Abstract
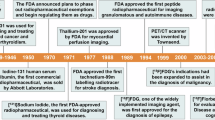
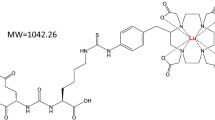
Theranostics utilizes tandem targeted diagnostic and therapeutic agents that are molecularly analogous. In a theranostic approach, the diagnostic agent is a tracer typically radiolabeled with a positron emission tomography radionuclide such as fluorine-18 or gallium-68. Utilizing the selectivity of the tracer, the therapeutic agent is subsequently radiolabeled with an ablative radionuclide such as the β− emitting lanthanide lutetium-177 (177Lu). 177Lu is typically incorporated into theranostics using the chelators 2,2′,2′′,2′′′-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA) and 2-(4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl)pentanedioic acid (DOTAGA) that are used to prepare the 177Lu-radiopharmaceutical [177Lu]Lu-DOTA-TATE, [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T. Here we describe the scalable and validated production for these 177Lu-radiopharmaceuticals and further include the necessary quality control protocols. The procedures can be generalized and support both carrier added and noncarrier added 177Lu sources for use in a clinical setting. With robust procedures that accommodate 177Lu activity levels from 5 to 100 GBq, the procedures ensure stability for up to 8 h postproduction and achieve an average activity yield of 98%. As proven in over 1,000 patient cycles, this methodology is adaptable to both centralized production facilities and regional centers, enabling versatile application across small and large-scale production settings.
Key points
-
The procedure outlines a method for labeling DOTA- and DOTAGA-modified peptides with a scalable range of [177Lu]LuCl3 to create theranostics that target cancer-specific biomarkers, including prostate-specific antigen. The procedure also includes validated quality control procedures for the radiopharmaceuticals to ensure they meet clinical-grade standards before administration.
-
These radiotheranostic agents can be used to treat patients with neuroendocrine and prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hofman, M. S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021).
Hofman, M. S. P. et al. Prostate-specific membrane antigen PET-CT imaging in patients with high risk prostate cancer before curative-intent surgery of radiotherapy (proPSMA): a prospective, randomised, multi-centre study. Lancet 395, 1208–1216 (2020).
Hofman, M. S. P. et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 19, 825–833 (2018).
Strosberg, J. et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Sartor, O. et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Hofman, M. S. et al. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 124, 5–13 (2019).
Schmitl, S. et al. Quality assurance investigations and impurity characterization during upscaling of [177Lu]Lu-PSMAI&T. Molecules 28, 7696 (2023).
Kraihammer, M. et al. Improved quality control of [177Lu]Lu-PSMA I&T. EJNMMI Radiopharm. Chem 8, 7 (2023).
Hooijman, E. L. et al. Radiolabeling and quality control of therapeutic radiopharmaceuticals: optimization, clinical implementation and comparison of radio-TLC/HPLC analysis, demonstrated by [177Lu]Lu-PSMA. EJNMMI Radiopharm. Chem. 7, 29 (2022).
Di Iorio, V. et al. Production and quality control of [177Lu]Lu-PSMA-I&T: development of an investigational medicinal product dossier for clinical trials. Molecules 27, 4143 (2022).
Wieczorek Villas Boas, C. A., Pereira Dias, L. A., Nakamura Matsuda, M. M. & Bortoleti De Araújo, E. Stability in the production and transport of 177Lu labelled PSMA. Braz. J. Radiat. Sci. https://doi.org/10.15392/bjrs.v9i1.1619 (2021).
Martin, S. et al. Identification, characterization, and suppression of side products formed during the synthesis of [177Lu]Lu-PSMA-617. J. Med. Chem. 64, 4960–4971 (2021).
Sørensen, M. A. et al. Automated synthesis of 68Ga/177Lu-PSMA on the Trasis miniAllinOne. J. Label. Comp. Radiopharm. 63, 393–403 (2020).
Das, T. et al. Clinical translation of 177Lu-labeled PSMA-617: initial experience in prostate cancer patients. Nucl. Med. Biol. 43, 296–302 (2016).
Weineisen, M. et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med 56, 1169–1176 (2015).
Mukherjee, A. et al. Single vial kit formulation of DOTATATE for preparation of 177Lu-labeled therapeutic radiopharmaceutical at hospital radiopharmacy. J. Label. Comp. Radiopharm. 58, 166–172 (2015).
Aslani, A. et al. Lutetium-177 DOTATATE production with an automated radiopharmaceutical synthesis system. Asia Ocean J. Nucl. Med. Biol. 3, 107–115 (2015).
Kostos, L. K. et al. AlphaBet: a phase I/II trial evaluating the combination of radium-223 and [177Lu]Lu-PSMA-I&T in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 41, TPS280-TPS280 (2023).
Kostos, L. K. et al. LuCAB: a phase I/II trial evaluating cabazitaxel in combination with [177Lu]Lu-PSMA-617 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 41, TPS278-TPS278 (2023).
Banerjee, S., Pillai, M. R. A. & Knapp, F. F. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem. Rev. 115, 2934–2974 (2015).
Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Parus, J. L., Pawlak, D., Mikolajczak, R. & Duatti, A. Chemistry and bifunctional chelating agents for binding 177Lu. Curr. Radiopharm. 8, 86–94 (2015).
Dvorakova, Z., Henkelmann, R., Lin, X., Türler, A. & Gerstenberg, H. Production of 177Lu at the new research reactor FRM-II: irradiation yield of 176Lu(n,γ)177Lu. Appl. Radiat. Isot. 66, 147–151 (2008).
Horwitz, E. P., McAlister, D. R., Bond, A. H., Barrans, R. E. & Williamson, J. M. A process for the separation of 177Lu from neutron irradiated 176Yb targets. Appl. Radiat. Isot. 63, 23–36 (2005).
Acknowledgements
The authors acknowledge S. Poniger, the Co-Founder and Chief Engineer at iPHASE technologies, for his assistance and support while establishing this protocol. M.S.H. acknowledges philanthropic/government grant support from the Prostate Cancer Foundation including CANICA Oslo Norway, Peter MacCallum Foundation and a NHMRC Investigator Grant.
Author information
Authors and Affiliations
Contributions
M.B.H. and P.D.R. designed and carried out the technical development of this protocol related to the production of theranostics. M.B.H., S.K. and W.N. designed and carried out the QC aspects of this protocol. W.W.H., M.L. U.K., B.E. and M.S.H. assisted with the implementation and write up of this protocol. M.S.H. leads the clinical work using theranostics produced by this protocol.
Corresponding author
Ethics declarations
Competing interests
M.S.F. reports research grant support (to Peter MacCallum Cancer Centre) from Novartis (including AAA and Endocyte), ANSTO, Bayer, Isotopia and MIM. Consulting fees for lectures or advisory boards are from Astellas, AstraZeneca and MSD in the last 2 years and Janssen and Mundipharma in the last 5 years. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Robert Ta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Hofman, S. M. et al. Lancet 397, 797 (2021): https://doi.org/10.1016/S0140-6736(21)00237-3
Azad, A. A. et al. Lancet Oncol. 25, 1267 (2024): https://doi.org/10.1016/S1470-2045(24)00440-6
Hofman, S. M. et al. Lancet Oncol. 25, 99 (2024): https://doi.org/10.1016/S1470-2045(23)00529-6
Kostos, L. et al. Front. Med. 9, 1059122 (2022): https://doi.org/10.3389/fmed.2022.1059122
Eapen, R. S. et al. Eur. Urol. 85, 227 (2024): https://doi.org/10.1016/j.eururo.2023.08.026
Supplementary information
Supplementary Information
Supplementary methods: HPLC and TLC methods and representative chromatograms. Supplementary tutorial: precursor preparation and calculations. Automation Recipe: used in the protocol for preparation of the Lu-radiopharmaceuticals using the iPHASE MultiSyn radiosynthesizer.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hunt, W.W., Long, M., Kamil, U. et al. A scalable protocol for the radiosynthesis of clinical grade lutetium-177-labeled theranostic agents. Nat Protoc 21, 60–78 (2026). https://doi.org/10.1038/s41596-025-01176-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-025-01176-2
This article is cited by
-
Multifactorial analysis of radiochemical purity in high-activity 177Lu-labeled theranostics: impact of precursor source, 177Lu form, and production parameters
EJNMMI Radiopharmacy and Chemistry (2025)
-
Optimization of the radiosynthesis of the PSMA-targeting drugs [177Lu]Lu-P17-087 and [177Lu]Lu-P17-088 on the MiniLu™ automated module
EJNMMI Radiopharmacy and Chemistry (2025)