normothermic machine perfusion: a prospective clinical trial. Ann. Surg. 270, 906–914 (2019).
Mergental, H. et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 11, 2939 (2020).
Watson, C. J. E. et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 18, 2005–2020 (2018).
Nasralla, D. et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018).
Chapman, W. C. et al. Normothermic machine perfusion of donor livers for transplantation in the United States—a randomized controlled trial. Ann. Surg. 278, e912–e921 (2023).
Markmann, J. F. et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: the OCS liver PROTECT randomized clinical trial. JAMA Surg. 157, 189–198 (2022).
Watson, C. J. E. et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation 101, 1084–1098 (2017).
Schlegel, A., Kron, P., Graf, R., Dutkowski, P. & Clavien, P.-A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 61, 1267–1275 (2014).
Westerkamp, A. C. et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation 100, 825–835 (2016).
Burlage, L. C. et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 19, 538–546 (2017).
Boteon, Y. L. et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl. 24, 1699–1715 (2018).
Minor, T., Efferz, P., Fox, M., Wohlschlaeger, J. & Lüer, B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am. J. Transpl. 13, 1450–1460 (2013).
van Leeuwen, O. B. et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transpl. 22, 1658–1670 (2022).
Hessheimer, A. J. et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 70, 658–665 (2019).
De Carlis, R. et al. How to preserve liver grafts from circulatory death with long warm ischemia? A retrospective italian cohort study with normothermic regional perfusion and hypothermic oxygenated perfusion. Transplantation 105, 2385–2396 (2021).
Sutton, M. E. et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS ONE 9, e110642 (2014).
Op Den Dries, S. et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am. J. Transpl. 13, 1327–1335 (2013).
Matton, A. P. M. et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation 103, 1405–1413 (2019).
van Leeuwen, O. B. et al. Donor hepatectomy time influences ischemia-reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery 168, 160–166 (2020).
Watson, C. J. E. et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transpl. 19, 1745–1758 (2019).
Lantinga, V. A., Buis, C. I., Porte, R. J., de Meijer, V. E. & van Leeuwen, O. B. Reducing cold ischemia time by donor liver ‘back-table’ preparation under continuous oxygenated machine perfusion of the portal vein. Clin. Transpl. 36, e14762 (2022).
Czigany, Z. et al. Hypothermic oxygenated machine perfusion reduces early allograft injury and improves post-transplant outcomes in extended criteria donation liver transplantation from donation after brain death: results from a multicenter randomized controlled trial (HOPE ECD-DBD). Ann. Surg. 274, 705–712 (2021).
Brüggenwirth, I. M. A. et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: a European observational cohort study. Am. J. Transpl. 22, 1842–1851 (2022).
Brüggenwirth, I. M. A. et al. Prolonged hypothermic machine perfusion enables daytime liver transplantation—an IDEAL stage 2 prospective clinical trial. EClinicalMedicine 68, 102411 (2024).
Schlegel, A., de Rougemont, O., Graf, R., Clavien, P.-A. & Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 58, 278–286 (2013).
Brüggenwirth, I. M. A. et al. The importance of adequate oxygenation during hypothermic machine perfusion. JHEP Rep. 3, 100194 (2021).
Rettich, T. R., Handa, Y. P., Battino, R. & Wilhelm, E. Solubility of gases in liquids. 13. High-precision determination of Henry’s constants for methane and ethane in liquid water at 275 to 328 K. J. Phys. Chem. 85, 3230–3237 (1981).
van Leeuwen, O. B., de Vries, Y., de Meijer, V. E. & Porte, R. J. Hypothermic machine perfusion before viability testing of previously discarded human livers. Nat. Commun. 12, 1008 (2021).
De Vries, Y. et al. Transplantation of high-risk donor livers after resuscitation and viability assessment using a combined protocol of oxygenated hypothermic, rewarming and normothermic machine perfusion: study protocol for a prospective, single-arm study (DHOPE–COR–NMP trial). BMJ Open 9, e028596 (2019).
de Vries, Y. et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am. J. Transpl. 19, 1202–1211 (2019).
Brüggenwirth, I. M. A., de Meijer, V. E., Porte, R. J. & Martins, P. N. Viability criteria assessment during liver machine perfusion. Nat. Biotechnol. 38, 1260–1262 (2020).
van Leeuwen, O. B., de Meijer, V. E. & Porte, R. J. Viability criteria for functional assessment of donor livers during normothermic machine perfusion. Liver Transpl. 24, 1333–1335 (2018).
Gaurav, R. et al. Bile biochemistry following liver reperfusion in the recipient and its association with cholangiopathy. Liver Transpl. 26, 1000–1009 (2020).
University Medical Center Groningen. Organ Perfusionist Educational Program. https://onderwijs.umcg.nl/-/educational-program-organ-perfusionist (2025).
Matsushima, H. et al. Too much, too little, or just right? The importance of allograft portal flow in deceased donor liver transplantation. Transplantation 104, 770–778 (2020).
Nair, A. et al. The prognostic utility of intraoperative allograft vascular inflow measurements in donation after circulatory death liver transplantation. Liver Transpl. 28, 65–74 (2021).
van Leeuwen, O. B., Bodewes, S. B., Porte, R. J. & de Meijer, V. E. Excellent long-term outcomes after sequential hypothermic and normothermic machine perfusion challenges the importance of functional donor warm ischemia time in DCD liver transplantation. J. Hepatol. 79, e244–e245 (2023).
Schlegel, A. et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J. Hepatol. 76, 371–382 (2021).
Author information
Authors and Affiliations
Contributions
O.B.v.L., V.A.L., V.E.d.M. and R.J.P. designed the protocol, wrote and critically revised the manuscript. B.L., A.M.T., S.B.B. and M.W.N. designed the protocol and critically revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Damiano Patrono and Renato Romagnoli for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
van Leeuwen, O. et al. Ann. Surg. 270, 906–914 (2019): https://doi.org/10.1097/SLA.0000000000003540
van Leeuwen, O. et al. Am. J. Transplant. 22, 1658–1670 (2022): https://doi.org/10.1111/ajt.17022
Supplementary information
Supplementary Information
Supplementary Material.
Supplementary Video 1
Supplementary Video 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Leeuwen, O.B., Lantinga, V.A., Lascaris, B. et al. ‘Back-to-base’ combined hypothermic and normothermic machine perfusion of human donor livers. Nat Protoc 20, 2151–2170 (2025). https://doi.org/10.1038/s41596-024-01130-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01130-8
This article is cited by
-
The IDEAL framework for machine perfusion in liver transplantation
Nature Reviews Gastroenterology & Hepatology (2025)