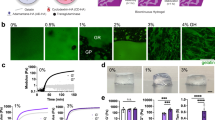
Abstract
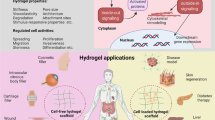
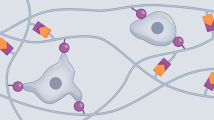
Three-dimensional (3D) cell culture models based on hydrogels are rapidly evolving into a prominent tool for tissue engineering, mechanobiology, disease modeling and drug screening. While a vast variety of synthetic gels have emerged in recent years, they fail to penetrate the market substantially for two major reasons: they poorly mimic the extracellular matrix or they are difficult to use in gel formation and cell extraction. Mimicking the complexity of nature is challenging: the extracellular matrix plays a crucial role in cell development and function, which goes well beyond simple mechanical support. Recently, we introduced polyisocyanide (PIC) hydrogels for 3D cell culture applications. The fibrous architecture and associated (non)linear mechanical behavior closely mimic the physical properties of biogels such as collagen and fibrin. As fully synthetic materials, PIC gels benefit from high tailorability and reproducibility. Moreover, the thermoresponsive properties of PIC gels make them easy to handle in the lab; the gels form instantly at 37 °C and cells are easily extracted after cooling to 5 °C. The potential of PIC gels has been demonstrated in a quickly expanding library of papers discussing different cell lines, primary cells and organoids, as well as in vivo experiments. This manuscript provides protocols on how to handle PIC gels in the chemistry and cell biology laboratories. Material preparation requires 72 h. Cell encapsulation takes 1 h and the time for downstream analysis depends on the (commercial) methods used. The protocols described are suitable for researchers with expertise in cell culture and molecular biology.
Key points
-
Protocol describing the use of polyisocyanide (PIC) as a model matrix for 3D cell culture. PIC hydrogels closely mimic the physical properties of biogels such as collagen and fibrin and, as fully synthetic materials, benefit from a high reproducibility.
-
The linear and nonlinear mechanics of PIC hydrogel can be controlled by tuning the polymer length or concentration, and the polymer can be biofunctionalized through azide-based click chemistry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sun, M. et al. 3D Cell culture—can it be as popular as 2D cell culture? Adv. NanoBiomed. Res. 1, 2000066 (2021).
Shamir, E. R. & Ewald, A. J. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15, 647–664 (2014).
FDA no longer needs to require animal tests before human drug trials. ScienceInsider https://doi.org/10.1126/science.adg6264 (2023).
3D Cell Culture Market (Reports and Data, 2022).
Kozlowski, M. T., Crook, C. J. & Ku, H. T. Towards organoid culture without Matrigel. Commun. Biol. 4, 1387 (2021).
Chen, Z., Sugimura, R., Zhang, Y. S., Ruan, C. & Wen, C. Organoids in concert: engineering in vitro models toward enhanced fidelity. Aggregate 5, e478 (2024).
Gan, Z., Qin, X., Liu, H., Liu, J. & Qin, J. Recent advances in defined hydrogels in organoid research. Bioact. Mater. 28, 386–401 (2023).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Liu, K., Wiendels, M., Yuan, H., Ruan, C. & Kouwer, P. Cell–matrix reciprocity in 3D culture models with nonlinear elasticity. Bioact. Mater. 9, 316–331 (2022).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Piechocka, I. K., Bacabac, R. G., Potters, M., MacKintosh, F. C. & Koenderink, G. H. Structural hierarchy governs fibrin gel mechanics. Biophys. J. 98, 2281–2289 (2010).
Kouwer, P. H. J. et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 493, 651–655 (2013).
Liu, K., Mihaila, S. M., Rowan, A., Oosterwijk, E. & Kouwer, P. H. J. Synthetic extracellular matrices with nonlinear elasticity regulate cellular organization. Biomacromolecules 20, 826–834 (2019).
Das, R. K., Gocheva, V., Hammink, R., Zouani, O. F. & Rowan, A. E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 15, 318–325 (2016).
Liu, K. et al. Synthetic extracellular matrices as a toolbox to tune stem cell secretome. ACS Appl. Mater. Interfaces 12, 56723–56730 (2020).
Yuan, H. et al. Synthetic fibrous hydrogels as a platform to decipher cell–matrix mechanical interactions. Proc. Natl. Acad. Sci. USA 120, e2216934120 (2023).
Zhang, Y. et al. Polyisocyanide hydrogels as a tunable platform for mammary gland organoid formation. Adv. Sci. 7, 2001797 (2020).
Schaafsma, P., Kracht, L., Baanstra, M., Jellema-de Bruin, A. L. & Coppes, R. P. Role of immediate early genes in the development of salivary gland organoids in polyisocyanopeptide hydrogels. Front. Mol. Biosci. 10, 1100541 (2023).
Liu, K. et al. Structure and applications of PIC-based polymers and hydrogels. Cell Rep. Phys. Sci. 5, 101834 (2024).
Zimoch, J. et al. Polyisocyanopeptide hydrogels: a novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 70, 129–139 (2018).
Van Velthoven, M. J. J. et al. Potential of estradiol‐functionalized polyisocyanide hydrogels for stimulating tissue regeneration of the pelvic floor. Adv. Ther. 7, 2300199 (2024).
Kumari, J., Wagener, F. A. D. T. G. & Kouwer, P. H. J. Novel synthetic polymer-based 3D contraction assay: a versatile preclinical research platform for fibrosis. ACS Appl. Mater. Interfaces 14, 19212–19225 (2022).
Kumari, J., Hammink, R., Baaij, J., Wagener, F. A. D. T. G. & Kouwer, P. H. J. Antifibrotic properties of hyaluronic acid crosslinked polyisocyanide hydrogels. Biomater. Adv. 156, 213705 (2024).
Van Velthoven, M. J. J. et al. An improved understanding of the pathophysiology of pelvic organ prolapse: a 3D in vitro model under static and mechanical loading conditions. Adv. Healthc. Mater. 13, 2302905 (2024).
Sun, R. et al. Microenvironment with NIR-controlled ROS and mechanical tensions for manipulating cell activities in wound healing. Nano Lett. 24, 3257–3266 (2024).
Zhang, Y. et al. Tunable hybrid matrices drive epithelial morphogenesis and YAP translocation. Adv. Sci. 8, 2003380 (2021).
Ma, C. et al. Synthetic extracellular matrices for 3D culture of Schwann cells, hepatocytes, and HUVECs. Bioengineering 9, 453 (2022).
Weiden, J. et al. Injectable biomimetic hydrogels as tools for efficient T cell expansion and delivery. Front. Immunol. 9, 2798 (2018).
Tiemeijer, B. M. et al. Probing single-cell macrophage polarization and heterogeneity using thermo-reversible hydrogels in droplet-based microfluidics. Front. Bioeng. Biotechnol. 9, 715408 (2021).
Vandaele, J. et al. Structural characterization of fibrous synthetic hydrogels using fluorescence microscopy. Soft Matter 16, 4210–4219 (2020).
Liu, Z. et al. Polyisocyanide hydrogels with tunable nonlinear elasticity mediate liver carcinoma cell functional response. Acta Biomater. 148, 152–162 (2022).
Cao, Z. et al. The preparation of biomineralized PIC/HA hybrid composites with strain‐stiffening and the effect on MC3T3‐E1 cells. Macromol. Rapid Commun. 43, 2200135 (2022).
Ye, S. et al. A chemically defined hydrogel for human liver organoid culture. Adv. Funct. Mater. 30, 2000893 (2020).
Jansen, K. A., Bacabac, R. G., Piechocka, I. K. & Koenderink, G. H. Cells actively stiffen fibrin networks by generating contractile stress. Biophys. J. 105, 2240–2251 (2013).
Kumari, J. et al. A Novel in vitro disease model for systemic sclerosis using polyisocyanide hydrogels. Adv. Ther. 6, 2200180 (2023).
Op ‘T Veld, R. C. et al. Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials 181, 392–401 (2018).
Yegappan, R. et al. Snake venom hydrogels as a rapid hemostatic agent for uncontrolled bleeding. Adv. Healthc. Mater. 11, 2200574 (2022).
Wang, B. et al. A tunable and injectable local drug delivery system for personalized periodontal application. J. Control. Release 324, 134–145 (2020).
Lou, J. & Mooney, D. J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 6, 726–744 (2022).
Kratochvil, M. J. et al. Engineered materials for organoid systems. Nat. Rev. Mater. 4, 606–622 (2019).
Koepf, M. et al. Preparation and characterization of non-linear poly(ethylene glycol) analogs from oligo(ethylene glycol) functionalized polyisocyanopeptides. Eur. Polym. J. 49, 1510–1522 (2013).
Jaspers, M. et al. Ultra-responsive soft matter from strain-stiffening hydrogels. Nat. Commun. 5, 5808 (2014).
Zhang, Z., Chen, W., Tiemessen, D. M., Oosterwijk, E. & Kouwer, P. H. J. A. Temperature‐based easy‐separable (TempEasy) 3D hydrogel coculture system. Adv. Healthc. Mater. 11, 2102389 (2022).
Jaspers, M., Rowan, A. E. & Kouwer, P. H. J. Tuning hydrogel mechanics using the hofmeister effect. Adv. Funct. Mater. 25, 6503–6510 (2015).
Kouwer, P. H. J. et al. Controlling the gelation temperature of biomimetic polyisocyanides. Chin. Chem. Lett. 29, 281–284 (2018).
Van Velthoven, M. J. J. et al. Growth factor immobilization to synthetic hydrogels: bioactive bFGF‐functionalized polyisocyanide hydrogels. Adv. Healthc. Mater. 12, 2301109 (2023).
Mandal, S. et al. Therapeutic nanoworms: towards novel synthetic dendritic cells for immunotherapy. Chem. Sci. 4, 4168 (2013).
Voerman, D. et al. Synthetic semiflexible and bioactive brushes. Biomacromolecules 20, 2587–2597 (2019).
Op’T Veld, R. et al. Monitoring 111 In-labelled polyisocyanopeptide (PIC) hydrogel wound dressings in full-thickness wounds. Biomater. Sci. 7, 3041–3050 (2019).
Plow, E. F., Haas, T. A., Zhang, L., Loftus, J. & Smith, J. W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 (2000).
Ligorio, C. & Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nat. Rev. Bioeng. 1, 518–536 (2023).
Schoenmakers, D. C., Rowan, A. E. & Kouwer, P. H. J. Crosslinking of fibrous hydrogels. Nat. Commun. 9, 2172 (2018).
Bruekers, S. M. C. et al. Fibrin-fiber architecture influences cell spreading and differentiation. Cell Adhes. Migr. 10, 495–504 (2016).
Jaspers, M. et al. Nonlinear mechanics of hybrid polymer networks that mimic the complex mechanical environment of cells. Nat. Commun. 8, 15478 (2017).
Kumari, J. et al. Conductive polyisocyanide hydrogels inhibit fibrosis and promote myogenesis. ACS Appl. Bio. Mater. 7, 3258–3270 (2024).
Chen, W. & Kouwer, P. H. J. Combining mechanical tuneability with function: biomimetic fibrous hydrogels with nanoparticle crosslinkers. Adv. Funct. Mater. 31, 2105713 (2021).
Chen, W., Kumari, J., Yuan, H., Yang, F. & Kouwer, P. H. J. Toward tissue‐like material properties: inducing in situ adaptive behavior in fibrous hydrogels. Adv. Mater. 34, 2202057 (2022).
Chen, W., Zhang, Y., Kumari, J., Engelkamp, H. & Kouwer, P. H. J. Magnetic stiffening in 3D cell culture matrices. Nano Lett. 21, 6740–6747 (2021).
Chen, W., Zhang, Z. & Kouwer, P. H. J. Magnetically driven hierarchical alignment in biomimetic fibrous hydrogels. Small 18, 2203033 (2022).
Gudde, A. N. et al. Vaginal fibroblast behavior as a function of stiffness changes in a polyisocyanide hydrogel for prolapse repair. ACS Appl. Bio. Mater. 6, 3759–3767 (2023).
Gudde, A. N., Van Velthoven, M. J. J., Roovers, J.-P. W. R., Kouwer, P. H. J. & Guler, Z. Polyisocyanides as a substrate to trigger vaginal fibroblast functioning in an in vitro model for prolapse repair. Biomater. Adv. 141, 213104 (2022).
Wang, Z. et al. Chemically defined organoid culture system for cholangiocyte differentiation. Adv. Healthc. Mater. 13, 2401511 (2024).
Nguyen-Ngoc, K.-V. et al. in Tissue Morphogenesis Vol. 1189 (ed. Nelson, C. M.) 135–162 (Springer, 2015).
Zhang, Z. et al. TempEasy 3D hydrogel coculture system provides mechanistic insights into prostate cancer bone metastasis. ACS Appl. Mater. Interfaces 16, 25773–25787 (2024).
Acknowledgements
We sincerely mourn the recent loss of R.J.M. Nolte and express our heartfelt gratitude for his instrumental contributions to the field of PIC polymers. We thank A. E. Rowan and all others who contributed to the development of PIC hydrogels, and W. Bonger for repeating the rheology experiments. In addition, the authors gratefully acknowledge the funding support from the National Key Research and Development Program of China (2023YFF1205500, H.Y.), the National Natural Science Foundation of China (32471368, H.Y; 32201097, K.L.), the Excellent Young Scientist Fund of the Natural Science Foundation of Hebei Province (B2022202027, H.Y.), the Shenzhen International Science and Technology Cooperation Project (grant no. GJHZ20240218112602004, K.L.), the Mainland–Hong Kong Joint Funding Scheme (grant no. 2023YFE0210500, K.L.), the Fonds Wetenschappelijk Onderzoek (12A2423N, H.Y.) and the funding from Dutch Organization for knowledge and innovation in health, healthcare and well-being (ZonMw, 01142052310003, TOP grant 91218030, P.H.J.K.), the Dutch Research Council (NWO, Demonstrator 19358, Take Off I 20941, P.H.J.K.). Figures were created with support of BioRender.
Author information
Authors and Affiliations
Contributions
All authors contributed to developing and writing the protocols in this manuscript. H.Y. and K.L. initiated the project, and drafted and edited the manuscript. P.H.J.K. supervised the project and edited the manuscript. H.Y. and K.L. contributed equally to this manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.Y., K.L. and P.H.J.K. have commercialized PIC polymers, which are available at SBMatrices B.V. (worldwide, under the commercial name Fybrix,) and at GTI Shenzhen Ltd. (in China). The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Kouwer, P. H. J. et al. Nature 493, 651–655 (2013): https://doi.org/10.1038/nature11839
Yuan, H. et al. Proc. Natl Acad. Sci. USA 120, e2216934120 (2023): https://doi.org/10.1073/pnas.2216934120
Van Velthoven, M. J. J. et al. Adv. Healthc. Mater. 12, 2301109 (2023): https://doi.org/10.1002/adhm.202301109
Zhang, Y. et al. Adv. Sci. 7, 2001797 (2020): https://doi.org/10.1002/advs.202001797
Liu, K. et al. Biomacromolecules 20, 826–834 (2019): https://doi.org/10.1021/acs.biomac.8b01445
Supplementary information
Supplementary Video 1
Overview of PIC preparation and cell encapsulation.
Supplementary Video 2
Overview of the procedure of fluorescence labeling PIC, matrix and cell encapsulation.
Supplementary Video 3
Overview of the cell retrieval procedure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, H., Liu, K., van Velthoven, M.J.J. et al. Fibrous polyisocyanide hydrogels for 3D cell culture applications. Nat Protoc 20, 3339–3360 (2025). https://doi.org/10.1038/s41596-025-01159-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-025-01159-3