Abstract
Bismuth titanate Bi4Ti3O12 (BiT), was one of the most promising lead-free high-temperature piezoelectric materials, due to high Curie temperature (675 °C) and large spontaneous polarization (50 µC/cm2); however, extensive studies had revealed that high leakage conductivity interferes with the poling process, hindering its practical applications. In this paper, an electrically insulating property was achieved by a low level Nb donor substitution to suppress a high level of holes associated with high oxygen vacancy concentration. Bi4Ti2.97Nb0.03O12 ceramic showed significant enhancements of electrical resistivity by more than three order of magnitude and activity energy with value >1.2 eV, which are significant for piezoelectric applications of BiT-based materials. However, pure and A2O3-excess (A = Bi, La and Nd; 3 at %) BiT ceramics, were mixed hole and oxygen ion conductors. Schottky barriers were both formed at grain boundary region and the sample-electrode interface, because of the existence of semiconducting bulk. Interestingly, the electron conduction could be suppressed in N2, as a consequence, they became oxide ion conductors with conductivity of about 4 × 10−4 S cm−1 at 600 °C.
Similar content being viewed by others
Introduction
Bismuth titanate Bi4Ti3O12 (BiT), has been widely studied as one of most important bismuth-layer-structured ferroelectrics (BLSFs), which consists of three-layer pseudo-perovskite (Bi2Ti2O10)2− units sandwiched between (Bi2O2)2+ layers along the c axis1,2,3,4,5. BiT shows a high Curie point (T c) of 675 °C and a large spontaneous polarization (P s) of about 50 µC/cm2 6, 7. It is well known that Bi2O3 vaporization with generations of VBi′′′ (bismuth vacancy) and VO •• (oxygen vacancy) is a severe problem in the preparation of BiT-based materials, which largely affect electrical properties of them. High leakage conductivity closely related to the existence of VO •• 8,9,10, would interfere with the poling process by an applied field. This, combined with a pinning effect on domain walls by point defects or defect dipoles [e.g., (VBi′′′-VO ••)′]11,12,13,14,15, results in a low remnant polarization (P r < 8 μC/cm2) for BiT thin films and ceramics16,17,18,19,20. Furthermore, a low poling field (<10 kV/cm) leads to a low piezoelectric activity (d 33 ≤ 8 pC/N), which has been an obstacle for piezoelectric applications of BiT-based materials4,5,6. Great efforts have been made to solve such problems in BiT materials as mentioned above. Isovalent substitution for Bi3+ by lanthanoid cations (La3+, Pr3+, Nd3+, etc.) at the perovskite A site has been found to be an effective way to reduce VO •• and VBi′′′ concentrations in the perovskite layers3, 21,22,23. On the other hand, B-site donor substitution such as W6+, Nb5+ or Ta5+, can significantly decrease the bulk conductivity and enhance ferroelectric and piezoelectric properties of the samples11, 12, 24,25,26,27. Also, excess raw oxides (e.g., Bi2O3 and CeO2) have been recommended by compensating the Bi2O3 vaporization28,29,30.
It is distinct that a problem that must be addressed in the development of BiT-based sensors is the origin of high leakage conductivity of materials. Takahashi et al. revealed that BiT single crystals were oxide ionic and p-type mixed conductors31. They suggested that VO •• and VBi′′′ preferentially existed in the perovskite layers, which could be effectively suppressed by the A-site La or the B-site donor substitution; thus the decreases in electronic and ionic conductivities were achieved, along the a(b) axis in BiT single crystals31,32,33. But for ceramics, the grain boundary is likely to act as a source or sink for defects (oxygen vacancies)34,35,36,37,38. Consequently the ceramics other than single crystals, are often electrically heterogeneous. BiT ceramics also showed oxide ionic and p-type mixed conduction, where the electrical properties of the boundaries were pronounced39, 40.
Impedance spectroscopy has been widely used to investigate AC conduction behaviors of crystalline, polycrystalline and amorphous materials41. Commonly, electrode, grain boundary and grain components together contribute to the conductions of polycrystalline materials41,42,43. In this paper, impedance data of BiT-based ceramics including BiT, BiT-A (3 at.% excess A2O3; A = Bi, La and Nd), BLT (Bi3.25La0.75Ti3O12) and BiT-Nb (Bi4Ti2.97Nb0.03O12), were studied systematically, to investigate the effects of different modifications on heterogeneous structure and electrical conductivity. Also, electrical conduction behaviors of them as functions of temperature and atmosphere were elaborated.
Results
Structure and compositional analysis
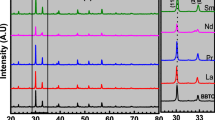
Powder diffraction refinement with a Le-Bail fit (GSAS-EXPGUI program)44, 45 was carried out to characterize crystal structures of the prepared oxides, as shown in Fig. 1. The orthorhombic Aba2 (ICSD #240210) transformed from B2cb (Aba2: abc = B2cb: b′c′a′) was used as an initial structural model. For each composition, the calculated data well agrees with the experimental ones, and the reliability factors (R wp, R p and reduced χ2; Table 1) are reasonable. However, the refined results reveal small amounts of Bi2O3 in BiT-A (e.g., BiT-Bi and BiT-La), corresponding to the small diffraction peak at 2θ = ∼28° (insets of Fig. 1b and c). In addition, the (0l0) diffraction peaks in BiT, BiT-Bi and BiT-La, e.g., (060), (080) and (0140), are abnormally intensive, while the major peak (171) is severely supressed (Fig. 1a–c). Absolutely, they were highly textured in the c axis direction. However, the texture is not pronounced in BiT-Nb (Fig. 1d). The degree of texture can be expressed by using Lotgering orientation factor (LOF), f 46. For (0l0) preferred orientation, f is defined as following equations:
where \(P=\sum {I}_{(0l0)}/\sum {I}_{(hkl)}\)(target sample) and \(P=\sum {I}_{(0l0)}/\sum {I}_{(hkl)}\) (standard random sample, PDF#35–0795). For BiT and BiT-A, highly c-oriented structure results in high f value, being around 0.9, much higher than that for BiT-Nb (0.54). Table 1 lists refined lattice parameters of BiT, BiT-A and BiT-Nb. BiT-Nb shows larger lattice parameters (a, b and c) and unit cell volume (V) than BiT, which should be the results of incorporation of Nb5+ with larger size at B site (Nb5+: N0.64 Å, Ti4+: 0.605 Å; 6 CN)47. BiT-Bi and BiT-La show increased V, however, this value for BiT-Nd decreases.
As indicated by surface SEM (scanning electron microscope) images (Fig. S1a–c; Supplementary Information), the great majority of grains in BiT-A are overlarge and laminated. The aspect ratio L/t (length/thickness) of the laminated grains ranges from 10 to 30. Probably, that stacking and laminating a few such grains leads to high degree of texture in them. In contrast, BiT-Nb shows size-smaller grains, which is consistent with the results of the Ta/Nb substituted BiT ceramics25. And no pores are found within grains, which are visible in BiT-A (cross-section SEM images; insets of Fig. S1a–d, Supplementary Information). It is presumed that the incorporation of Nb5+ into the lattices leads to a lower grain-growth rate in the a-b plane due to low VO •• concentration ([VO ••]) in the perovskite layers. Figure 2a and b show microstructure of the grain boundary region of the polished BiT-Bi and BiT-La samples, respectively. It is clear that heterogeneous structure is observed at the grain boundaries. As indicated by EDS (energy-dispersive x-ray spectroscopy) line scans, BiT-Bi and BiT-La show no obvious variation in Bi and Ti intensities along the line 1 → 3, whereas a composition deviation with respect to oxygen is observed at the position 2 of grain boundary (Fig. 2c and d). This position may correspond to the grain-boundary surface layer, which is possibly created by the accumulation of space charges. In addition, grain composition for them is analyzed by EDS surface scans of five entire grains, and the average atomic ratios are normalized and listed in Table 2. The measured compositions of BiT-Bi and BiT-La are close to the theoretical ratios in BiT within instrument resolution and standard deviations. However, stoichiometric composition shows somewhat deviation at the position 2 of grain boundary, where the O concentration and the ratio of 2[O]/(3[Bi] + 4[Ti] + 3[La]) are both higher than those in grain (about 63% and 1, respectively). The experiments also reveal about 0.55 at. % La in the bulk phase, close to nominally excess level (0.62 at. %). Presumably, these nonstoichiometric compositions at the grain boundary regions are not associated with Bi2O3 or La2O3. TEM (Transmission electron microscopy) image reveals an evidence of some Bi-rich regions at the triple points in BiT-Bi, as shown in Fig. 3a. The relevant EDS line scan reveals the absence of element Ti in Bi-rich phase (Fig. 3b), which is likely ascribed to be Bi2O3.
Impedance spectra and component response
Figure 4 demonstrates variable-temperature impedance diagrams of the air-, O2- and N2-processed BiT-Bi samples. For the three samples, two complete arcs at high and medium frequencies and, an incomplete one at low frequencies (0.1–1 Hz) due to the limited measurement range of the instrument, are observed in the 250 °C diagrams (Fig. 4a). The 250 °C capacitance (C′) plots of BiT-Bi show a plateau with capacitance values of ∼5 nF/cm at low frequencies (Fig. 5). Therefore, the incomplete arc can be ascribed to a grain boundary (GB) response48. Previously, BiT single crystals showed two resolved impedance semicircles, which were considered as the results of the effects of crystalline plate (CP) and plate boundary (PB), respectively43. In this study, BiT-Bi ceramic also presents mica-like grains, where CP and PB are visible (inset of Fig. S1a; Supplementary Information). Therefore, the two complete arcs as mentioned above are likely to be associated with the responses of CP and PB, respectively. As indicated by inset of Fig. 4a and the frame inside Fig. 4e, two component parts are observed at the CP region. In BiT-Bi, the electrical properties of the (Bi2O2)2+ layers should be pronounced due to high degree of texture in the c axis direction. Therefore, it is likely that they correspond to the AC responses of the (Bi2O2)2+ layers (higher frequency) and the pseudo-perovskite blocks (lower frequency), respectively49. It is reasonable that the former has a lower electrical capacitance than the latter, because of the polarization vector of BiT along the crystallographic a-axis (within the pseudo-perovskite blocks).
(a–d) 250–700 °C complex Z* plots of the air-, O2- and N2-processed BiT-Bi samples, and insets showing regionally enlarged drawings (the symbols: experimental data; the solid lines: fitting spectra). (e–h) 250–700 °C Z″/M″ plots of air-, O2- and N2-processed BiT-Bi samples. “CP” crystalline plate, “PB” plate boundary, “GB” grain boundary, “ED” electrode impedance, “W” Warburg impedance.
When temperature is elevated, the grain boundary arc becomes complete, and another arc (the 4th arc; inset of Fig. 4b) is visible in the 400 °C air/O2 impedance diagrams, at low frequencies (Fig. 4b). The same data presented as Z″ plots (Fig. 4f) also exhibit an additional peak at low frequencies. The 400 °C C′ plot of the O2 sample shows a high capacitance plateau (10−7–10−6 F/cm) in the same frequency range (Fig. 5a). These experiments suggest that the most likely origin of the 4th arc is ascribed to the electrode effect (discussed later)38, 42. However, a clear Warburg impedance contribution can be observed below 1 Hz in the 400 °C Z* plot of the N2 sample (Fig. 4b). To further increasing temperature, such a Warburg response presents an intensive inclined spike at 550 °C and 700 °C (Fig. 4c and d), due to limited ionic diffusion into a partially blocking electrodes42, 50. This, combined with steeply increased capacitance at low frequencies (Fig. 5b), indicates that the N2 sample shows prominent ionic conduction, and the principal conducting species could be O2− ions51. In contrast, the air/O2 samples only show a tail associated with a weak trace of Warburg impedance at the lowest frequencies, due to significantly enhanced electronic conduction53, 54. The ionic transference number (t ion) can be evaluated from these Warburg impedances in the case of ion blocking electrode condition, which was described in the literature52,53,54. t ion in N2 is roughly evaluated to be about 0.85 at 550 °C, which are much higher than those in air and O2 (<0.1). High t ion in N2 is apparently associated with high [VO ••] in the sample.
In general, the time constant difference for relaxation of each component is large at low temperature, so one can see separated semicircles for each component (see Fig. 4a and b)55. However, at high temperature, the time constants for different component are very close and/or relatively small for some of the components, so we normally observe two component semicircles at 700 °C. Like electrolytes and solid-state ionic conducting materials56, 57, a modified Randles equivalent circuit was used to calculate the impedance data of the N2 sample, as shown in Fig. 6a An equivalent circuit including several (R//CPE)s in series (Fig. 6b) was used to calculate the impedance data of the air/O2 samples. Here, the bulk is integrated and considered as a (R//CPE) component. The parameters and errors for the equivalent circuits are reasonable, and the calculated data well agree with the experiment ones. Similar impedance behaviors as functions of atmosphere and temperature are found for BiT-La and BiT-Nd, which are not shown here. For BiT-La with Pt and Ag electrodes, there are both four overlapping arcs in the 450 °C Z* plots (Fig. S2, Supplementary Information). The resistance (the 4th arc) and the capacitance associated with the electrode effect in the low frequency range are clearly affected by the electrode materials, because of different work functions of them. However, the bulk response that dominates the high frequency data remains unchanged. These experiment results are consistent with those for CaCu3Ti4O12 ceramics38. Slightly affected boundary responses may be ascribed to two different preparation conditions for Ag and Pt electrodes.
In contrast, BLT and BiT-Nb show dissimilar AC responses, as shown in Fig. 7. In Fig. 7a, the N2-processed BLT sample shows a small inclined spike at 400 °C, which turns into a distorted arc at 750 °C (inset of Fig. 7a). While no obvious Warburg characteristic is found in BiT-Nb at both 550 °C and 750 °C (Fig. 7c and inset of Fig. 7c), which is an indication of a very small contribution of ion conduction to the electrical conduction. This could be related to low [VO ••] and highly stabilized oxygen ion in it. In addition, two principal semicircles are observed in the complex Z* plots of BLT and BiT-Nb under O2 and N2. The same data presented as C′ (Fig. 7b and d) and Z″ (insets of Fig. 7b and d) plots show two capacitance plateaus and two Z″ peaks, respectively. It means that the resistances and the capacitances of BLT and BiT-Nb are principally derived from two regions, i.e., grain (high frequency) and GB (low frequency), respectively.
(a) 400 °C and 750 °C (inset) Z* plots of the O2- and N2-processed BLT samples. (b) 400 °C and 750 °C C′ plots with an inset showing 400 °C Z″ plots, of the O2- and N2-processed BLT samples. (c) 550 °C and 750 °C (inset) Z* plots of the O2- and N2- processed BiT-Nb samples. (d) 550 °C and 750 °C C′ plots with an inset showing 550 °C Z″ plots, of the O2- and N2-processed BiT-Nb samples.
pO2 dependences of resistivity(ρ)and conductivity(σ)
Figure 8 shows ρ bulk (bulk resistivity) and ρ GB (GB resistivity) Arrhenius plots for BiT-Bi, BLT and BiT-Nb measured under different atmospheres. As shown in Fig. 8a, BLT shows slightly higher ρ bulk than BiT-Bi. While its ρ GB increases obviously, by about two orders of magnitude (air-processed) (Fig. 8b). By comparison, BiT-Nb shows excellent electrical insulating property, whose ρ bulk and ρ GB are both much higher than those of BiT-Bi by approximately three orders of magnitude. In addition, the ρ GB of BiT-Bi and BLT is strongly atmosphere-dependent, whereas the ρ bulk of them is nearly independent of pO2. By and large, both ρ bulk and ρ GB of the samples increase with decreasing pO2, indicating that p-type conduction is predominant in them. However, n-type conduction dominates BiT-Nb above T c, and therefore the ρ bulk and ρ GB of the N2 sample are much lower than those of the air/O2 samples above 650 °C. BiT-Bi and BLT show comparable activation energy (E a) for the bulk and the grain boundary barrier, being 0.5–0.6 eV and 1.0–1.2 eV, respectively. The two values of BiT-Nb become much higher, being 1.37–1.71 and 1.28–1.83 eV, respectively.
Figure 9 shows σ bulk (bulk conductivity) and σ GB (GB conductivity) as a function of pO2 for BiT-Bi and BiT-Nb at different temperatures. In Fig. 9a, the σ bulk and σ GB of BiT-Bi both increase with increasing pO2, and logσ bulk vs log pO2 and logσ GB vs log pO2 both present nonlinear variations. It is clear that ionic conductivity is approximately independent of pO2, while hole conductivity linearly increases with increasing pO2 31, 32. These nonlinear variations suggest that BiT-Bi is an oxide ionic and p-type mixed conductor. By comparison, σ bulk showed a very smaller dependence of pO2, indicating that ion conduction dominates the bulk. As indicated by Fig. 9b, the σbulk and σGB of BiT-Nb show strong dependence of oxygen activity, which is in response to a prominent electronic conduction. In the present oxides, oxygen vacancies mainly arise from the evaporation of Bi2O3 during sintering, and the incorporation of oxygen into the VO •• sites follows after cooling, expressed by the following reaction.
Each VO •• is compensated by two holes (h •) to satisfy the electroneutrality condition, and consequently the sample presents p-type conduction. When the reaction by Eq. 2 is dominant, Takahashi, et al. suggested that hole conductivity (or hole concentration [h •]) follows the 1/6 power dependence of pO2 31, 32. The σGB nearly proportional pO2 1/6 in the measured pO2 range at 500 °C, implies that p-type conduction is predominant at the GB of BiT-Nb. However at 750 °C, the two conductivities both increase with decreasing pO2, which is directly opposite to the experiments exhibited at 500 °C. This change is consistent with the results of YMnO3 ceramics58. It is presumed that BiT-Nb above T c is an oxide ionic and n-type mixed conductor. Under reducing conditions the required electrons (e′) are created through the release of oxygen from the lattices, expressed by the following equation.
The reduction of Ti4+ to Ti3+ is likely to be the source of conduction. And the electrons are probably trapped by the donor defects such VO •• and NbTi •, leading to high E a (>2.0 eV) for the N2 sample in the high temperature range (see Fig. 8). In addition, good fittings for the σ bulk and σ GB of BiT-Nb at 750 °C can be obtained by an equation of σ bulk (σ GB) = σ ion + σ electronic (pO2)m (m = −1/6). In the case of both 500 °C (BiT-Nb) and 600 °C (BiT-Bi), the exponent m is equal to 1/6. For the O2-processed BiT-Bi sample at 600 °C (pO2 = 1 atm), the calculated σ ion and σ electronic (hole) in the bulk are 3.02 × 10−4 and 2.21 × 10−4 S cm−1, respectively, and they are estimated to be 0.68 × 10−3 and 1.64 × 10−3 S cm−1 at the GB region, respectively.
Phase transition and dielectric relaxation
Figure 10a shows temperature dependence of dielectric permittivity (ε′) at several frequencies for the air-, O2- and N2-processed BiT samples. High-and-sharp dielectric permittivity peak corresponds to ferroelectric-to-paraelectric phase transition. It is observed that the atmosphere has no influence on T c of BiT, which is around 673 °C and the same as those reported in the literature6, 40. Notably, an anomaly associated with two primarily high permittivity regions is detected below T c, which agrees with the results of Shulman et al.12. Also, there are several peaks observed in the tan δ-T plots (Fig. 10b), corresponding to the steep increases of dielectric permittivities in the ε′ vs. T plots (Fig. 10a), which all move toward higher temperature with increasing frequency. The dielectric relaxation and the loss peak in the low temperature range (region I) are not almost affected by altering pO2, indicating an intrinsically physical nature, i.e., the bulk response. In contrast, the medium- and high-temperature dielectric relaxations in regions II and III, respectively, are strongly affected. They are likely in response to the boundary capacitance and the electrode capacitance, respectively, being referred to the results of Fig. 4 and ref. 38. The notable peak between 450 and 550 °C in the dielectric loss tangent (inset of Fig. 10b) for the air/O2 samples seems to be attributed to the conduction loss.
(a) Log ε′ vs. T and (b) log tanδ vs. T plots for the air-, O2- and N2-processed BiT samples, and tanδ vs. T plots for the air-, O2- and N2-processed BiT as an inset in (b). (c) ε′ vs. T and (d) tanδ vs. T plots for the air-processed BiT, BiT-A, BLT and BiT-Nb samples, with an inset showing regionally enlarged drawing for ε′ vs. T plots in (c).
As shown in Fig. 10c and d, the Nb donor substitution suppresses the loss and the relaxation process dramatically, and a sharp transition peak is observed at T c. However, the A2O3 additions are not critical to the dielectric data. In addition, the T c of BiT-Bi and BiT-Nb is close to that of BiT, while the T c of BiT-La and BiT-Nd decreases to 638 °C and 647 °C, respectively (Fig. 10c). For A-site bismuth-containing Aurivillius compounds, the polarity Bi3+ with 6 s2 lone pair electrons causes the deformation from the prototype structure59. Therefore, it is presumable that non-polarity La3+ and Nd3+ were incorporated into the lattices by replace the A-site Bi3+, and thus the T c of BiT-La and BiT-Nd decreases significantly. Newnham et al. proposed that T c vs. x in Bi4−x RExTi3O12 follows a linear relationship60. In this study, the substitution content x in BiT-La and BiT-Nd is roughly estimated to be about 0.1 (Fig. S3; Supplementary Information), close to nominal A-site excess level (Bi4A0.12Ti3O12.18). Furthermore, the change trend of T c is in accord with that of structural orthorhombicity (see Table 1), and higher T c corresponds to higher orthorhombicity value.
Discussion
Commonly, Schottky barriers can form in electrically heterogeneous ceramics at insulating grain boundaries between semiconducting grains, which is referred to as an internal barrier layer capacitor (IBLC) effect61. Also, Schottky barriers can occur between “leaky” or semiconducting ceramics and metal electrodes, acting as a non-ohmic electrode effect62. The formation of these barriers can be ascribed to compositional variations and/or a mismatch between Fermi energy levels between the two materials that meet at the interface38, 48. Polarization effects at these barrier layers can generate nonintrinsic and colossal dielectric permittivities in ceramics such as CaCu3Ti4O12 (CCTO)38. In this study, the bulks of BiT-A clearly are semiconducting, whose resistivity are lower than 105 Ω cm above 300 °C (Fig. 8a). Thus the Schottky barriers are possibly generated at the grain boundary regions in them, which is consistent with the results of CCTO and Pb(Fe1/2Nb1/2)O3 ceramics37, 38. The results of Figs 8 and 9 indicate that BiT-Bi is an oxygen ion and hole mixed conductor. It is presumed that the bulk of BiT-Bi is dominated by positive defects (h • and VO ••), which can be charge-compensated by grain boundary acceptor surface charges. Such a barrier layer may be associated with the depletion of VO •• at the grain boundary region during oxidative cooling63, as sketched in Fig. 11a.
It is possible that the electrodes nominally “blocking” for ions, allow for a certain ionic leakage, while the electrodes nominally “reversible” for electrons, still represent a certain interfacial resistance52, 64. In the case of the air/O2 BiT-Bi samples, it is believed that weak Warburg impedance at the lowest frequencies corresponds to the limited ionic diffusion into a partially blocking electrode (see Fig. 4). The additional arc between the boundary impedance and the Warburg one is presumably attributed to the electrode effect due to deviation from electronic reversibility. Two types of electrode effects together with the boundary barrier effects that dominate the intermediate frequency data, induce two high dielectric permittivity regions for BiT-A. Therefore, the following successions of layers starting from the inside are possibly: bulk/plate boundary/grain boundary/electrode.
One can speculate that oxide ion conduction may be a common feature of perovskite materials with a high [VO ••]. Furthermore, high oxygen ion conductivity has been recorded in the literature for intergrowths of Aurivillius with Brownmillerite structure, and cubic δ-Bi2O3 is known as a fast ion oxygen conductor11, 65, 66. For BiT-A, it is reasonable that predominant ionic conduction in the bulk is associated with the textured structure along the c-axis direction, in addition to high [VO ••] in the perovskite blocks. However, hole conduction is dominant at the boundaries, and thus the ρ GB or σGB is apparently dependent of pO2 (see Figs 8b and 9a). A detailed structure analysis for pure BiT showed that some Bi ions in the perovskite layers are overbonded with a valence state of >3+67. A Pb2+ → Pb3+ hopping conduction has been proposed in PZT perovskite68. Presumably in BiT-Bi, hole conduction is associated with oxidation of Bi3+ to Bi4+, and the relevant E a is probably close to that for the trapping of holes by Pb2+ ions, 0.26–0.3 eV11, 68, 69. The reported E a for the mobility of VO •• is about 1.0 eV70, 71. The E a of about 0.6 eV for the bulk of BiT and BiT-A can be attributed to a compromise of oxide ion conduction and hole conduction. Higher E a (1.0–1.2 eV) for the boundary barriers may be attributed to trapping of holes (h •) by the grain-boundary acceptor surface charges (A′)52.
In order to achieve predominant oxygen ion conduction, it is necessary to avoid samples picking up oxygen from high pO2 environments. Otherwise, the rich-oxygen atmosphere encourages the incorporation of foreign oxygen into the lattices (Eq. 1); as a consequence, the samples transform into p-type electronic conductors with higher conductivity and lower activation energy. As indicated by the XPS (X-ray photoelectron spectroscopy) results (Fig. 12a), the increased pO2 leads to the increases in the nominal valence states of Bi and Ti, which is related to both a decrease in [VO ••] and an increase in [h •]72. Therefore, BiT-A show a transition from the dominant oxide ion conduction to the dominant p-type semiconduction with increasing pO2.
(a) Bi 4f and Ti 2p (inset) XPS spectra of the air, O2 and N2 BiT-Bi samples. (b) compared O 1 s and Ti 2p (inset) XPS spectra of the air BiT and BiT-Nb, where the O 1 s spectra were deconvoluted into two natural components by Gauss-Lorentz method (solid lines are experimental data, and dotted lines are fitting peaks).
As shown in Fig. S4 (Supplementary Information), BiT shows similar conduction behaviors as functions of atmosphere and temperature. BiT-A relative to BiT shows slightly increased ρ bulk, and the variations in ρ PB (PB resistivity) and ρ GB are slight and irregular (Fig. S5; Supplementary Information). This, combined with the evolutions in V and T c (see Table 1 and Fig. 10c), indicates that A3+ could be mainly diffused into the lattices by substituting the A-site Bi3+, which limited the predicted compensation effect of excess A2O3 on the VBi′′′ and VO •• sites. Excess or substituted bismuth (in fact, Bi2O3), should act as sintering aids because of low melting point (about 830 °C), and consequently BiT-A and BiT show comparable grains and texture factor values. La isovalent substitution with a high concentration can induce an increase in the formation energy of VO •• in the perovskite blocks, where the decreases in [VO ••] and [h •] are induced33, 73. Thus in BLT, hole conduction and oxygen ion conduction are both suppressed. As sketched in Fig. 11b, BiT-Nb relative to BiT-Bi becomes rather more insulating. Two situations can be considered by the Nb substitution for the Ti site (B site), as expressed by the following equations.
Clearly the Nb5+ donor substitution can decrease efficiently [h •] and [VO ••] in the perovskite blocks, which supports significantly increased ρ bulk of BiT-Nb (see Fig. 9). As indicted by the results of Figs 8 and 9, for BiT-Bi (>350 °C) and BiT-Nb, the grain resistivity of is lower than the grain resistivity. However, it does not mean that grain boundaries are electrically more conduction than bulk grains. In fact, grain boundaries in this study are electrically more insulating as shown in Fig. 11b, because the thicknesses of them are far smaller than the grain sizes of samples. This is consistent with the results of other electroceramics73. The XPS results of Fig. 12b indicates that BiT-Nb shows lower [VO ••] and stronger Ti-O band than BiT-Bi, in the perovskite blocks. In addition, the donor centrals (Nb5+) may affect the movement of VO •• 11, 12. This combined with low [VO ••], should result in much higher potential barrier energy for the jump of VO •• in BiT-Nb than those in BiT and BiT-A, as sketched in Fig. 13. Therefore, the oxygen ion conduction is weak in BiT-Nb, of which the electrical conduction is primarily attributed to an electronic mechanism. Previous researches revealed that additional polarization associated with the electrode-sample interface was readily observed for CCTO ceramics with relatively low ρ GB 38. If ρ GB was large, the electrode polarization was obscured by sample-related effects. Thus, the absence of the electrode effect in BLT and BiT-Nb may be associated with high ρ GB of them, which are much higher than those of BiT and BiT-A (see Fig. 8b).
Conclusions
Structure and electrical properties of modified BiT-based ceramics could be tailored readily by changing chemical compositions, including the perovskite A and B sites. As a consequence, different electrical conductions and dielectric properties among them were found. Excess A2O3 had no obvious effects on electrical resistivity and conduction mechanism. BiT and BiT-A all showed prominent oxide ion conduction under N2, which was likely associated with high [VO ••] in the perovskite blocks and the pronounced texture in the c-axis direction. However, the hole conduction was prominent in the air/O2 samples, because of the absorption of oxygen by them. In addition to the semiconducting bulk, impedance data revealed that Schottky barriers had been formed both at grain boundary and at the sample-electrode interface in heterogeneous BiT and BiT-A, which contributed to two abnormally high ε′ regions below T c. BLT with 0.75 La substitution at A site and BiT-Nb with 0.03 Nb substitution at B site, both showed a suppression in electrical conduction including ionic and electronic. Especially, a low level of Nb donor substitution led to the significant decreases in [VO ••] and [h •]; thus BiT-Nb showed much higher ρ and E a than BiT and BiT-A. The results in this study are significant for BiT-base high-temperature piezoelectric sensors in solving the origin of high leakage conductivity. Also, they provided some probability for the future work that BiT-based ceramics are considered as new oxide ion conductors by adjusting appropriately chemical compositions.
Methods
Materials
BiT-based ceramics, BiT, BiT-A (A = Bi, La and Nd), BLT and BiT-Nb, were prepared by using traditional solid state reaction. Appropriate amounts of starting materials [Bi2O3 (99.5%), TiO2 (99.99%), La2O3 (99.99%), Nd2O3 (99.99%), and Nb2O5 (99.99%); Sinopharm Chemical Reagent Co., Ltd, CN] were mixed and milled in ethanol for 24 h. The mixtures were dried and then calcined at 800 °C for 4 h. The calcined powders were remilled in ethanol for 24 h, dried, ground and cold isostatically pressed into pellets at 300 MPa. The pressed pellets were then sintered at 980–1000 °C for 2 h.
Characterization and measurements
XRD data were collected by using an automated diffractometer (X’Pert PRO MPD, Philips, Eindhoven, The Netherlands) with a nickel filter (Cu Kα radiation) at room temperature. SEM images were observed by using a field-emission scanning electron microscopy (JEOL-6700F, Japan Electron Co., Tokyo, Japan) instrument equipped with an EDS system, at room temperature. TEM image and compositional analysis were performed by using a transmission electron microscopy (Tecnai F30, FEI, Hillsboro, OR, USA) instrument equipped with a high angle annular dark-field (HAADF) detector and an EDS system, at room temperature. XPS data were performed with a spectrometer (VG ESCALAB220i-XL, Thermo Scientific, Surrey, UK) with Al Kα (E = 1486.6 eV) radiation, at room temperature. Temperature dependences of dielectric permittivity and dielectric loss were measured by using an LCR meter (4284 A, Agilent, CA, USA). Impedance data were performed by using an Impedance Analyzer (Solartron, SI 1260, Hampshire, U.K.), with a frequency range of 0.1–1 M Hz and an AC measuring voltage of 0.3 V. The same sample was used for electrical property measurements firstly in air, subsequently in N2, and finally in O2, at a slow cooling rate. pO2 dependence of conductivity was measured in the range of 10−6–1 atm, which was controlled by mixing O2 and N2 gases and monitored by a zirconia oxygen sensor. Ag and Pt electrodes for the measurements of the electrical properties were made of fired-on silver paste at 850 °C and sputtered at room temperature (after removing Ag), respectively.
References
Aurivillius, B. Mixed Bismuth Oxides with Lauer Lattices: I. Ark. Kemi. 1, 463–471 (1949).
Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices: II. Ark. Kemi. 1, 499 (1950).
Park, B. H. et al. Lanthanum-Substituted Bismuth Titanate for Use in Non-volatile Memories. Nature 401, 682 (1999).
Damjanovic, D. Ferroelectric, Dielectric and Piezoelectric Properties of Ferroelectric Thin Films and Ceramics. Rep. Prog. Phys. 61, 1267 (1998).
Damjanovic, D. Materials for High Temperature Piezoelectric Transducers. Curr. Opin. Solid–State Mater. Sci. 3, 469 (1998).
Yan, H. X., Zhang, H. T., Reece, M. J. & Dong, X. L. Thermal Depoling of High Curie Point Aurivillius Phase Ferroelectric Ceramics. Appl. Phys. Lett. 87, 0829111 (2005).
Cummins, S. E. & Cross, L. E. Electric and Optical Properties of Ferroelectric Bi4Ti3O12 Single Crystals. J. Appl. Phys. 39, 2268 (1968).
Irie, H., Saito, H., Ohkoshi, S. & Hashimoto, K. Enhanced Ferroelectric Properties of Nitrogen-Doped Bi4Ti3O12 Thin Films. Adv. Mater. 17, 491–494 (2005).
Takahashi, R. et al. Perfect Bi4Ti3O12 Single-Crystal Films via Flux-Mediated Epitaxy. Adv. Fun. Mater 16, 485–491 (2006).
Noguchi, Y., Yamamoto, K., Kitanaka, Y. Z. & Miyayama, M. Effects of Mn doping on the polarization and leakage current properties in Bi4Ti3O12 single crystals. J. Eur. Ceram. Soc. 27(13–15), 4081 (2007).
Shulman, H. S., Testorf, M., Damjanovic, D. & Setter, N. Microstructure, Electrical Conductivity, and Piezoelectric Properties of Bismuth Titanate. J. Am. Ceram. Soc 79, 3124–3128 (1996).
Shulman, H. S., Damjanovic, D. & Setter, N. Niobium Doping and Dielectric Anomalies in Bismuth Titanate. J. Am. Ceram. Soc. 83, 528–32 (2000).
Park, C. H. & Chadi, D. J. Microscopic study of oxygen-vacancy defects in ferroelectric perovskites. Phys. ReV. B 57(R13), 961 (1998).
Scott, J. F. & Dawber, M. Oxygen-vacancy ordering as a fatigue mechanism in perovskite ferroelectrics. Appl. Phys. Lett. 76, 3801 (2000).
Park, B. H. et al. Differences in nature of defects between SrBi2Ta2O9 and Bi4Ti3O12. Appl. Phys. Lett. 74, 1907–1909 (1999).
Joshi, P. C., Krupanidhi, S. B. & Mansingh, A. Rapid Thermally Processed Ferroelectric Bi4Ti3O12 Thin Films. J. Appl. Phys. 72, 5517–5519 (1992).
Du, X. F. & Chen, I. W. Ferroelectric Thin Films of Bismuth-Containing Layered Perovskites: Part I, Bi4Ti3O12. J. Am. Ceram. Soc. 81, 3253–3259 (1998).
Nagata, H., Chikushi, N. & Takenaka, T. Ferroelectric Properties of Bismuth Layer-Structured Compound SrxBi4−xTi3−xTaxO12 (0 ≤ x ≤ 2). Jpn. J. Appl. Phys. 38(1A), 5497–5499 (1999).
Kijima, T., Ushikubo, M. & Matsunaga, H. New Low-Temperature Processing of Metalorganic Chemical Vapor Deposition-Bi4Ti3O12 Thin Films Using BiO x Buffer Layer. Jpn. J. Appl. Phys. 38(1A), 127–130 (1999).
Noguchi, Y., Miwa, I. & Goshima, Y. Defect Control for Large Remanent Polarization in Bismuth Titanate Ferroelectrics—Doping Effect of Higher-Valent Cations—. Jpn. J. Appl. Phys. 39, 1259–1262 (2000).
Chon, U., Kim, K. B., Jang, H. M. & Yi, G. C. Fatigue-Free Samarium-Modified Bismuth Titanate (Bi4−xSmxTi3O12) Film Capacitors Having Large Spontaneous Polarizations. Appl. Phys. Lett. 79, 3137 (2001).
Du, H. C., Wohlrab, S. & Kaskel, S. Synthesis, Structure, and Properties of Bi3.25Pr0.75Ti2.97V0.03O12 Ferroelectric Ceramics. J. Phys. Chem. C. 111, 11095–11103 (2007).
Xue, K. H., A-Paz de Araujo, C. & Celinska, J. A comparative study on Bi4Ti3O12 and Bi3.25La0.75Ti3O12 ferroelectric thin films derived by metal organic decomposition. J. Appl. Phys. 107(104123), 1–5 (2010).
Hong, S. H., McKinstry, S. T. & Messing, G. L. Dielectric and Electromechanical Properties of Textured Niobium-Doped Bismuth Titanate Ceramics. J. Am. Ceram. Soc 83, 113 (2000).
Hou, J. G., Kumar, R. V., Qu, Y. F. & Krsmanovic, D. B-site Doping Effect on Electrical Properties of Bi4Ti3−2xNbxTaxO12 Ceramics. Scr. Mater 61, 664 (2009).
Du, H. C., Tang, L. J. & Kaskel, S. Preparation, Microstructure, and Ferroelectric Properties of Bi3.25La0.75Ti3−x M x O12 (M = Mo, W, Nb, V) Ceramics. J. Phys. Chem. C. 113, 1329–1339 (2009).
Li, W., Gu, J. & Song, C. H. B-site doping effect on ferroelectric property of bismuth titanate ceramic. J. Appl. Phys. 98(114104), 1–4 (2005).
Gao, X. S., Zhou, Z. H., Xue, J. M. & Wang, J. Effects of Excess Bi2O3 on the Ferroelectric Behavior of Nd-Doped Bi4Ti3O12 Thin Films. J. Am. Ceram. Soc. 88, 1037–1040 (2005).
Wang, C. M. et al. Cerium-Modified Aurivillius-Type Sodium Lanthanum Bismuth Titanate with Enhanced Piezoactivities. Mater. Sci. Eng. B 163, 179–183 (2009).
Wang, C. M., Wang, J. F. & Gai, Z. G. Enhancement of Dielectric and Piezoelectric Properties of M0.5Bi4.5Ti4O15 (M = Na, K, Li) Ceramics by Ce Doping. Scr. Mater 57, 789–792 (2007).
Takahashi, M., Nguchi, Y. & Miyayama, M. Electrical Conduction Mechanism in Bi4Ti3O12 Single Crystal. Jpn. J. Appl. Phys. 41, 7053–7056 (2002).
Takahashi, M., Nguchi, Y. & Miyayama, M. Effects of V-Doping on Mixed Conduction Properties of Bismuth Titanate Single Crystals. Jpn. J. Appl. Phys. 42, 6222–6225 (2003).
Takahashi, M., Nguchi, Y. & Miyayama, M. Electrical conduction properties of La-substituted bismuth titanate single crystals. Journal of Ceramic Processing Research 6, 281–285 (2005).
Smyth, D. M. Defect structure in perovskite titanates. Curr. Opin. Solid–State Mater. Sci 1, 692–697 (1996).
Raymond, M. V. & Smyth, D. M. Defects and charge transport in perovskite ferroelectrics. J. Phys. Chem. Solids 57, 1507–1511 (1996).
Krohns, S., Lunkenheimer, P., Ebbinghaus, S. G. & Loidl, A. Colossal Dielectric Constants in Single-Crystalline and Ceramic CaCu3Ti4O12 Investigated by Broadband Dielectric Spectroscopy. J. Appl. Phys. 103, 084107 (2008).
Raymond, O. et al. Frequency-temperature response of ferroelectromagnetic Pb(Fe1/2Nb1/2)O3 ceramics obtained by different precursors. Part II. Impedance spectroscopy characterization. J. Appl. Phys. 97, 084108 (2005).
Li, M. et al. Origins of the apparent high permittivity in CaCu3Ti4O12 ceramics: clarification on the contributions from internal barrier layer capacitor and sample-electrode contact effects. J. Appl. Phys. 106, 104106 (2009).
Takahashi, M., Nguchi, Y. & Miyayama, M. Estimation of ionic and hole conductivity in bismuth titanate polycrystals at high temperatures. Solid State Ionics 172, 325–329 (2004).
Macedo, Z. S., Ferrari, C. R. & Hernandes, A. C. Impedance spectroscopy of Bi4Ti3O12 ceramic produced by self-propagating high-temperature synthesis technique. J. Euro. Ceram. Soc. 24, 2567–2574 (2004).
Cheng, H. F. Modeling of Electrical Response for Semiconducting Ferrite. J. Appl. Phys. 56, 1831 (1984).
Irvine, J. T. S., Sinclair, D. C. & West, A. R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 2, 132 (1990).
Huanosta, A., Alvarez–Fregoso, O. & Amano, E. AC Impedance Analysis on Crystalline Layered and Polycrystalline Bismuth Titanate. J. Appl. Phys. 69, 404 (1991).
Larson, A. C. & Von Dreele, R. B. General Structure Analysis System (GSAS), 79–92 (Report No. LAUR-86-748) (Los Alamos National Laboratory 2000).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 34, 210–213 (2001).
Lotgering, F. K. Topotactical reactions with ferrimagnetic oxides having hexagonal crystal structures. J. Inorg. Nucl. Chem 9, 113 (1959).
Shannon, R. D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A: Cryst. Phys. Diffr. Theor. Gen. Crystallogr A32, 751–767 (1976).
Li, M. & Sinclair, D. C. The extrinsic Origins of High Permittivity and its Temperature and Frequency Dependence in Y0.5Ca0.5MnO3 and La1.5Sr0.5NiO4 Ceramics. J. Appl. Phys. 111(054106), 1–8 (2012).
Kim, S. K., Miyayama, M. & Yanagida, H. Electrical anisotropy and a plausible explanation for dielectric anomaly of Bi4Ti3O12 single crystal. Mater. Res. Bull. 31, 121–131 (1996).
Jamnik, J., Maier, J. & Pejovnik, S. A powerful electrical network model for the impedance of mixed conductors. Electrochimica Acta 44, 4139–4145 (1999).
Maso, N. & West, A. R. Electrical Properties of Ca-Doped BiFeO3 Ceramics: From p-Type Semiconduction to Oxide-Ion Conduction. Chem. Mater. 24, 2127–2132 (2012).
Yoon, S. H., Randall, C. A. & Hur, K. H. Effect of Acceptor (Mg) Concentration on the Resistance Degradation Behavior in Acceptor (Mg)-Doped BaTiO3 Bulk Ceramics: I. Impedance Analysis. J. Am. Ceram. Soc. 92(8), 1758–1765 (2009).
Yoon, S. H., Randall, C. A. & Hur, K. H. Effect of acceptor concentration on the bulk electrical conduction in acceptor Mg-doped BaTiO3. J. Appl. Phys. 107, 103721 (2010).
Yoon, S. H., Randall, C. A. & Hur, K. H. Difference between resistance degradation of fixed valence acceptor (Mg) and variable valence acceptor (Mn)-doped BaTiO3 ceramics. J. Appl. Phys. 108, 064101 (2010).
Lee, S. & Randall, C. A. Determination of electronic and ionic conductivity in mixed ionic conductors: HiTEC and in-situ impedance spectroscopy analysis of isovalent and aliovalent doped BaTiO3. Solid State Ionics 249–250, 86–92 (2013).
Funabiki, A., Inaba, M. & Ogumi, Z. Ac Impedance Analysis of Electrochemical Lithium Intercalation into Highly Oriented Pyrolytic Graphite. J. Power Sources 68, 227–231 (1997).
Stromme Mattsson, M., Niklasson, G. A. & Granqvist, C. G. Li Diffusion in Ti Oxyfluoride Films: Thermal Activation Energy and Jump Length Derived From Impedance Spectroscopy. J. Appl. Phys. 80(4), 2169 (1996).
Ren, P. R., Fan, H. Q. & Wang, X. Bulk conduction and nonlinear behaviour in multiferroic YMnO3. Appl. Phys. Lett. 103, 152905 (2013).
Frit, B. & Mercurio, J. P. The crystal chemistry and dielectric properties of the Aurivillius family of complex bismuth oxides with perovskite-like layered structures. J. Alloys Comp. 188, 27–35 (1992).
Wolfe, R. W. & Newnham, R. E. Rare-earth bismuth titanates. J. Electrochem. Soc. 116, 832–835 (1969).
Greuter, F. & Blatter, G. Electrical properties of grain boundaries in polycrystalline compound semiconductors. Semicond. Sci. Technol. 5, 111 (1990).
Yang, C. F. An equivalent cicuit for CuO modified surface barrier layer capacitors. Jpn. J. Appl. Phys. Part 1 36, 188 (1997).
Chiang., Y. & Takag, T. Grain-Boundary Chemistry of Barium Titanate and Strontium Titanate: II, Origin of Electrical Barriers in Positive-Temperature-Coefficient Thermistors. J. Am. Ceram. Soc. 73(11), 3286–3291 (1990).
Jamnik, J. & Maier, J. Treatment of the Impedance of Mixed Conductors: Equivalent Circuit Model and Explicit Approximate Solutions. J. Electrochem. Soc. 146(11), 4183–4188 (1999).
Thomas, J. M., Anderson, M. E., Krause, W. E. & Loye, H. Z. Oxygen-Ion Conductivity in a New Class of Layered Bismuth Oxide Compounds. Mater. Res. Soc. Symp. Proc 293, 295–300 (1993).
Hapase, M. G., Tare, V. B. & Biswas, A. B. Electrical Properties and Defect Structure of Bi2O3. Indian J. Pure Appl. Phys. 5, 401–404 (1967).
Withers, R. L., Thompson, J. G. & Rae, A. D. The Crystal Chemistry Underlying Ferroelectricity in Bi4Ti3O12, Bi3TiNbO9, and Bi2WO6. J. Solid State Chem. 94(191), 404–417.
Raymond, M. V. & Smyth, D. M. Science and Technology of Electroceramic Thin Films (ed. Aucrello, O. & Waser, R.) 315–325 (Kluwer 1995).
Prisedsky, V. V., Shishkovosky, V. I. & Klimov, V. V. High-Temperature Electrical Conductivity and Point Defects in Lead Zirconate-Titanate. Ferrelectrics 17, 465 (1978).
Yoo, H. I., Chang, M. W. & Oh, T. S. Electrocoloration and oxygen vacancy mobility of BaTiO3. J. Appl. Phys. 102, 093701 (2007).
Yoo, H. I., Song, C. R. & Lee, D. K. BaTiO3−δ: Defect Structure, Electrical Conductivity, Chemical Diffusivity, Thermoelectric Power, and Oxygen Nonstoichiometry. J. Electroceram 8, 5 (2002).
Park, B. H., Hyun, S. J. & Bu, S. D. Differences in nature of defects between SrBi2Ta2O9 and Bi4Ti3O12. Appl. Phys. Lett. 74(13), 1907–1909 (1999).
Yoon, S. H. & Kim, H. Effect of donor (Nb) concentration on the bulk electrical resistivity of Nb-doped barium titanate. J. Appl. Phys. 92, 1039–1047 (2002).
Acknowledgements
This work was supported by the National Natural Science Foundation (51502346, 51672220), the 111 Program (B08040) of MOE, the National Defence Science Foundation (32102060303) of China, the National Basic Research Program of China (2015Cb057400), and the National Key Research and Development Plan (2016YFB1102600). We also thank Dr. Weifeng He & Dr. Liucheng zhou for TEM measurements.
Author information
Authors and Affiliations
Contributions
L.C. designed the experiments, wrote the manuscript. H.F. directed the work. H.F., L.C. and C.Q. performed the experiments and analyzed data. All authors discussed and interpreted the results and have approved the final version of the manuscript. H.F. and L.C. supervised the project.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Long, C., Chang, Q. & Fan, H. Differences in nature of electrical conductions among Bi4Ti3O12-based ferroelectric polycrystalline ceramics. Sci Rep 7, 4193 (2017). https://doi.org/10.1038/s41598-017-03266-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03266-y
This article is cited by
-
Effect of the doping concentration of Eu3+ on the properties of the Bi4 − xEuxTi3O12 films
Journal of Materials Science: Materials in Electronics (2025)
-
Effects of the Bi3+ substitution on the structural, vibrational, and magnetic properties of bismuth layer-structured ferroelectrics
Applied Physics A (2020)
-
Significant ion conduction in Cu acceptor-substituted bismuth titanate polycrystalline ceramics
Journal of Materials Science (2020)
-
Crystallization evolution and ferroelectric behavior of Bi3.25La0.75Ti3O12-based thin films prepared by rf-magnetron sputtering
Journal of Materials Science: Materials in Electronics (2019)
-
Growing Oriented Layers of Bi4Ti3O12 in Bi2O3/TiO2/SiO2/Nd2O3/Al2O3 Glass-Ceramics by Melt Quenching
Scientific Reports (2018)