Abstract
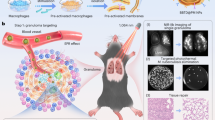
Phototheranostics, which allows simultaneous diagnosis and therapy, offers notable advantages in terms of noninvasiveness, controllability and negligible drug resistance, presenting a promising approach for disease treatment. By integrating second near-infrared (NIR-II, 1,000–1,700 nm) phototheranostic agents characterized by aggregation-induced emission (AIE) and cell membranes with specific targeting capacity, we have developed a versatile type of biomimetic nanoparticle (NP) for precise phototheranostics of pulmonary tuberculosis (TB). Coating the phototheranostic agents with preactivated macrophage membranes results in the formation of biomimetic NPs, which exhibit specific binding to TB through a lesion–pathogen dual-targeting strategy, allowing the accurate detection of Mycobacterium tuberculosis via NIR-II fluorescence imaging and precise photothermal therapy using the irradiation of a 1,064 nm laser. In comparison with traditional treatments, small individual granulomas (0.2 mm in diameter) in TB-infected mice are visualized, and improved antibacterial effects are achieved upon NP administration. Here we present a standardized workflow for the synthesis of the NIR-II AIE agents, their use for the fabrication of the biomimetic NPs and their in vitro and in vivo applications as phototheranostics against M. tuberculosis. The preparation and characterization of the NIR-II AIE agents requires ~8 d, the synthesis and characterization of the phototheranostic NPs requires ~8 d, the validation of in vitro targeting capacity and photothermal eradication requires ~26 d, and the in vivo NIR-II fluorescence imaging and imaging-guided photothermal therapy requires ~74 d. All procedures are straightforward and suitable for clinicians or researchers with prior training in organic synthesis and biomedical engineering.
Key points
-
The procedure covers the synthesis of the photothermal agents, the preactivation of macrophages, membrane extraction and coextrusion to create the final biomimetic nanoparticles, as well as validation of the in vitro and in vivo image-guided photothermal therapy of pulmonary TB.
-
Alternative clinical approaches include chemotherapy, immunotherapy, photodynamic therapy and vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research paper45. The raw datasets are provided in the Source Data file. The online version also contains a Supplementary Information PDF file. All other data are available for research purposes from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Dheda, K., Barry, C. E. & Maartens, G. Tuberculosis. Lancet 387, 1211–1226 (2016).
Pai, M. et al. Tuberculosis. Nat. Rev. Dis. Primers 2, 16076 (2016).
Getahun, H., Matteelli, A., Chaisson Richard, E. & Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 372, 2127–2135 (2015).
Global Tuberculosis Report 2022 https://www.who.int/publications/i/item/9789240061729 (World Health Organization, 2022).
Fenaroli, F. et al. Enhanced permeability and retention-like extravasation of nanoparticles from the vasculature into tuberculosis granulomas in zebrafish and mouse models. ACS Nano 12, 8646–8661 (2018).
Prabhu, P. et al. Mannose-conjugated chitosan nanoparticles for delivery of rifampicin to Osteoarticular tuberculosis. Drug Deliv. Transl. Res. 11, 1509–1519 (2021).
Khawbung, J. L., Nath, D. & Chakraborty, S. Drug resistant tuberculosis: a review. Comp. Immunol. Microb. 74, 101574 (2021).
Hoagland, D. T., Liu, J., Lee, R. B. & Lee, R. E. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 102, 55–72 (2016).
Diacon Andreas, H. et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 371, 723–732 (2014).
Conradie, F. et al. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 382, 893–902 (2020).
Zhang, Z. et al. The fast-growing field of photo-driven theranostics based on aggregation-induced emission. Chem. Soc. Rev. 51, 1983–2030 (2022).
Feng, G., Zhang, G. Q. & Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 49, 8179–8234 (2020).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Hu, W., Prasad, P. N. & Huang, W. Manipulating the dynamics of dark excited states in organic materials for phototheranostics. Acc. Chem. Res. 54, 697–706 (2021).
Yang, Z. & Chen, X. Semiconducting perylene diimide nanostructure: multifunctional phototheranostic nanoplatform. Acc. Chem. Res. 52, 1245–1254 (2019).
Li, H., Kim, Y., Jung, H., Hyun, J. Y. & Shin, I. Near-infrared (NIR) fluorescence-emitting small organic molecules for cancer imaging and therapy. Chem. Soc. Rev. 51, 8957–9008 (2022).
Cheng, P. & Pu, K. Molecular imaging and disease theranostics with renal-clearable optical agents. Nat. Rev. Mater. 6, 1095–1113 (2021).
Zhen, X. et al. Macrotheranostic probe with disease-activated near-infrared fluorescence, photoacoustic, and photothermal signals for imaging-guided therapy. Angew. Chem. Int. Ed. 57, 7804–7808 (2018).
Cai, Y. et al. Organic dye based nanoparticles for cancer phototheranostics. Small 14, 1704247 (2018).
Wang, F., Zhong, Y., Bruns, O., Liang, Y. & Dai, H. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photonics 18, 535–547 (2024).
Wang, F. et al. In vivo non-invasive confocal fluorescence imaging beyond 1,700 nm using superconducting nanowire single-photon detectors. Nat. Nanotechnol. 17, 653–660 (2022).
Chen, Y., Wang, S. & Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 1, 60–78 (2023).
Zhao, H. et al. Near-infrared II fluorescence-guided glioblastoma surgery targeting monocarboxylate transporter 4 combined with photothermal therapy. eBioMedicine 106, 10524 (2024).
Yan, D. et al. An all-rounder for NIR-II phototheranostics: well-tailored 1064 nm-excitable molecule for photothermal combating of orthotopic breast cancer. Angew. Chem. Int. Ed. 63, e202401877 (2024).
Chen, Y., Yang, Y. & Zhang, F. Noninvasive in vivo microscopy of single neutrophils in the mouse brain via NIR-II fluorescent nanomaterials. Nat. Protoc. 19, 2386–2407 (2024).
Caspar, J. V. & Meyer, T. J. Application of the energy gap law to nonradiative, excited-state decay. J. Phys. Chem. 87, 952–957 (1983).
Wei, Y.-C. et al. Overcoming the energy gap law in near-infrared OLEDs by exciton–vibration decoupling. Nat. Photonics 14, 570–577 (2020).
Ding, Q. et al. Diverse interactions between AIEgens and biomolecules/organisms: advancing from strategic design to precision theranostics. Chem 10, 2031–2073 (2024).
Wang, H. et al. Aggregation-induced emission (AIE), life and health. ACS Nano 17, 14347–14405 (2023).
Kenry & Liu, B. Enhancing the theranostic performance of organic photosensitizers with aggregation-induced emission. Acc. Mater. Res. 3, 721–734 (2022).
Kang, M. et al. Aggregation‐enhanced theranostics: AIE sparkles in biomedical field. Aggregate 1, 80–106 (2020).
Mei, J., Leung, N. L., Kwok, R. T., Lam, J. W. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Gui, Y. et al. Thiophene π-bridge manipulation of NIR-II AIEgens for multimodal tumor phototheranostics. Angew. Chem. Int. Ed. 63, e202318609 (2024).
Xu, C. et al. Molecular motion and nonradiative decay: towards efficient photothermal and photoacoustic systems. Angew. Chem. Int. Ed. 61, e202204604 (2022).
Yan, D. et al. Adding flying wings: butterfly-shaped NIR-II AIEgens with multiple molecular rotors for photothermal combating of bacterial biofilms. J. Am. Chem. Soc. 145, 25705–25715 (2023).
Gao, H. et al. Boosting photoacoustic effect via intramolecular motions amplifying thermal-to-acoustic conversion efficiency for adaptive image-guided cancer surgery. Angew. Chem. Int. Ed. 60, 21047–21055 (2021).
Fang, R. H., Kroll, A. V., Gao, W. & Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 30, 1706759 (2018).
Hu, C.-M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Zeng, Z. & Pu, K. Improving cancer immunotherapy by cell membrane-camouflaged nanoparticles. Adv. Funct. Mater. 30, 2004397 (2020).
Li, J. et al. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano 12, 8520–8530 (2018).
Krishnan, N. et al. A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nat. Nanotechnol. 19, 345–353 (2024).
Li, Z., Tang, B. Z. & Wang, D. Bioinspired AIE nanomedicine: a burgeoning technology for fluorescence bioimaging and phototheranostics. Adv. Mater. 36, 2406047 (2024).
Dai, J. et al. Red blood cell membrane-camouflaged nanoparticles loaded with AIEgen and Poly(I : C) for enhanced tumoral photodynamic-immunotherapy. Natl Sci. Rev. 8, nwab03 (2021).
Cui, J. et al. ‘Trojan horse’ phototheranostics: fine-engineering NIR-II AIEgen camouflaged by cancer cell membrane for homologous-targeting multimodal imaging-guided phototherapy. Adv. Mater. 35, 2302639 (2023).
Li, B. et al. Photothermal therapy of tuberculosis using targeting pre-activated macrophage membrane-coated nanoparticles. Nat. Nanotechnol. 19, 834–845 (2024).
Jiang, Z. M. et al. Development of genetically engineered iNKT cells expressing TCRs specific for the M. tuberculosis 38-kDa antigen. J. Transl. Med. 13, 141 (2015).
Shi, C. et al. Lipophilic AIEgens as the ‘trojan horse’ with discrepant efficacy in tracking and treatment of mycobacterial infection. Adv. Healthc. Mater. 13, 2301746 (2023).
Fan, S. et al. Photothermal and host immune activated therapy of cutaneous tuberculosis using macrophage targeted mesoporous polydopamine nanoparticles. Mater. Today Bio 28, 101232 (2024).
Coler, R. N. et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. npj Vaccines 3, 34 (2018).
Wang, H. et al. NIR-II AIE luminogen-based erythrocyte-like nanoparticles with granuloma-targeting and self-oxygenation characteristics for combined phototherapy of tuberculosis. Adv. Mater. 36, 2406143 (2024).
Xu, Z. et al. Nanofiber-mediated sequential photothermal antibacteria and macrophage polarization for healing MRSA-infected diabetic wounds. J. Nanobiotechnol. 19, 404 (2021).
Faa, G., Gerosa, C., Fanni, D., Lachowicz, J. I. & Nurchi, V. M. Gold-old drug with new potentials. Curr. Med. Chem. 25, 75–84 (2018).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Wang, W. et al. Macrophage-derived biomimetic nanoparticles for light-driven theranostics toward Mpox. Matter 7, 1187–1206 (2024).
Gawne, P. J., Ferreira, M., Papaluca, M., Grimm, J. & Decuzzi, P. New opportunities and old challenges in the clinical translation of nanotheranostics. Nat. Rev. Mater. 8, 783–798 (2023).
Okuda, Y., Lakshmikantham, M. V. & Cava, M. P. A new route to 1,3-disubstituted benzo[c]thiophenes. J. Org. Chem. 56, 6024–6026 (1991).
Yan, D. et al. Multimodal imaging-guided photothermal immunotherapy based on a versatile NIR-II aggregation-induced emission luminogen. Angew. Chem. Int. Ed. 61, e202202614 (2022).
Wang, M. et al. A versatile 980 nm absorbing aggregation-induced emission luminogen for NIR-II imaging-guided synergistic photo-immunotherapy against advanced pancreatic cancer. Adv. Funct. Mater. 32, 2205371 (2022).
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (52122317, 22175120, 22307080), the Developmental Fund for Science and Technology of Shenzhen Government (RCYX20200714114525101), The Shenzhen Science and Technology Program (RCBS20221008093224016, JCYJ20220531101201003, 20220809130438001), the Guangdong Basic and Applied Basic Research Fund (2023A1515010558), the Pearl River Talent Recruitment Program (2019QN01Y103), the Research Team Cultivation Program of Shenzhen University (2023QNT003), the Medical-Engineering Interdisciplinary Research Foundation of Shenzhen University (2023YG021) and the Medicine Plus Program of Shenzhen University (2024YG004). The authors also acknowledge the Instrumental Analysis Center of Shenzhen University.
Author information
Authors and Affiliations
Contributions
D.Y., D.W. and B.Z.T. conceived and initiated the project. D.Y., H.W. and B.L. contributed to the experimental work displayed in this protocol. D.Y., X.L., H.W., Y.L., W.W. and D.W. wrote the protocol. D.W. and B.Z.T. supervised the study and the manuscript preparation. All authors reviewed and edited the manuscript and approved the final draft. Lab links: https://www.kmmc.cn/Pages_2592_53259.aspx; https://tangbenz.people.ust.hk/; https://cmse.szu.edu.cn/szdw1/jsml/gfzclygc/j_s/wd.htm.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Kanyi Pu and Fan Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Li, B. et al. Nat. Nanotechnol. 19, 834–845 (2024): https://doi.org/10.1038/s41565-024-01618-0
Wang, W. et al. Matter 7, 1187–1206 (2024): https://doi.org/10.1016/j.matt.2024.01.004
Li, B. et al. Adv. Sci. 8, 2003556 (2021): https://doi.org/10.1002/advs.202003556
Wang, M. et al. Adv. Funct. Mater. 32, 2205371 (2022): https://doi.org/10.1002/adfm.202205371
Yan, D. et al. Angew. Chem. Int. Ed. 60, 26769–26776 (2021): https://doi.org/10.1002/anie.202111767
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and procedures for characterizing the intermediates and TPE-BT-BBTD, NIR-II imaging and biotoxicity evaluation.
Source data
Source Data Figs. 4 and 5
Statistical Source Data.
Source Data Fig. 4
Parent Figure.
Source Data Supplementary Fig. 11
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, D., Li, X., Wang, H. et al. NIR-II aggregation-induced emission nanoparticles camouflaged with preactivated macrophage membranes for phototheranostics of pulmonary tuberculosis. Nat Protoc 20, 2560–2585 (2025). https://doi.org/10.1038/s41596-025-01146-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-025-01146-8