Abstract
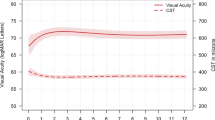
To describe the surgical outcomes of using human amniotic membrane (hAM) grafts in the management of retinal breaks in diabetic tractional detachment (TRD) and combined tractional and rhegmatogenous retinal detachment (CTRRD). A retrospective case series of 10 eyes with TRD or CTRRD receiving pars plana vitrectomy with hAM grafts implantation, compared with 13 controls receiving the same surgery without hAM grafts. Best-corrected visual acuity (BCVA) and re-detachment rate were compared between two groups. Postoperatively, all eyes in the hAM group had retina attachment without recurrence, while 9 eyes in the control group had retina re-detachment and required additional surgery (0% vs 69.2%, p = 0.003). The BCVA significantly improved in the hAM group (from 1.96 ± 0.95 to 1.44 ± 0.77 in log MAR, p = 0.03), but not improved in control group (p = 0.20). Postoperative optical coherence tomography of the eyes receiving hAM grafts demonstrated glial tissue regeneration and restoration of ellipsoid zone. In diabetic TRD or CTRRD, hAM grafts could be an effective method, with promising outcome. Compared to standard surgery, it could result in higher retina reattachment rate and significant visual improvement. Moreover, it may offer the adjunctive benefit in tissue regeneration and fasten ellipsoid zone restoration.
Similar content being viewed by others
Introduction
Diabetic tractional retinal detachment (TRD) now still remains a surgical challenge to vitreoretinal surgeons. To manage the fibrovascular membrane, iatrogenic breaks are often encountered during membrane segmentation and delamination and could develop at any location, including the posterior pole1,2. Moreover, some patients would have combined tractional and rhegmatogenous retinal detachment (CTRRD) preoperatively due to the preexisting retinal breaks caused by tractional force of the fibrovascular membrane. Conventional laser retinopexy may sometimes be inappropriate to apply because of the location of breaks within the posterior pole or may be difficult to apply because of the incomplete drainage of chronic subretinal fluid or rigid nearby retina due to long-term traction, even after complete membrane removal.
Human amniotic membrane (hAM) has been used in ophthalmic surgery for many years, mainly in ocular surface diseases. It can support epithelialization; promote tissue healing; and has antifibrotic, anti-inflammatory features3,4. Recently, hAM plugs have been reported to treat recurrent macular hole and high myopia-associated retinal detachment, with good functional and anatomical outcomes5,6,7,8,9. But little is known about the outcomes of using hAM grafts in diabetic TRD eyes. Therefore, this study aimed to report the surgical outcome of using hAM grafts to repair retinal breaks in the eyes of those with diabetic TRD and CTRRD. Moreover, the surgical results were compared with those of patients receiving standard surgery of TRD/CTRRD alone.
Materials and methods
This was a retrospective, consecutive case series study conducted at Changhua Christian Hospital. This study was approved by the Ethics Committee and Research Board of Changhua Christian Hospital, and all procedures were conducted according to the Declaration of Helsinki. A written informed consent was obtained from each patient enrolled in the study.
Patients with diabetic TRD or CTRRD and receiving hAM grafts implantation from January 2019 to July 2020 were included in the study. Patients with TRD/CTRRD receiving the standard surgery without hAM grafts within this period were included as the control group. The eyes with simple rhegmatogenous retinal detachment or macular hole-associated retinal detachment and with follow-up less than 3 months were excluded from the study. The eyes with concomitant ophthalmic diseases including glaucoma or uveitis were also excluded.
All included patients underwent thorough ophthalmological examinations including slit-lamp and indirect ophthalmoscopy examinations, ultra-wide-field color fundus photography (Optos California, Optos PLC, Dunfermline, United Kingdom), and spectral-domain optical coherence tomography (OCT) (Heidelberg Spectralis, Germany) 1 week, 1 month, 3 months and 6 months after surgery. Data including age, gender, and best-corrected visual acuity (BCVA) were recorded. All of the patients were followed up at the outpatient clinics for at least 6 months after the surgery. Outcome measures included BCVA, BCVA improvement and re-detachment rate at the end of follow-up.
Surgical technique
All of the cases underwent standard three-port 25-gauge pars plana vitrectomy (Alcon Laboratories, Inc., Fort Worth, TX). After core vitrectomy, the fibrovascular membrane over the retina was removed by using either cutter or forceps or scissors. In cases with CTRRD or strongly adherent fibrovascular membrane on the retina, a bimanual technique was used. To implant hAM over the retinal breaks, the soft tip was first used to drain the subretinal fluid through the breaks to flatten the retina to remove the chronic viscous subretinal fluid. Then the cryopreserved hAM from the tissue bank of our hospital was used and defrosted before the implantation. The amniotic membrane was cut under the microscope to create the small hAM pieces, and it was inserted into all the identified retinal breaks through the trocar. The trocar was removed in some cases for the larger-sized hAM. Which side should face the retinal pigment epithelium was not specifically identified. To implant the hAM, the hAM was generally directly inserted into the retinal breaks. For the larger retinal breaks, or eyes with more bullous retinal detachment, assisted perfluorocarbon liquid was used; thus, the graft could be more easily flattened and positioned under the breaks. After securing the graft, air–fluid exchange was performed, accompanied by either room air, sulfur hexafluoride (SF6), octafluoropropane (C3F8), or silicone oil tamponade. No laser retinopexy around the breaks were performed except for panretinal photocoagulation at the peripheral retina. All patients were asked to maintain the prone position for 1 or 2 weeks. Silicone oil was removed 3 months after surgery, and patients with cataract formation underwent lens extraction and intraocular lens implantation. All operations were performed by a single experienced vitreoretinal surgeon (SN Chen).
Statistical analysis
Snellen BCVA was converted to the logarithm of minimal angle of resolution (logMAR) for statistical analysis and expressed as mean values and standard deviations. Preoperative and postoperative BCVA values were compared using the Wilcoxon signed-rank test. Besides, Mann–Whitney test was performed to compare the results between the hAM and control groups. All analyses were performed using MedCalc Statistical Software version 19.6 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Results
A total of 10 eyes from 10 patients were included in the hAM group. Five patients were men, and five patients were women. Thirteen eyes were included in the control group. Seven patients were men, and six patients were women. No significant differences were found between the two groups regarding the baseline age, gender and follow-up period. Postoperatively, all eyes in the hAM group had complete retinal attachment without the need for additional surgery, whereas 9 eyes (69.2%) in the control group needed additional surgery because of retinal re-detachment. Regarding BCVA, although baseline and final BCVA did not differ significantly between the two groups, the eyes in the hAM group were found to have significant visual improvement but not the eyes in the control group (p = 0.03) (Table 1).
For patients in the hAM group, eight cases had TRD with retinal breaks made during membrane delamination. Two cases had preoperative CTRRD. Table 2 shows the demographic data of the patients. Five eyes received silicone oil endotamponade, two eyes had 24% SF6 infusion, two eyes had 13% C3F8 infusion, and one eye had room air infusion. The mean follow-up duration was 6.78 months. Postoperatively, the retina was successfully reattached in all of the eyes. For five eyes with silicone oil tamponade, silicone oil was removed smoothly without recurrence after an average of 4.4 months. Fundus examinations, fundus photography, and serial OCT confirmed that the retinal breaks had been sealed by hAM grafts. Serial OCT revealed that all the hAM grafts stayed in place without dislocation. The graft size also seemed to be stationary without lysis. Moreover, from OCT, partial tissue regeneration was also observed over the retinal breaks (Fig. 1). In another patient (Fig. 2, case 7), the tissue regeneration effect was even more marked with the large retinal break all covered by regenerated glial and possible retinal tissue along the surface of the hAM graft. No postoperative major adverse event was found. An accident happened in one patient (Fig. 2D,E, case7); the break was too large, and the first hAM graft dislocated into the subretinal space during manipulation; thus, the second piece of the hAM was implanted, and the graft was positioned well into place under perfluorocarbon liquid. Postoperatively, the displaced subretinal hAM plug did not cause inflammation or retinal atrophy during serial postoperative OCT scans. Surprisingly, from the OCT, the partial recovery of the ellipsoid zone of the originally detached retina was observed.
Postoperative color fundus photograph and serial optical coherence tomography (OCT) exams of case 1. (A) Color fundus photograph showed the two retinal breaks sealed securely by two human amniotic membrane (hAM) grafts (arrow and arrow head). (B,C) Two months after surgery, OCT scans over the location of superior temporal break (arrow) and inferior temporal break (arrow head) showed the retina breaks well sealed by the grafts. (D,E) One year later, OCT scan over the same location showed that the grafts stayed in place without graft dislocation or lysis. Besides, some retina and glial tissue regeneration were observed at the retina break edges(arrows).
Postoperative color fundus photograph and serial optical coherence tomography (OCT) exams of case 7. (A) Color fundus photograph showed the one large retinal break securely covered by a human amniotic membrane (hAM) graft (arrow head). Also, there was one pice of hAM dislocated at the subretinal space within the vascular arcade intraoperatively (arrow). (B) Ten days after surgery, OCT scans over the location of retinal break showed the hAM graft covering the large break. (C) Six months later, follow-up OCT showed marked retina and glial tissue growing to cover the retina defect (arrow). And the graft remained stable without change in size. (D) Ten days after surgery, OCT scans of the dislocated subretinal hAM graft showed a sheet of hAM under the retina. (E) Six months later, the follow-up OCT revealed that there was no atrophy of the retina over the amniotic membrane. The retina structure remained intact and even more partial recovery of ellipsoid zone of the previous detached retina was observed.
Discussion
Diabetic TRD and CTRRD are serious complications in patients with diabetic retinopathy, posing a challenge to vitreoretinal surgeons due to the guarded surgical prognosis. In these complicated cases, iatrogenic retinal breaks are often encountered during the surgery. Additionally, the chronic subretinal fluid is generally viscous and the retina is rigid, which make the retina difficult to flatten with either air–fluid exchange or perfluorocarbon liquid intraoperatively, making laser retinopexy difficult to apply around the break and requiring heavy energy. The heavy laser may either increase the postoperative inflammation or incite fibrous tissue proliferation around the breaks. Furthermore, the retinal break may not be securely sealed that reopening of the breaks may happen postoperatively. Besides, for some breaks develop very posteriorly within the vascular arcade, laser photocoagulation at posterior poles would lead to permanent visual field defects10,11, and progressive enlargement of laser scars with subsequent involvement of the central visual field is another concern.
Recently, free flap technique was reported using either free ILM flaps or lens capsular flaps to treat posterior breaks related to rhegmatogenous retinal detachment without laser retinopexy with successful surgical outcome12,13,14,15. Compared with laser retinopexy, which creates a barricade around the retinal breaks, free flaps could truly close the breaks, thus decreasing the chance of proliferative vitreoretinopathy development due to intravitreal migration of retinal pigment epithelial cells from exposed retinal pigment epithelium from the retinal breaks16. However, ILM or lens capsular flaps are not always available, especially for patients who underwent previous cataract surgery or ILM peeling surgery. Besides, ILM and lens capsular flaps are inappropriate to use for large retinal breaks because of the difficulty in harvesting a large sheet of flap at one time.
Human amniotic membrane grafts, however, could overcome the limitation from ILM or lens capsular flaps, owing to the rich tissue resources. In the present case series, hAM grafts were found to be an effective surgical technique in managing retinal breaks in either diabetic TRD or CTRRD cases with encouraging outcomes. Complete retinal reattachment with significant visual acuity improvement was observed after following for 6 months. Compared with the eyes with standard surgery using laser retinopexy alone without hAM grafts, hAM grafts would achieve higher retinal reattachment rate, decrease the need for additional surgery, and result in significant visual improvement.
Caporossi et al. recently reported on the surgical outcome of using hAM graft in the management of retinal detachment caused by paravascular retinal break and large macular tear7,8. From their results, all patients achieved complete retinal reattachment. Similar to our reports, they also observed the partial regrowth of the retinal tissue covering the retinal breaks. The regeneration effect is not often observed in cases receiving laser retinopexy or ILM/capsular flap, indicating the tissue healing effect of the amniotic membrane. However, more studies are needed to confirm this assumption.
Compared with ILM or lens capsular flaps, hAM grafts had several advantages in managing patients with diabetic TRD: First, the size of hAM could be tailored according to any size of the breaks. And unlike ILM or lens capsule, there is no worry about the sufficiency of the graft tissue, especially for patients with multiple or large breaks. Second, because of the physical property and thickness of the hAM graft, the graft is more easily manipulated and secured within the breaks, with less chance dislocating to the vitreous cavity. Thus, no graft dislocation was noted after the surgery in any of our patients. Third, in diabetic retinopathy, excessive inflammatory cytokines or growth factors secondary to the changes in vascular permeability and retinal ischemia contribute to the formation of epiretinal fibrous membrane formation17,18, whereas the anti-inflammatory and antifibrotic properties of hAM19 may help eliminate the condition. From the eyes in hAM group, 5 eyes had received partial laser treatment before. The other 5 eyes had no laser before. During surgery, PRP was performed in conventional fashion and no eyes received addition steroid at the end of the surgery. There was no marked inflammation observed, and from the serial follow-up OCT, there was no cystoid macular edema nor new epiretinal membrane development. This may be contributed to the anti-inflammatory effect of the intraocular hAM, but more studies may be needed to investigate this issue.
For the complication related to this technique, none of our cases had retinal atrophy over the retinal breaks implanted by hAM graft. This is in contrast with the recently published report by Tsai et al. that retinal atrophy and pigment disturbance developed adjacent to the hAM graft20. The different result may lie in that all patients in the report of Tsai et al. had highly myopic eyes, which were more vulnerable to the surgical-induced trauma from graft insertion rather than the hazardous effect of the graft. From our case series, one case had a piece of hAM graft dislocated into the subretinal space close to the macular area during flap manipulation intraoperatively. To prevent this, an early instillation of perfluorocarbon liquid to attach the retina adjacent to the retinal breaks in the eyes of more bullous retinal detachment before excessive graft manipulation may help prevent this complication. Interestingly, serial OCT was used to evaluate the potential effect of subretinal amniotic membrane, but no adverse reaction was noted related to the graft. Neither retinal atrophy nor inflammation was noted. Instead, a partially recovered ellipsoid zone was noted at the originally detached retina overlying the hAM graft, along with the adjacent retina. By this observation, it seemed that subretinal hAM does not have an adverse effect on the overlying retina, which may also explain the good surgical outcome from the reports of Caporossi et al.6,7,8,9. The persistence of hAM may further facilitate the detached retina restored ellipsoid zone function. This may also explain the better BCVA improvement compared with that of the eyes without hAM graft implantation. However, whether hAM may contribute to this finding, more study and longer-term follow-up are necessary.
In our serial follow-up of the hAM graft using OCT, the size of hAM was noted to stay stationary from 6 months to 1 year of follow-up. This is different from the hAM graft transplantation in ocular surface disease, in which the hAM generally dissolves soon postoperatively21. This may be because that tissue degradation behaves differently intraocularly and extraocularly.
Overall, this report showed encouraging results of using hAM in managing retinal breaks in PDR with TRD or CTTRD. However, this technique has some limitations: First, hAM is a thick tissue; thus, it is not ideal for tiny retinal breaks because the tissue is difficult to insert inside the retinal breaks. Second, because the amniotic membrane from our tissue bank was used and was trimmed under the microscope, the thickness of hAM was not uniform. The thick hAM graft may protrude above the retinal surface and may cause difficulty for the retinal tissue around the retinal breaks to realign over the hAM graft. A commercialized hAM graft may better fit the purpose. Third, which side of the graft should face the retinal pigmented epithelium was not identified; the different effects from the stromal layer or epithelial layer of the amniotic membrane could have different effects on surgical results.
Moreover, this study has several limitations. First, the sample size is small, and the follow-up duration is short; a larger patient cohort with longer period of follow-up is required to investigate the possible long-term adverse effect of the hAM graft to the retinal tissue. Second, this is a retrospective study. Although the results were compared with those of patients receiving standard surgery, a prospective, comparative study is necessary in the future to confirm whether hAM is better than traditional laser retinopexy in sealing retinal breaks and ensuring a better visual and anatomic prognosis or not.
In conclusion, for complicated diabetic TRD or CTRRD cases with multiple or large retinal breaks, hAM graft may successfully close the retinal defects with the successful retinal reattachment and significant visual acuity improvement, especially useful under conditions when laser retinopexy was inappropriate to apply. Compared with other free flap techniques, it offers the advantages of rich graft source, tissue regeneration effects, and facilitate ellipsoid zone restoration, which may in turn lead to promising surgical outcomes in managing retinal breaks in TRD and CTRRD.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
23 March 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-022-08884-9
References
Newman, D. K. Surgical management of the late complications of proliferative diabetic retinopathy. Eye (Lond)24(3), 441–449 (2010).
Stewart, M. W., Browning, D. J. & Landers, M. B. Current management of diabetic tractional retinal detachments. Indian J. Ophthalmol.66(12), 1751–1762 (2018).
Tseng, S. C. Amniotic membrane transplantation for ocular surface reconstruction. Biosci. Rep.21(4), 481–489 (2001).
Tseng, S. C. et al. How does amniotic membrane work?. Ocul. Surf.2(3), 177–187 (2004).
Rizzo, S. et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina39(Suppl 1), S95–S103 (2019).
Caporossi, T. et al. Human amniotic membrane to close recurrent, high myopic macuar holes in pathologic myopia with axial length of ≥30 mm. Retina40(10), 1946–1954 (2020).
Caporossi, T. et al. Amniotic membrane for retinal detachment due to paravascular retinal breaks over patchy chorioretinal atrophy in pathologic myopia. Eur. J. Ophthalmol.30(2), 392–395 (2020).
Caporossi, T., De Angelis, L., Pacini, B. & Rizzo, S. A human amniotic membrane plug to repair retinal detachment associated with large macular tear. Acta Ophthalmol.97(8), 821–823 (2019).
Caporossi, T. et al. A human Amniotic Membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol.98(2), e252–e256 (2020).
Subash, M. et al. The effect of multispot laser panretinal photocoagulation on retinal sensitivity and driving eligibility in patients with diabetic retinopathy. JAMA Ophthalmol.134(6), 666–672 (2016).
Fong, D. S., Girach, A. & Boney, A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina27(7), 816–824 (2007).
Rizzo, S. et al. Autologous internal limiting membrane fragment transplantation for rhegmatogenous retinal detachment due to paravascular or juxtapapillary retinal breaks over patchy chorioretinal atrophy in pathologic myopia. Retina38(1), 198–202 (2018).
Rossi, T. et al. Autologous internal limiting membrane flap for retinal detachment due to posterior retinal tears over choroidal atrophy in highly myopic eyes. Br. J. Ophthalmol.103(8), 1133–1136 (2019).
Chen, Y. C., Yang, C. M. & Chen, S. N. Internal limiting membrane flap in the management of retinal detachment due to paracentral retinal breaks. J. Ophthalmol.2019, 4303056 (2019).
Chen, Y. C., Yang, C. M. & Chen, S. N. Lens capsular flap in the management of posterior retinal hole associated retinal detachment in high myopic eyes with previous internal limiting membrane peeling: 3 case reports. Medicine (Baltimore)98(29), e16422 (2019).
Tosi, G. M., Marigliani, D., Romeo, N. & Toti, P. Disease pathways in proliferative vitreoretinopathy: an ongoing challenge. J. Cell Physiol.229(11), 577–1583 (2014).
Yoshida, S. et al. Differential association of elevated inflammatory cytokines with postoperative fibrous proliferation and neovascularization after unsuccessful vitrectomy in eyes with proliferative diabetic retinopathy. Clin. Ophthalmol.11, 1697–1705 (2017).
Kuo, B. I., Yang, C. M. & Hsieh, Y. T. Lamellar macular hole in diabetic retinopathy. Eur. J. Ophthalmol.https://doi.org/10.1177/1120672119879665 (2019).
Navas, A. et al. Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl. Med.7(12), 906–917 (2018).
Tsai, D. C., Huang, Y. H. & Chen, S. J. Parafoveal atrophy after human amniotic membrane graft for macular hole in patients with high myopia. Br. J. Ophthalmol.https://doi.org/10.1136/bjophthalmol-2019-315603 (2020).
Tosi, G. M. et al. Amniotic membrane graft: histopathological findings in five cases. J. Cell Physiol.202(3), 852–857 (2005).
Author information
Authors and Affiliations
Contributions
S.N.C. designed and conceived the study. Y.C.C. collected and analyzed the data. S.N.C., Y.C.C. discussed and interpreted the results. Y.C.C. wrote the manuscript. S.N.C. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-022-08884-9"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YC., Chen, SN. RETRACTED ARTICLE: Human amniotic membrane grafts for retinal breaks in diabetic tractional retinal detachment and combined tractional and rhegmatogenous retinal detachment. Sci Rep 11, 8035 (2021). https://doi.org/10.1038/s41598-021-86804-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-86804-z