Abstract
In patients with exertional limb symptoms and normal ankle-brachial index (ABI) at rest, exercise testing can be used to diagnose lower extremity arterial disease (LEAD). Post-exercise ABI decrease or Exercise transcutaneous oxygen pressure measurement (Exercise-TcPO2) can be used to diagnose LEAD. Objectives were (i) to assess the agreement between both methods (ii) to define the variables associated with the discordance, and (iii) to present results of healthy subjects. In this prospective cross-sectional study, patients with exertional limb symptoms and normal rest ABI were consecutively included. ABI was measured at rest and after standardized exercise protocol as well as Exercise-TcPO2. A kappa coefficient with a 95% confidence interval was used to assess the agreement between the two methods. Logistic regression analysis was performed to outline variables potentially responsible for discordance. Ninety-six patients were included. The agreement between the tests was weak with a k value of 0.23 [0.04–0.41]. Logistic regression analysis found that a medical history of lower extremity arterial stenting (odds ratio 5.85[1.68–20.44]) and age (odds ratio 1.06[1.01–1.11]) were the main cause of discordance. This study suggests that post-exercise ABI and Exercise-TcPO2 cannot be used interchangeably for the diagnosis of LEAD in patients with exertional symptoms and normal rest ABI.
Similar content being viewed by others
Introduction
Lower extremity arterial disease (LEAD) is a highly prevalent disease with more than 200 million patients affected worldwide1 with only half presenting exertional limb symptoms2. The measurement of the ankle-brachial pressure index at rest (ABI) is the gold standard method for diagnosis of LEAD, with a value greater than 0.90 being considered normal3,4. However, in some cases, ABI can be normal in spite of the presence of exertional limb symptoms. In these cases, international guidelines recommend to perform other tests, including exercise tests3,4,5.
Post-Exercise ABI decrease (Post-Exercise ABI > 20% compared with baseline values or Post-Exercise pressure decrease >30 mmHg) has been proposed by the American Heart Association (AHA) as a second line tool to diagnose LEAD3,4,5. However, several studies have questioned the validity of the cut-off values currently used6,7,8,9.
Exercise transcutaneous oxygen pressure (Exercise TcPO2) is an alternative to Post-Exercise ABI to diagnose LEAD8,10,11,12. In the eighties, several groups have performed TcPO2 after exercise and during exercise without standardized methodology13,14,15,16. In 2003, Abraham and colleagues have proposed to perform Exercise TcPO2 with a standardized methodology (i.e. the places of the probe were defined, the room temperature was controlled and the duration of the pre-test heating period was defined)10. This standardized methodology associated with the use of the delta from rest of oxygen pressure (DROP) cut-off has improved the reliability of this procedure that was confirmed by two studies17,18. Finally, two different teams found similar cut-off values of the DROP8,10,12,19.
Stivalet et al. have recently shown that Post-Exercise ABI and Exercise-TcPO2 have similar accuracies to diagnose LEAD in patients with a normal resting ABI using computed tomography angiography (CTA) as a reference8. Indeed, the accuracy for the Post-exercise ABI was 67%[53–78] with a sensitivity of 71%[48–89] and specificity of 64%[47–79] whereas the accuracy of Exercise TcPO2 was 72%[59–83] with a sensitivity of 48%[26–70%]and a specificity of 85%[70–94]8.
A recent study has suggested that discordance of diagnosis of LEAD (20.8%) exits between exercise tests (Exercise TcPO2 and post-exercise pressure measurement) and ABI at rest in patients with normal and abnormal ABI20. However, there are several issues about the study: i) the authors did not detail how they did the pressure measurements at rest and after exercise; ii) they did not clarify if the ABI at rest was performed on the same day of the exercise tests; iii) they did not use the same exercise duration for the two exercise tests and, iv) they mixed the results of post-exercise ABI and post-exercise ankle pressure in the analyses. For all these reasons, additional study is required to assess the concordance between Exercise-TcPO2 and post-exercise pressure measurements in patients with exertional limb symptoms and normal ankle-brachial index (ABI).
Furthermore, although several studies have assessed the interest of exercise tests in suspected LEAD patients10,12,21,22, no study has reported healthy subject results of exercise tests (Exercise TcPO2 and post-exercise ABI after a treadmill test (10% slope and 3.2 km/h)).
Therefore, the aims of this study were i) to assess the concordance of Post-Exercise ABI and Exercise-TcPO2 drops to diagnose LEAD in patients with exertional limb symptoms and normal resting ABI, ii) to define the variables associated independently with the discordance, and iii) to show exercise test results obtained in healthy subjects.
Results
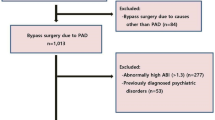
Among 265 consecutive patients who visited the clinic for standard care, 96 patients with exertional limb symptoms and normal ABI (>0.90) at rest on both limbs and who had performed both exercise tests were included (Fig. 1). Patient characteristics are presented in Table 1. The average age and body mass index were 61 +/−13 years, 26.2 +/−4.5 kg/m2, respectively. In this population, median estimated walking distance was 500 m [250–2500]. Median maximal walking distance on treadmill was 258 m [139–525] and 213 m [122–524] for Exercise-TcPO2 and for Post-Exercise ABI, respectively (p < 0.05). Mean heart rate before the first test (Exercise-TcPO2) was 76[68–86] beats per minute (bpm) and 81[71–93] bpm before the second test (Post-Exercise ABI) (p < 0.05).
In the suspected LEAD population, the mean post-exercise ABI for the symptomatic leg was 0.87 +/−0.32. The mean post-exercise ABI decrease for the symptomatic leg was 21.6%+/−20.2%. The maximum post-exercise increase for the symptomatic leg was 14.3% and the maximal post-exercise decrease was 66.3%. Regarding Exercise TcPO2 values, the mean right DROP was −13 +/−12 mmHg whereas the mean left DROP was −13 +/−13 mmHg. The lowest DROP value was −79 mmHg and the maximal DROP value was 11 mmHg.
Concordance between both tests
The prevalence of LEAD based on Post-Exercise ABI decrease was 50% whereas the prevalence was 34% based on Exercise-TcPO2 results. The agreement between both tests was weak with only a fair rating with a k value of 0.23[0.04–0.41] (Table 2). The characteristics of the concordant and discordant patients are presented in Table 3. Figure 2 depicts the results of both tests in the included population.
Results of Post-Exercise ABI and Exercise TcPO2 in patients with exertional limb symptoms and normal ABI (>0.90). Horizontal line represents the cut-off value (DROP: delta from rest of oxygen pressure) of exercise transcutaneous oxygen pressure measurement (−15 mmHg). Vertical line represents the cut-off value of Post-exercise ABI (ankle-brachial index) decrease (18.5%).
Variables associated with the discordance between the exercise tests
Variables tested in the univariate analysis are presented in Table 4. Variables with a p value < 0.20 that were considered for the step-by-step logistic regression were gender, age, diabetes, dyslipidemia, hypertension, coronary disease, stent in the lower limbs, coronary artery bypass graft. Step-by-step logistic regression found that the presence of a stent in the lower extremities (OR: 5.85 [1.68;20.44; p < 0.01] and age (1.06 [1.01;1.11]; p < 0.05) were the variables independently associated with the discordance in this population (Table 4). Indeed, both tests had more risk to be discordant when a patient had an arterial stent or was old.
Results of the healthy subjects
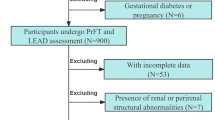
We included 37 healthy subjects that walked 15 minutes on the treadmill without any symptoms. The mean age of the healthy population was 64 +/−6 years old. There was a majority of men (62%) and mean body mass index was 23.9 +/−3.0 kg/m2. None of them was active smoker.
In this population the mean right and left ABI at rest was 1.19 +/−0.07 and 1.18 +/−0.08 respectively. The mean post-exercise ABI for the right leg and the left leg was 1.17 +/−0.11 and 1.16 +/−0.11, respectively. The mean post-exercise ABI decrease for the right and left legs were 1.8%+/−9.2% to and 1.1%+/−9.6%, respectively. The maximum post-exercise increase for the right leg was 15.2% and the maximal post-exercise decrease was 23.3%. The maximum post-exercise increase for the left leg was 15.7% and the maximal post-exercise decrease was 22.0%. Two right legs and two left legs had a value lower than >18.5% in this healthy population. Regarding Exercise TcPO2 values, the mean right DROP was −5 +/−3 mmHg whereas the mean left DROP was −6 +/−3 mmHg. The lowest DROP value was −13 mmHg in this healthy population and the maximal DROP value was 1 mmHg. No subject at the leg level had a DROP value lower or equal to −15 mmHg.
Discussion
Post-exercise pressures are key elements for the diagnosis of LEAD in patients with exertional limb symptoms and normal ABI as suggested by the international recommendations3,5. One previous study has analyzed the concordance between exercise tests in a population of LEAD with or without abnormal ABI at rest20. However, no study has specifically studied the concordance between exercise tests in patients with normal ABI at rest. A good concordance between tests would suggest the tests are interchangeable, whereas discordance would indicate that a specific test is more suited to a specific patient population. To our knowledge, this is the first study to assess the concordance between Post-exercise ABI and Exercise-TcPO2 in patients with exertional limb symptoms and normal ABI. This study has demonstrated that the concordance between two exercise tests is only fair implying that Post-Exercise ABI cannot be replaced by Exercise-TcPO2 and vice-versa.
Post-exercise ABI is the most widely used test in clinical routine when ABI is normal at rest. However, there are no guidelines on how to perform the measurement23,24 and cut-off values have not yet reach consensus7,8,23. Mahe et al. have shown that approximately 1 in 5 patients were classified differently depending on which of the two AHA criteria was used7. Several authors have suggested that absolute Post-Exercise ABI decrease is less accurate than relative Post-Exercise ABI to diagnose LEAD6,23. One of the main advantages of Post-exercise ABI is that it is inexpensive and easy to perform in a clinical setting. Conversely, exercise-TcPO2 requires expensive equipment and dedicated software to perform the test21,25. Currently, only a few hospitals have this system available, although this may change in the near future as software to calculate the DROP freely available at no expense25.
The results of the current study suggest that there is discordance between the two tests used to diagnose LEAD. Indeed, we found a discordance between post-exercise ABI decrease >18.5% and the DROP value. This confirms previous results showing discrepancies between AHA post-exercise criteria and DROP value20,26. Several points can explain these discrepancies as suggested by the logistic regression. Age is one of the variables associated independently with the discordance in the present study. As Post-exercise ABI measures the variation of blood pressure in the main arteries, unlike Exercise-TcPO2 which measures the variation of transcutaneous oxygen pressure at capillary level5,27, arterial stiffness is a major source of error in ABI measurement. As shown in many studies, the main determinant of arterial stiffness is age28,29. Thus, discordance being prominent among older patients suggests that the very nature of the parameter being measured can explain the discrepancies. Furthermore, the presence of a stent was also associated with a discrepancy between the two tests. Proportionally, more patients considered as LEAD patients using Post-Exercise ABI (38.5%) than patients considered as suffering from LEAD using Exercise TcPO2 (18.2%) had a medical history of lower limb arterial stenting. One explanation could be that post-exercise ABI is difficult to assess in patients who are more severely affected by arterial disease, as it is when measuring ABI at rest30. Indeed, even with a marked on the skin it could be difficult to find the artery and detect arterial pressure23,30, additional delay in the measurement potentially resulting in inaccuracy. Also, the presence of a stent means the presence of stenosis. Stenosis can be a cause of a drop of downstream perfusion and pressure. A decrease of blood perfusion would affect both measurements while a drop of downstream pressure would only affect ABI measurement. A decrease of downstream pressure while perfusion is preserved occurs when a collateral arterial network is present. Thus, the discrepancies observed among patients with a medical history of arterial stenting could be due to the presence or not of a collateral arterial network. Apart from the variables found by the logistic regression several points can also explain the discrepancies. First, Exercise-TcPO2 is a measurement during exercise whereas Post-exercise ABI is a measure after the end of exercise. It has been suggested in animals and humans that exercise training induces collateral vessel development and enlargement during exercise31,32,33. This might explain the lower prevalence of LEAD found with Exercise-TcPO2 than with Post-exercise ABI in this study. Second, the Clark electrode only allows a small area of skin oxygen pressure measurement. We cannot exclude that this small area might have been well perfused by a collateral or inversely might have been poorly perfused by a partially occluded vessel. Third, systemic hypoxemia can modify Exercise-TcPO2 results whereas this will have no impact on Post-exercise ABI34,35. Additionally, several patients were closed to the cut-off values for Exercise-TcPO2 and Post-exercise ABI meaning that errors in diagnosis may be the consequence of each method precision.
The final interest of the present study is the results of the healthy subjects about Exercise-TcPO2 and Post-exercise ABI. Indeed, to the best of our knowledge, it is the first time that such results obtained with a treadmill test (3.2 km/h and 10% slope) that is currently used in clinical practice are presented. Using Post-exercise criterion, 4 legs out of 74 might be considered as positive for LEAD. However, it is important to underscore that the criterion of a post-exercise ABI decrease greater than 18.5% was validated in patients who experienced pain during walking that was not the case in these healthy subjects8. Furthermore, none of these healthy subjects who walked for 15 minutes had a DROP value lower than −15 mmHg suggesting that the DROP value previously validated is accurate8,10,12,19.
Limitations
This study has several limitations. Firstly, the exercise tests were not randomized. This was due to time constraints during clinical practice. As a result, that rest conditions were not exactly the same in spite of the fact that the Post-exercise ABI walking test was performed after pain had disappeared and TcPO2 values had returned to baseline. Secondly, this study cannot confirm which test is the most accurate. Due to the fact that a disagreement of the magnitude detected was not expected, the computed tomography angiography data as a reference to determine tests accuracy was not acquired. Further work should be conducted to study this issue. Thirdly, it was not possible to assess the reliability of the tests in this study. We do not have our own reliability for the exercise tests but the intra-observer coefficient of variation (CV) for the ABI at rest in our vascular laboratory is 9.4% (typical error of the estimate is 0.06)36. Furthermore, the reliability of the exercise tests has been previously reported by several studies: i) Van Langen et al. showed that the inter-observer variability of the post-exercise ABI was 21%37; ii) intra-test and test-retest reliability of exercise TcPO2 using a similar protocol that we used has been reported by Abraham and colleagues as excellent17,18. Indeed, the correlation coefficient between two tests was r2 = 0.80 at distal level and intra-class correlation coefficients for the intra test-reliability and test-retest reliability were 0.920[0.846–0.967] and 0.807[0.723–0.868] respectively17,18.
Conclusion
This study suggests that Post-Exercise ABI and Exercise-TcPO2 are not equivalent tests. The utility of each test in the management of LEAD must be defined in future studies.
Methods
Ethical standards
The data that support the findings of this study are available from the corresponding author upon reasonable request. The corresponding author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Study design and populations
Suspected lower extremity artery disease patients with normal ABI at rest and exertional limb symptoms:
This is a prospective, monocentric study on consecutive patients referred to the vascular unit of University Hospital, Rennes, France for exertional limb symptoms and normal ABI (>0.90).
The study was conducted from May 2016 to May 2019 and approved by an institutional review board (IRB). All participants gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The Exercise PAD study was registered with the American National Institutes of Health database under reference n° NCT03186391.
Healthy subjects
Healthy subjects were analyzed to present the physiologic range of the responses of exercise tests. Subjects were contacted in our healthy database. To be included healthy subjects had to be able to complete a 15-minute walk on a treadmill (3.2 km/h, 10% slope) without any symptom. Exclusion criteria were:
-ABI < 1.00 or >1.40
-Presence of hypertension, heart failure, angina pectoris, diabetes, chronic obstructive pulmonary disease, supported by the presence of a medical treatment and the medical history;
-Presence of conditions likely to cause a functional limitation in walking and/or significant modification of physiological responses to the exercise: current or former smoker from less than 6 months, cancer (ongoing), Parkinson’s disease, renal failure (ongoing), supported by the presence of a medical treatment and the medical history.
-History of cardiovascular disease (heart failure, stroke, myocardial infarction) reported by the patient.
The part of this study including healthy subjects was conducted between January 2019 and December 2019 and approved by an institutional review board (CPP Ouest II- Angers, France). All participants gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The ELECTROPAD study was registered with the American National Institutes of Health database under reference n° NCT 03795103.
Patient demographic characteristics
Variables collected included age, gender, body mass index, smoking status, comorbidities, and medications (statins, anti-hypertension treatment, antiplatelet, antidiabetic oral treatment or insulin).
ABI measurement
After careful clinical evaluation, a measurement of ABI was performed according to AHA recommendations using a hand-held Doppler probe (8 MHz; Basic Atys Medical, Soucieu en Jarrest, France) by a trained vascular medicine physician4, with the exception of brachial blood pressures being measured using an automated oscillometric blood pressure monitor (Carescape Dinamap V100; GE Healthcare) in order to have the same procedure to measure the pressures at rest and after exercise and ensure almost simultaneous measures of upper and lower limb pressures8. The patient was at rest for 10 minutes in the supine position, relaxed, head and heels supported, in a temperature controlled room (21 °C)30. The following counterclockwise sequence was used: right brachial artery, right posterior tibial artery, right dorsalis pedis artery, left posterior tibial artery, left dorsalis pedis artery, left brachial artery, and right brachial artery. The ABI was calculated by dividing the highest pressure of the limb (dorsalis pedis or posterior tibial pressures) by the highest arm pressure as recommended4,30.
Treadmill test
A treadmill test (3.2 km/h, 10% slope) was used for both the Exercise-TcPO2, which was performed first, and for the Post-Exercise ABI measurements as previously described by Stivalet and colleagues8. A minimum of 10 minutes was required between the two exercise tests. The patients were asked to inform the physician when and where (buttock, thigh, calf or other) the pain appeared8. Exercise was stopped for both studies according to patient limitation or up to a maximal distance of 525 m (over a period of 10 minutes)8.
Exercise-TcPO2 test
Measurement of TcPO2 was performed using calibrated TcPO2 electrodes (TCOM/TcPO2; PF 6000TcPO2/CO2 Unit; Perimed; Jarfalla, Sweden). A reference electrode (chest electrode) was placed between the scapulae to measure systemic changes in TcPO2 during exercise10,11,12,21. One electrode was positioned on each calf. The measurement from the TcPO2 electrodes were used to calculate the Delta from Resting Oxygen Pressure (DROP) index, which was expressed in mmHg, and was the absolute change in TcPO2 from resting value in each of the 2 limb electrodes corrected for the absolute change in TcPO2 at the chest electrode. DROP was recorded in real time by the in-house Oxymonitor (version 2019.01.05) free Software (https://imagemed.univ-rennes1.fr/en/oxymonitor/download.php) as previously described8,25. As defined in previous studies, we considered a DROP ≤ −15 mmHg accurate to diagnose arterial stenoses of ≥50% assessed by computed tomography angiography (CTA) as a gold standard8,10,12.
Post-exercise ABI
Two trained physicians performed these measurements: one at the brachial level with the automatic blood pressure device (Carescape Dinamap V100; GE Healthcare) and one at the limb level with the handheld Doppler. Post-Exercise brachial pressure was assessed on the same artery as it was for the ABI measurement at rest. When the resting ABI was measured, a black pen was used to mark the skin area where the highest limb pressure had been recorded with a hand-held Doppler23,27 in order to shorten the measurement process post exercise. As defined in our previous study, we considered Post-exercise ABI decrease ≥18.5% accurate to diagnose arterial stenoses of ≥50% assessed by computed tomography angiography (CTA) as a gold standard8.
Statistical analyses
Data analysis
Continuous variables were described as mean ± standard deviation (sd) or median and interquartile range (IQR) values, and categorical variables were expressed as numbers (percentages). Baseline characteristics were compared between groups using t-test or U Mann-Whitney for quantitative variables, and chi-squared test or Fisher’s exact test were used for qualitative variables, depending on the distribution. Analysis was performed per patient, rather than per limb and only the symptomatic leg was included in the analysis. In case of both limbs symptomatic, the most symptomatic was analyzed. The concordance between Exercise-TcPO2 and Post-exercise ABI was assessed using the Kappa coefficient with a confidence interval of 95%. The Landis and Koch interpretation of kappa values was used: 0.21–0.40: fair; 0.41–0.60: moderate, 0.61–0.80: substantial; >0.80: almost perfect38. Logistic univariate regressions were performed first to define the variables independently associated with the discordance. Then variables with p value <0.20 were included in multivariate analysis. A backward stepwise procedure was used. A significance threshold of 0.05 was used in all statistical tests. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC, USA) (https://www.sas.com/en_us/home.html).
References
Fowkes, F. G. R. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet Lond. Engl. 382, 1329–1340 (2013).
Hirsch, A. T. et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113, e463–654 (2006).
Gerhard-Herman, M. D. et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135, e726–e779 (2017).
Aboyans, V. et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 126, 2890–2909 (2012).
Aboyans, V. et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 39, 763–816 (2018).
Aday, A. W., Kinlay, S. & Gerhard-Herman, M. D. Comparison of different exercise ankle pressure indices in the diagnosis of peripheral artery disease. Vasc. Med. Lond. Engl., https://doi.org/10.1177/1358863X18781723 (2018).
Mahe, G. et al. Discordant Diagnosis of Lower Extremity Peripheral Artery Disease Using American Heart Association Postexercise Guidelines. Medicine (Baltimore) 94, e1277 (2015).
Stivalet, O. et al. Exercise testing criteria to diagnose lower extremity peripheral artery disease assessed by computed-tomography angiography. PloS One 14, e0219082 (2019).
Tehan, P. E., Barwick, A. L., Sebastian, M. & Chuter, V. H. Diagnostic accuracy of the postexercise ankle-brachial index for detecting peripheral artery disease in suspected claudicants with and without diabetes. Vasc. Med. Lond. Engl. 23, 116–125 (2018).
Abraham, P. et al. Transcutaneous oxygen pressure measurements on the buttocks during exercise to detect proximal arterial ischemia: comparison with arteriography. Circulation 107, 1896–1900 (2003).
Mahe, G., Kalra, M., Abraham, P., Liedl, D. A. & Wennberg, P. W. Application of exercise transcutaneous oxygen pressure measurements for detection of proximal lower extremity arterial disease: a case report. Vasc. Med. Lond. Engl. 20, 251–255 (2015).
Koch, C. et al. Exercise transcutaneous oxygen pressure measurement has good sensitivity and specificity to detect lower extremity arterial stenosis assessed by computed tomography angiography. Medicine (Baltimore) 95, e4522 (2016).
de Groote, P. et al. Comparative diagnostic value of ankle-to-brachial index and transcutaneous oxygen tension at rest and after exercise in patients with intermittent claudication. Angiology 46, 115–122 (1995).
Christensen, K. S., Larsen, J. F. & Klaerke, M. Transcutaneous oxygen tension response to exercise in health and in occlusive arterial disease. Acta Chir. Scand. 152, 657–660 (1986).
Larsen, J. F., Christensen, K. S. & Egeblad, K. Assessment of intermittent claudication by means of the transcutaneous oxygen tension exercise profile. Eur. J. Vasc. Surg. 4, 409–412 (1990).
Schmidt, J. A., Bracht, C., Leyhe, A. & von Wichert, P. Transcutaneous measurement of oxygen and carbon dioxide tension (TcPO2 and TcPCO2) during treadmill exercise in patients with arterial occlusive disease (AOD)–stages I and II. Angiology 41, 547–552 (1990).
Henni, S. et al. Intra-test and test-retest reliability of exercise oximetry in arterial claudication. Microvasc. Res. 117, 44–49 (2018).
Bouyé, P. et al. Reproducibility of proximal and distal transcutaneous oxygen pressure measurements during exercise in stage 2 arterial claudication. Int. Angiol. J. Int. Union Angiol. 23, 114–121 (2004).
Audonnet, M. et al. Exercise Transcutaneous Oximetry of the Buttocks- External Validation With Computed Tomography Angiography. Circ. J. Off. J. Jpn. Circ. Soc. 81, 1123–1128 (2017).
Abraham, P. et al. Comparison of exercise oximetry and ankle pressure measurements for patients with intermittent claudication: an observational study of 433 patients. Pflugers Arch. https://doi.org/10.1007/s00424-019-02340-w (2020).
Mahé, G., Kaladji, A., Le Faucheur, A. & Jaquinandi, V. Internal Iliac Artery Stenosis: Diagnosis and How to Manage it in 2015. Front. Cardiovasc. Med. 2, 33 (2015).
Mahé, G. et al. Exercise treadmill testing in patients with claudication, with and without diabetes. Diabet. Med. J. Br. Diabet. Assoc. 28, 356–362 (2011).
Stivalet, O., Laneelle, D., Omarjee, L. & Mahe, G. Post-exercise criteria to diagnose lower extremity peripheral artery disease: Which one should I use in my practice? Vasc. Med. Lond. Engl. 24, 76–77 (2019).
Stivalet, O., Lanéelle, D., Mahé, G., Jaquinandi, V. & Omarjee, L. Time to redefine post-exercise pressure decrease and post-exercise ankle-brachial index to diagnose peripheral artery disease. Scand. J. Med. Sci. Sports 28, 2457–2458 (2018).
Poulin, A. et al. Validation of a software to perform exercise oximetry to diagnose arterial stenosis of the lower limbs. Atherosclerosis 278, 325–327 (2018).
Mahe, G. et al. Confirmation of discrepancies between exercise oximetry and American Heart Association post-exercise criteria to diagnose peripheral artery disease in patients with normal ankle-brachial index at rest. Pflüg. Arch. - Eur. J. Physiol. (2020).
Mahé, G. & Jaquinandi, V. Diagnosis of lower limb peripheral artery disease. Presse Medicale Paris Fr. 1983 https://doi.org/10.1016/j.lpm.2017.09.021 (2017).
Nam Su, C. et al. Factors Affecting the Validity of Ankle-Brachial Index in the Diagnosis of Peripheral Arterial Obstructive Disease. Angiology 61, 392–396 (2010).
O’Rourke, M. F., Safar, M. E. & Dzau, V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc. Med. Lond. Engl. 15, 461–468 (2010).
Chaudru, S. et al. Training to Perform Ankle-Brachial Index: Systematic Review and Perspectives to Improve Teaching and Learning. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 51, 240–247 (2016).
Cohoon, K., Mahe, G., Liedl, D., Rooke, T. & Wennberg, P. Discrepancies in Prevalence of Peripheral Arterial Disease between Lower Extremities at Rest and Postexercise. Int. J. Angiol. 26, 179–185 (2017).
Prior, B. M. et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am. J. Physiol. Heart Circ. Physiol. 287, H2434–2447 (2004).
Ziegler, M. A. et al. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirc. N. Y. N 1994 17, 3–20 (2010).
Ouedraogo, N. et al. Chest tcpO2 changes during constant-load treadmill walking tests in patients with claudication. Physiol. Meas. 32, 181–194 (2011).
Bruneau, A. et al. The walking-induced transient hack concept is valid & relies on a transient early-exercise hypoxemia. PloS One 8, e62651 (2013).
Donnou, C. et al. How to become proficient in performance of the resting ankle–brachial index: Results of the first randomized controlled trial. Vasc. Med., https://doi.org/10.1177/1358863X17740993 (2017).
van Langen, H., van Gurp, J. & Rubbens, L. Interobserver variability of ankle–brachial index measurements at rest and post exercise in patients with intermittent claudication. Vasc. Med. 14, 221–226 (2009).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Acknowledgements
Several parts of the method section of this manuscript are similar to method sections described in our previous studies8,11,12.
Author information
Authors and Affiliations
Contributions
A.L.F. and G.M.: Conception and design of the protocol. F.C., Q.T., P.J., A.G., G.L., L.O. and G.M.: Data Acquisition. G.M., F.C. and E.L.P.: Data Analysis and writing the report. G.M., F.C. and A.L.F.: Data interpretation and drafted the work. Each author revised the report and approved the submitted version of the manuscript. Each author has agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahé, G., Catillon, F., Tollenaere, Q. et al. Discordance of peripheral artery disease diagnosis using exercise transcutaneous oxygen pressure measurement and post-exercise ankle-brachial index. Sci Rep 10, 7419 (2020). https://doi.org/10.1038/s41598-020-64276-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-64276-x