Abstract
The incidence of pulmonary and venous thromboembolism is increased during the first trimester of pregnancies after assisted reproductive technology (ART) compared to spontaneous conception. We previously found that haemostatic plasma variables changed but within normal limits during controlled ovarian hyperstimulation (COH) concomitant with a major increase in plasma microvesicles (MVs) and markers indicating cell activation. We now explored the proteome of these MVs. Thirty-one women undergoing ART were blood sampled at down-regulation (DR) of oestrogen and at high level stimulation (HLS) with its 10–100-fold increased oestrogen level. Samples were analysed by liquid chromatography and tandem mass spectrometry to identify and quantify the proteome. We identified 306 proteins in the MVs and 72 had changed significantly at HLS compared to DR and more than 20% of them were associated with haemostasis. Thus, proteins related to both haemostasis and complement activation altered in plasma MVs in parallel with MV activation during COH. This needs to be further explored in the clinical context.
Similar content being viewed by others
Introduction
Assisted reproductive technology (ART) is increasingly used to treat infertility since 1978. In vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) are the most applied of the ARTs. ICSI was developed to treat male factor infertility i.e. when the cause was sperm-related. ICSI has today in many clinics replaced IVF irrespective of the cause of infertility. To date more than ten million people have been born after IVF and ICSI1.The method is considered effective and safe, with approximately a third of the embryo transfers resulting in a pregnancy2. In 2013 we found that the incidence of venous thromboembolism (VTE) increased during the first trimester of ART pregnancies with a sevenfold increase in pulmonary embolism (PE) as compared to matched pregnant women3. It is of course of immense importance to understand the pathogenesis of this increased incidence of potentially lethal PE. PE is indeed a dominating cause of maternal mortality4. During the controlled ovarian hyperstimulation (COH) phase of ART there is a huge increase in endogenous oestrogen levels. It is thus plausible that oestrogen could have a causal role related to the increased incidence of VTE and PE5.
We and others have identified changes in haemostatic variables in plasma during COH. In our studies both thrombin generation and fibrin formation were altered in the direction of a procoagulable state though still within reference values6. To assess platelet function in vivo and in vitro is methodologically demanding and there is thus a lack of knowledge of changes in cell-based haemostasis during ART. Microvesicles (MVs) are small vesicles generated and released from cells undergoing cell activation or apoptosis. The cellular origin of MVs can be determined through analysis of membrane bound surface markers with specific monoclonal antibodies. Besides surface markers, MVs can harbour various cytokines, growth factors and proteases connecting them to thrombosis, inflammation and immune responses. We have previously found elevated levels of MVs in patients with acute coronary syndrome7,8, type 1 diabetes9 and peripheral arterial occlusive disease10. Circulating MVs might thus be conceivable carriers of cellular candidate biomarkers that could help to identify high-risk patients or to assess disease activity in patients.
In a previous study we found that circulating cell-derived MVs, with platelet-derived MVs in particular, increased during the COH phase of ART (Table 1)11. Besides an increase in total numbers of MVs, we found that MVs with specific markers of activation and inflammation increased significantly. Conceivable mechanistic explanations might be identified by studying changes in the protein composition of MVs during COH. We thus probed modifications of plasma MVs during ART by performing a proteomic analysis of MV-enriched pellets. The protein content of such pellets represents less than 0.003% of the total amount of plasma proteins and changes in this fraction could thus not be detected if plasma was analysed. The plasma MV fraction was dominated by platelet-derived MVs and thus offers a chance to study platelet modification. However, flow cytometric studies are restricted to capture quantitative and qualitative changes of membrane bound proteins, whilst proteomic analysis offers a possibility to capture modifications in the whole proteome of the MVs.
Our hypothesis is that the increased incidence of PE and deep venous thrombosis (DVT) during the first trimester of pregnancies after ART is initiated by the profound increase in endogenous oestrogen levels during COH. Our approach was thus to explore the cellular response to endogenous oestrogen by assessment of the protein composition of MVs and the qualitative and quantitative changes in their protein constituents during ovarian stimulation. The objective was to enable a better understanding of cellular mechanisms and pathways, both physiological and pathological, affected during ART, which could help to explore potential mechanisms explaining the increased incidence of PE and DVT in ART-pregnancies.
Results
We identified 1,199 proteins in the MV fraction of plasma during the COH phase of ART. Out of these identified proteins, 306 were quantified in more than 50% of all samples and were used in further analysis using moderated t-tests for paired comparison of protein abundance at high level stimulation (HLS), with its 10–100-fold increased oestradiol (E2) levels, to the abundance at down-regulation (DR), with E2 levels less than 150 pmol/L6. T-test originated P-values were corrected for multiple comparisons by the Benjamini–Hochberg method. Proteins with corrected P-values less than 0.01 were considered significantly changed and we thus found 72 significantly changed proteins; 28 of those were up-regulated and 44 were down-regulated. (Fig. 1, Table 2, Supplementary Table S1). We found that the dominating cellular compartment of the enriched proteins were vesicle bound with more than half of the up-regulated proteins annotated as blood microvesicle proteins (GO:0072562; Fig. 2)12. Furthermore, the most abundant protein in plasma, albumin, was not abundant in any of our samples and these results indicate that the samples constituted a representative MV pellet.
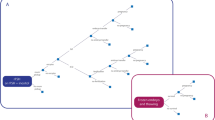
Protein-to-protein interaction showing in red colour the proteins identified as blood microvesicle-bound (n = 162 of 206 recognized). The network diagram was originally obtained with STRING database12.
A large number of the altered proteins are associated to the coagulation cascade but also to the complement cascade. Most of these proteins can be seen in the picture using the KEGG database (Fig. 3)13. Dominating pathways and most enriched biological processes were those of blood coagulation and fibrinolysis, including more than a third of the identified pathways.
Coagulation and complement cascade proteins significantly altered (P < 0.01) during controlled ovarian hyperstimulation (n = 18). Red denotes up-regulated proteins. Blue denotes down-regulated proteins. The diagram was originally obtained from the KEGG database13.
Discussion
We found significant alterations in the MV proteome related to coagulation. The potential implications of these findings and other identified proteins that might be of relevance will be discussed.
We detected several shifts in protein levels that might be anticipated following the increased oestrogen level during COH. One example is the up-regulation of oestrogen sensitive Sex Hormone Binding Globulin (SHBG), which is the main carrier of E2 in the circulation. This has previously been found in plasma during COH14. Furthermore, the acute phase protein alpha-1-antitrypsin (SERPINA1) was up-regulated and has previously been found to increase in pregnancy and to decrease in post-menopausal women15. It has been speculated that this protein could be related to a successful pregnancy16. Moreover, oestrogen might trigger secretion of von Willebrand Factor (VWF) from endothelial cells and in line with this we found VWF to be up-regulated in MVs at HLS. Other MV proteins decreased concurrently with the increase in oestrogen levels. We found decreased antithrombin, which has previously been found to decrease in plasma following the increased oestrogen level during COH17.
Furthermore, we found alterations in coagulation proteins, as mentioned and illustrated in Fig. 3, with an up-regulation in MVs of VWF and fibrinogen, which are both essential for clot formation in the conversion of fibrinogen to fibrin when the coagulation system is activated. VWF is a large multimeric, adhesive protein important to normal haemostasis adhering both to the subendothelium, the site of injury, and to platelet-surface-receptors. VWF in plasma increases in response to inflammation and is released from the endothelium as well as from platelets upon activation.
We found that kininogen-1 (KNG1), vitronectin (VTN) and alpha-1-antitrypsin (SERPINA1) were up-regulated, while the natural anticoagulants, antithrombin (SERPINC1) and protein S (PROS1), were down-regulated. High-molecular-weight-kininogen can be expressed by activated platelets on their surface membrane and the protein is crucial to the intrinsic system of coagulation as a co-factor for prekallikrein, FIX and FXII, positioning them for facilitation of activation. VTN has been shown to be incorporated in the fibrin clot18. It is a cofactor of plasminogen activator inhibitor (PAI-1), binds to and stabilises PAI-1, one of the most important inhibitors of fibrinolysis, through inhibition of plasminogen activator (tPA). Thus, increased levels of VTN might decrease fibrinolysis. Furthermore, VTN has been shown to augment platelet adhesion and platelet aggregation in the formation a blood clot19.
Apart from the proteins shown in Fig. 3, there were other proteins with substantial alterations that might affect haemostasis, such as histidine-rich glycoprotein (HRG) and tetranectin (CLEC3B). These were found to be down-regulated. HRG circulates in plasma and is found in the alpha-granules of platelets which are released during platelet activation. HRG binds to FXIIa and inhibits contact activation and can also bind to fibrinogen and plasminogen and thus inhibit both coagulation and fibrinolysis20. Animal studies have shown that HRG-deficient mice have an accelerated arterial thrombus formation21. These findings suggest that HRG may act both as an anticoagulant and as an antifibrinolytic modifier and might thus control platelet function in vivo. CLEC3B binds to the kringle 4 region of plasminogen and activates the change of plasminogen to plasmin and might thus have a potential to interact and decrease fibrinolysis. Decreased levels have been shown in coronary artery disease22.
We also found nine significantly down-regulated proteins associated to the complement system during COH (P < 0.01). The complement system is in turn closely connected to the similarly structured coagulation system with an interaction during inflammation, healing of tissue damage and in the development of a thrombosis. We found down-regulation of C1-inhibitor (C1INH), which acts by binding to FXII and kallikrein and thus inhibits activation of the intrinsic system. It further inhibits thrombin directly and plasmin. C1INH inhibits the first component of the complement system, i.e. complement C1r and C1s. Decreased levels of complement factors have been found in human follicular fluid during ART and complement factor I has been suggested to be a biomarker for fertilisation23.
Fetuin-B (FETUB) was also found to increase. FETUB is a member of the Cystatin superfamily and is increased in females. This protein is required for egg fertilisation and loss of FETUB decreases fertility due to hardening of the Zona pellucida membrane that surrounds the oocyte, thereby hindering binding and penetration of the sperm. A recent study indicated an association of high FETUB concentrations in serum with fertilisation rate in ART24. FETUB has not been extensively studied but has been found to increase in patients with acute myocardial infarction25 and in other states of increased cardiovascular risk such as in females treated by oestrogen in Women´s Health Initiative26. FETUB is thus both related to fertility and cardiovascular risk and should indeed be further studied.
In addition, we also detected further alterations in potential risk markers of thrombosis. Fibulin-1 (FBLN1) was up-regulated in MVs. This is a protein linked to thrombosis by its binding to fibrinogen, mediating platelet adhesion27, and has been proposed to be a biomarker for cardiovascular disease in patients with diabetes and kidney disease28.
As described above we found changes in MVs of several proteins previously known to change during COH, but also some sparsely detected protein changes. A link between the supraphysiologic oestrogen levels during COH and a procoagulant and proinflammatory state was strengthened by the present results identified in MVs.
With regard to the limitations of our study, it is worth noting that the population of study was small. We detected MV proteome alterations with statistical significance since we compared samples before and after COH with each woman being her own control. The changes found might though be of clinical relevance and help to reveal mechanisms explaining the increased incidence of VTE and PE in ART-pregnancies and open up for further studies. VTE is a rare event afflicting about one to two out of 1,000 pregnant women. Despite the major relative increase during the first trimester—sevenfold as concerns PE—in ART-pregnancies3 any associations between thromboembolic events and changes in the MV proteome should require a rather large study.
We analysed MV-enriched samples with the majority of those being platelet-derived MVs, but in the present study we could not derive these proteins to a specific parent cell. The whole proteome and alterations of the proteome before and after COH were analysed, thus including both membrane-bound and proteins found within the MV. Furthermore, our technique has a limit in the detection of low abundance proteins.
Conclusion
Haemostatic pathway proteins were identified and shown to alter in plasma MVs during the controlled ovarian hyperstimulation phase of ART. These changes thus occurred in the phase of the ART procedure that directly precedes the first trimester with its increased incidence of PE and VTE. It is of utmost importance to elucidate the potential mechanisms behind this serious side effect of ART. Such findings might be potential biomarkers that could be used to identify women at risk of VTE and might indicate a need of adequate prophylactic treatment. Identification of biomarkers might lead to early detection of increased risk and offer a possibility to prevent VTE and potentially lethal PE. Identification of the pathophysiological mechanisms might in the future lead to effective treatment and prophylaxis.
Some of the proteome alterations found might also be useful as potential biomarkers of successful ART; reflecting the processes of oocyte maturation, fertilisation, embryogenesis and implantation, and other alterations might be biomarkers reflecting detrimental processes during ART such as those causing a procoagulant, prothrombotic state.
The clinical usefulness of identified changes in protein levels will necessitate longitudinal studies with large patient cohorts and thus enough cardiovascular events to investigate their potential in the prediction and prevention of events such as PE and VTE in women undergoing ART.
Methods
Study population
The present study population and sampling has previously been described. It comprised thirty-one women aged 25–38 years who were treated by ART at the Fertility Unit of Karolinska University Hospital, Stockholm (Table 1)6. The study was approved by the Regional Ethical Review Board in Stockholm, reference number 2006/1223-31/1. All patients gave their informed consent to participate. The research was conducted in accordance with the Declaration of Helsinki on ethical principles in medical research. Women with a family history of arterial or venous thrombosis, polycystic ovarian syndrome or ongoing anticoagulation were excluded and no woman was afflicted by arterial hypertension, diabetes mellitus or dyslipidaemia. As described the power exceeded 80% to detect a 20% difference at a two-sided alpha level of 0.056.
As previously described, the long protocol for the ovarian stimulation phase of ART was applied6. This was initiated by the gonadotropin releasing hormone (GnRH) agonist, Buserelin (Suprecur, Aventis Pharma, Frankfurt, Germany), to down-regulate oestrogen synthesis. GnRH was given in the form of a nasal spray, 300 µg three times daily, starting on the 21st day of the menstrual cycle. E2 levels were assessed after the menstrual bleeding two weeks later. Ovarian stimulation started when E2 was less than 150 pmol/L. Recombinant follicular stimulating hormone (FSH) was initiated as daily subcutaneous injections of 75–300 IU daily of follitropin alpha, Gonal-f (Merck Serono, Stockholm, Sweden) or follitropin beta, Puregon (Schering-Plough, Gothenburg, Sweden). Concomitantly, the GnRH-agonist was reduced to twice daily. E2 was assessed after 6 days and an ultrasound of the ovaries was performed at day 9–10 after initiation of FSH as previously described6.
The women were fasting when they left blood samples, first at the time point maximal down-regulation (DR) and the second time at high level stimulation (HLS), immediately before they received human chorionic gonadotropin (hCG). Samples were taken by direct venepuncture into a medium of buffered sodium citrate (nine parts blood and one part of sodium citrate, 0.129 mol/L; pH 7.4). The samples were centrifuged at 2000g for 20 min at room temperature (RT) to obtain platelet-poor plasma and plasma aliquots were stored at the temperature of − 80 °C.
Microvesicle enrichment
To prepare a MV-enrich pellet for proteomic analysis, a series of centrifugation steps were performed, as described elsewhere29. Briefly, the stored PPP (500 µl) was thawed at 37 °C for approximately 5 min. After thawing, the plasma was centrifuged at 2000g for 20 min at RT to exclude any large particles or cells that might interfere with the analysis. The supernatant was then centrifuged at 20,800g for 45 min at RT. The upper part of the supernatant was discarded (450 µL) and the remaining 50 µL pellet was mixed with 450 µl of PBS (Phosphate-buffered saline, pH 7.4) and centrifuged again at 20,800g for 45 min in RT. After centrifugation, the upper part of the supernatant was discarded (450 µL) and the remaining 50 µl of MV-enriched pellet was vortexed for 10–15 s. The prepared MV-samples was then stored in − 80 °C until proteomics analysis.
Microvesicle proteomics: protein digestion
The MV enriched plasma samples were thawed on ice and homogenised by vortexing. Small aliquots were mixed 1:8 with 0.5 M Urea and 2.5% methanol and the protein concentration was determined using Micro BCA from Pierce (Thermo Fisher Scientific Inc). From each sample 2 × 10 µg of protein was dissolved in a final concentration of 0.1% ProteaseMax (Thermo Fisher Scientific Inc), 0.05 M Urea, 50 mM ammonium bicarbonate and 10% methanol in a total volume of 80 µL. The resulting protein solutions were sonicated (2 cycles, each cycle 20 s with 40% amplitude) on ice using a probe (Vibra-Cell CV18, Sonics & Materials, Newtown, CT, USA) followed by incubation for 30 min at 37 °C while shaking. Samples were centrifuged and directly subjected to a tryptic digestion protocol carried out by a liquid handling robot (MultiProbe II, Perkin Elmer) as previously described30. This included protein reduction in 5 mM DTT at 56 °C and alkylation in 15 mM iodacetamide for 30 min at RT in the dark. Trypsin was added in an enzyme to protein ratio of 1:30 and digestion was carried out over night at 37 °C. Samples were acidified by adding 6 µL concentrated formic acid, incubated for 30 min at RT and centrifuged for 20 min at 1,000g to remove undigested material.
Microvesicle proteomics: liquid chromatography tandem mass spectrometry
Proteomic analysis was initiated with a pilot study of four samples, of which one sample was run in two replicates. The results were consistent with previous findings in the research group, that the biological variation between individuals was larger than the technical variation between samples due to handling of the samples and technique.
As previously described, tryptic peptides were cleaned and injected into an UltiMate Nano system (Thermo Scientific, Bremen, Germany) in-line coupled to a Q Exactive mass spectrometer (Thermo Scientific, Bremen, Germany)30. The chromatographic separation of the peptides was achieved using a 25 cm long, ID 150 µm in-house packed column (C18-AQ ReproSil-Pur, Dr. Maisch GmbH, Germany) at 55 °C with the following gradient: 4 − 30% acetonitrile in 89 min, 30 − 95% ACN for 5 min and 95% ACN for 8 min all at a flow rate of 250 nL/min, as previously described30.
As also previously described, the MS-acquisition method comprised of one survey full scan ranging from m/z 300 to m/z 1,650 acquired with a resolution of R = 140,000 at m/z 200 and with a target value of 5e6, followed by data-dependent higher-energy collisional dissociation fragmentation scans from maximum sixteen most intense precursor ions with a charge state greater than or equal to 230. Sequencing was accomplished with a target value of 2e5 ions as determined by predictive automatic gain control, for which the isolation of precursors was performed with a window of 4 m/z. Scans were acquired as profile data with a resolution of R = 17,500 and normalised collision energy was set to 26.
Data analysis
MaxQuant software (version 1.5.3.30) was used for analysis of MS raw data31. We used a false discovery rate (FDR) of 0.01 for proteins and peptides and required a minimum peptide length of six amino acids as described in a previous study32. We improved the mass accuracy of precursor ions by the MaxQuant time-dependent recalibration algorithm and as described in the previously mentioned study32 we used the Andromeda search engine to search MS/MS spectra against the Uniprot human database combined with 262 common contaminants and concatenated with reversed versions of all sequences. Enzyme specificity was set to trypsin specificity and further modifications were cysteine carbamidomethylation (fixed) as well as protein N-terminal acetylation, asparagine and glutamine deamidation and methionine oxidation (variable) as previously described32. MaxQuant was used for identification of the peptides and a maximum of two missed cleavages was allowed. Peptide identification was based on a search with an initial mass deviation of the precursor ion of up to 7 ppm. The fragment mass tolerance was set to 20 ppm. Only proteins quantified with at least two peptides were considered for quantitation. As shown by Lyutvinskiy et al. accurate relative quantification does not require internal standard33.
Normalisation of the data was performed by calculating the summed intensities of all proteins in each sample and the median of all these summed intensities over the entire sample set. Each quantitative value was multiplied by the median/summed intensity and the resulting values were log2-transformed. Differences in relative protein abundances between DR and HLS were assessed by moderated paired t-test using limma package34. Benjamini–Hochberg corrections for multiple comparisons were used. Gene ontology enrichment analyses was performed in software databases; DAVID35, STRING12, PANTHER36 and KEGG13.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Fauser, B. C. Towards the global coverage of a unified registry of IVF outcomes. Reprod. Biomed. Online38, 133–137. https://doi.org/10.1016/j.rbmo.2018.12.001 (2019).
Kupka, M. S. et al. Assisted reproductive technology in Europe, 2010: Results generated from European registers by ESHREdagger. Hum. Reprod.29, 2099–2113. https://doi.org/10.1093/humrep/deu175 (2014).
Henriksson, P. et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: Cross sectional study. Br. Med. J.346, e8632. https://doi.org/10.1136/bmj.e8632 (2013).
Berg, C. J., Chang, J., Callaghan, W. M. & Whitehead, S. J. Pregnancy-related mortality in the United States, 1991–1997. Obstet. Gynecol.101, 289–296. https://doi.org/10.1016/s0029-7844(02)02587-5 (2003).
Olausson, N. et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilization with fresh respectively frozen-thawed embryo transfer: Nationwide cohort study. J. Thromb. Haemost. https://doi.org/10.1111/jth.14840 (2020).
Westerlund, E. et al. Detection of a procoagulable state during controlled ovarian hyperstimulation for in vitro fertilization with global assays of haemostasis. Thromb. Res.130, 649–653. https://doi.org/10.1016/j.thromres.2011.11.024 (2012).
Skeppholm, M., Mobarrez, F., Malmqvist, K. & Wallen, H. Platelet-derived microparticles during and after acute coronary syndrome. Thromb. Haemost.107, 1122–1129. https://doi.org/10.1160/th11-11-0779 (2012).
Kafian, S., Mobarrez, F., Wallen, H. & Samad, B. Association between platelet reactivity and circulating platelet-derived microvesicles in patients with acute coronary syndrome. Platelets26, 467–473. https://doi.org/10.3109/09537104.2014.940304 (2015).
Tehrani, S. et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb. Res.126, e225-231. https://doi.org/10.1016/j.thromres.2010.05.023 (2010).
Mobarrez, F. et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thromb. Haemost.106, 344–352. https://doi.org/10.1160/th10-12-0810 (2011).
Olausson, N. et al. Microparticles reveal cell activation during IVF—A possible early marker of a prothrombotic state during the first trimester. Thromb. Haemost.116, 517–523. https://doi.org/10.1160/th15-12-0970 (2016).
Szklarczyk, D. et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res.45, D362–D368. https://doi.org/10.1093/nar/gkw937 (2017).
Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res.47, D590–D595. https://doi.org/10.1093/nar/gky962 (2019).
Shiina, Y. Changes of estradiol (E2) concentration and sex hormone binding globulin (SHBG) concentration in serum during controlled ovarian hyperstimulation (COH) in pregnant and non-pregnant women. Gynecol. Obstet.4, 1–4. https://doi.org/10.4172/2161-0932.1000215 (2014).
Hashimoto, S., Miwa, M., Akasofu, K. & Nishida, E. Changes in 40 serum proteins of post-menopausal women. Maturitas13, 23–33 (1991).
Madar, T. et al. Low levels of circulating alpha-1 antitrypsin are associated with spontaneous abortions. J. Matern. Fetal Neonatal Med.26, 1782–1787. https://doi.org/10.3109/14767058.2013.801955 (2013).
Bremme, K., Wramsby, H., Andersson, O., Wallin, M. & Blomback, M. Do lowered factor VII levels at extremely high endogenous oestradiol levels protect against thrombin formation?. Blood Coagul. Fibrinolysis5, 205–210 (1994).
Wu, Y. P. et al. Fibrin-incorporated vitronectin is involved in platelet adhesion and thrombus formation through homotypic interactions with platelet-associated vitronectin. Blood104, 1034–1041. https://doi.org/10.1182/blood-2003-12-4293 (2004).
Preissner, K. T. & Reuning, U. Vitronectin in vascular context: Facets of a multitalented matricellular protein. Semin. Thromb. Hemost.37, 408–424. https://doi.org/10.1055/s-0031-1276590 (2011).
MacQuarrie, J. L. et al. Histidine-rich glycoprotein binds factor XIIa with high affinity and inhibits contact-initiated coagulation. Blood117, 4134–4141. https://doi.org/10.1182/blood-2010-07-290551 (2011).
Vu, T. T. et al. Arterial thrombosis is accelerated in mice deficient in histidine-rich glycoprotein. Blood125, 2712–2719. https://doi.org/10.1182/blood-2014-11-611319 (2015).
Chen, Y. et al. Tetranectin as a potential biomarker for stable coronary artery disease. Sci. Rep.5, 17632. https://doi.org/10.1038/srep17632 (2015).
Estes, S. J. et al. A proteomic analysis of IVF follicular fluid in women ≤ 32 years old. Fertil. Steril.92, 1569–1578. https://doi.org/10.1016/j.fertnstert.2008.08.120 (2009).
Floehr, J. et al. Association of high fetuin-B concentrations in serum with fertilization rate in IVF: a cross-sectional pilot study. Hum. Reprod.31, 630–637. https://doi.org/10.1093/humrep/dev340 (2016).
Jung, S. H. et al. The serum protein fetuin-B is involved in the development of acute myocardial infarction. Clin. Sci. (Lond)129, 27–38. https://doi.org/10.1042/cs20140462 (2015).
Katayama, H. et al. Application of serum proteomics to the Women’s Health Initiative conjugated equine estrogens trial reveals a multitude of effects relevant to clinical findings. Genome Med.1, 47. https://doi.org/10.1186/gm47 (2009).
Godyna, S., Diaz-Ricart, M. & Argraves, W. S. Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood88, 2569–2577 (1996).
Scholze, A. et al. Plasma concentrations of extracellular matrix protein fibulin-1 are related to cardiovascular risk markers in chronic kidney disease and diabetes. Cardiovasc. Diabetol.12, 6. https://doi.org/10.1186/1475-2840-12-6 (2013).
Mobarrez, F. et al. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb. Res.125, e110-116. https://doi.org/10.1016/j.thromres.2009.10.006 (2010).
Lee, R. F. S. et al. Expression proteomics study to determine metallodrug targets and optimal drug combinations. Sci. Rep.7, 1590. https://doi.org/10.1038/s41598-017-01643-1 (2017).
Wichmann, C. et al. MaxQuant.Live enables global targeting of more than 25,000 peptides. Mol. Cell. Proteom.18, 982–994. https://doi.org/10.1074/mcp.TIR118.001131 (2019).
Song, J. et al. An improvement of shotgun proteomics analysis by adding next-generation sequencing transcriptome data in orange. PLoS ONE7, e39494. https://doi.org/10.1371/journal.pone.0039494 (2012).
Lyutvinskiy, Y., Yang, H., Rutishauser, D. & Zubarev, R. A. In silico instrumental response correction improves precision of label-free proteomics and accuracy of proteomics-based predictive models. Mol. Cell. Proteomics12, 2324–2331. https://doi.org/10.1074/mcp.O112.023804 (2013).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res.43, e47. https://doi.org/10.1093/nar/gkv007 (2015).
da Huang, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res.37, 1–13. https://doi.org/10.1093/nar/gkn923 (2009).
Thomas, P. D. et al. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res.13, 2129–2141. https://doi.org/10.1101/gr.772403 (2003).
Acknowledgements
We thank Karin Persdotter Eberg for assisting in patient inclusion at the ART Unit of Karolinska University Hospital. The study was funded mainly by the Swedish Heart-Lung Foundation, the Swedish Research Council funding for Clinical Research in Medicine, the Regional Agreement on Medical Training and Clinical Research between Stockholm County Council and Karolinska Institutet. Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
P.H., principal investigator, has performed the main part of the design of the study, analyses of data, writing parts of the article and approved the article. N.O. has taken part in the analyses the data, written the article draft, taken part in revising all parts of the article and approved the article. R.Z. and his laboratory have performed the LC–MS/MS analysis, mainly written the methodology part on the proteome analysis, revised parts of the article and approved the article. D.R. has been involved in the proteome analysis, revised parts of the article and approved the article. A.C. has been involved in the proteome analysis, revised parts of the article and approved the article. F.M. has taken part in the design of the study, performed analyses, of data, written the methodology part on microvesicle analysis, revised all parts of the article and approved the article. H.W. has taken part in designing the study, analysing of the results, revised parts of the article and approved the article. E.W. has taken part in designing the study, included the patients and collected the data, revised parts of the article and approved the article. O.H. has provided her expertise in designing the study, revised parts of the article and approved the article. K.B. has taken part in the discussion, revised parts of the article and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olausson, N., Mobarrez, F., Zubarev, R. et al. Changes in the plasma microvesicle proteome during the ovarian hyperstimulation phase of assisted reproductive technology. Sci Rep 10, 13645 (2020). https://doi.org/10.1038/s41598-020-70541-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-70541-w