Abstract
An easily accessible biomarker with good diagnostic power for patients with traumatic brain injury (TBI) was needed to predict the short-term mortality. Studies have shown that platelet-to-lymphocyte ratio (PLR) is a biomarker for patients with tumor. This study aimed to identify the relationship between PLR and short-term mortality in patients with moderate to severe TBI. This is a retrospective cohort study. We selected patients with moderate to severe TBI who were admitted to the emergency department of The First Affiliated Hospital of Zhengzhou University. Biomarkers were collected within 24 h after admission. To investigate their relationship with short-term mortality, Cox proportional hazards regression and ROC curve analysis were performed. A total number of 170 patients was included. 47 (27.6%) patients had died and 123 (72.4%) patients were survived by the end of the study. Patients with different Rotterdam CT score (HR = 1.571, 95%CI 1.232–2.002, p < 0.001) or PLR levels (HR = 1.523, 95%CI 1.110–2.090, p = 0.009) had significant different mortality rates. The AUC curve analysis showed that the AUC of Rotterdam CT score and PLR groups were 0.729 (95%CI 0.638–0.821, p < 0.001) and 0.711 (95%CI 0.618–0.803 p < 0.001), respectively. PLR level is an independent biomarker with great diagnostic power for short-term mortality in patients with moderate to severe brain injury.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) has become one of the main causes of death and disability related to traumatic diseases. As of 2016, the number of patients worldwide reached 27 million, an increase of 47% over 19901,2. Traumatic brain injury includes primary injury and secondary injury. The primary injury is mainly caused by mechanical impact, while the mechanism of secondary injury is complicated, which includes a series of injuries such as neuroinflammation, hypoxia, and hypoperfusion. Neuroinflammation is a complicated, non-specific response that can occur in the acute phase of traumatic brain injury. It may last from minutes to years after injury, resulting in persistent neurological damage and poor prognosis3,4,5.
The severity of traumatic brain injury is ranked as mild, moderate, and severe according to the Glasgow Coma Score (GCS). As high as 30–40% of the mortality rate of patients with severe brain injury has been reported6,7,8,9,10. A large number of literatures have identified biomarkers for the prognosis of traumatic brain injury. They mainly focus on those in blood and/or cerebrospinal fluid, such as: S100β, GFAP, NSE, UCH-L1, NF, Tau, MBP, etc.11,12,13,14,15,16,17. However, in the majority of these studies, patients are with either mild or severity-undefined brain injuries. Studies about the prognosis of patients with moderate or severe traumatic brain injury, especially short-term mortality related, are still limited. More importantly, these blood and/or cerebrospinal fluid biomarkers test are hospital grade- and test level-dependent, which tremendously limit their clinical application. The repeated operations of lumbar puncture increase the cost as well as the trauma of patients. Therefore, there is an urgent need for an easily accessible biomarker with better or comparable diagnostic power.
Platelet-To-Lymphocyte ratio (PLR) has good generalizability and could be calculated and obtained by routine laboratory test at admission without further bothering the patients. Studies have shown that PLR was associated with systemic non-specific inflammation, and high PLR implied a poor prognosis for colorectal cancer and non-small cell lung cancer18,19,20. This study identified PLR as an independent biomarker with great diagnostic power for short-term mortality in patients with moderate to severe traumatic brain injury.
Methods
Participants
This was a retrospective cohort study. Selected patients with moderate to severe TBI who were admitted to the emergency department of The First Affiliated Hospital of Zhengzhou University from January 2020 to December.
2021, and whose first admission department was Neuro-Intensive Care Unit (NICU). Inclusion criteria: (1) There was a clear history of injury, and the time of injury was within 24 h; (2) Patients with moderate to severe TBI with a GCS score of 3–12 points on admission, severe (GCS score 3–8), moderate (GCS score 9–12); (3) All patients were confirmed by head CT at emergency department. Exclusion criteria: (1) Patients with Abbreviate Injury Score (AIS) ≥ 3 points in organs other than head and neck; (2) Patients also are diagnosed with pregnancy, heart failure, renal failure, tumor, blood system disease; (3) Patients taking drugs that may affect platelet and lymphocyte count and function (antiplatelet aggregation drugs, glucocorticoids, contraceptives etc.); (4) Patients with hospitalization time < 24 h. This study was approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University (2019-KY-220) and in accordance with the Declaration of Helsinki. All patients and their legally authorized representative signed written informed consent on admission.
The outcome of this study was short-term mortality, which referred to 30-day all-cause mortality; the diagnosis of hypertension was defined according to the 2018 ESC/ESH guidelines for the management of hypertension, and the diagnosis of diabetes was defined according to the American Diabetes Association diagnostic criteria. The time of injury was defined as the time from injury to admission. There were four categories of injury, including traffic accident, motorcycle, fall from height, and fall. CT was performed immediately at emergency department, and the CT score was defined by radiologist and NICU specialists according to the Rotterdam CT scoring standard; the GCS score and pupillary were determined by at least two NICU physicians after admission; asymmetry of pupillary was defined as a measurement difference of 5 mm or more in diameter of pupils between two sides; neurosurgery referred to decompression and/or removed of hematoma, ventricular drainage. Blood transfusion included red blood cells, plasma, cryoprecipitate and/or platelets. All patients included in this study did not receive platelet transfusions within 24 h after admission.
Clinical data
The baseline characteristics of the patients included age, gender, history of hypertension, diabetes, time of injury, categories of injury, Rotterdam CT score,
GCS score, neurosurgery, blood transfusion, the platelet and lymphocyte count within 24 h after admission. Blood was drawn and sent to the emergency laboratory of The First Affiliated Hospital of Zhengzhou University immediately after admission, where Coulter LH750 (Beckman-Coulter company in Calif. USA) was used for testing. GCS score was divided into two groups: moderate (9–12), severe (3–8); PLR = platelet /lymphocyte.
Data analysis
Continuous variables were shown as mean ± standard deviation. t test was performed to compare the difference between survivors and non-survivors. Variance analysis was used to compare different PLR groups; skewed distribution of continuous variables was presented as median (p25, p75), using Mann–Whitney U test for statistics. We presented categorical variables as number and percentage, compared by Chi-square test or Fisher test. Baseline PLR was a continuous variable, divided into four groups according to the interquartile range: PLR group1 (< 128.0), PLR group2 (128.0–207.6), PLR group3 (207.6–330.7), PLR group4 (> 330.7).
Kaplan–Meier survival analysis was used to generate survival curves and we used the Log-rank test to examine the differences between the survival curves. Univariate and multivariate Cox proportional hazards regression models were applied to analyze the association between PLR groups and 30-day mortality by calculating hazard ratios (HR) and 95% confidence intervals (CI). The multivariate Cox proportional hazards regression model included variables which were identified significant association with 30-day mortality in univariate analysis (p \(<0.05\)) or conventional confounding factors (p \(<0.2\)). SPSS 25.0 software was used for data analysis, and p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This trial has the ethical approval of the ethics committee of The First Affiliated Hospital of Zhengzhou University (2019-KY-220) and in accordance with the Declaration of Helsinki. All patients and their legally authorized representative signed written informed consent on admission.
Results
Baseline data
The flow chart of this cohort study is shown in Fig. 1. 170 patients were included, with 136 males, accounting for 80%. The mean age was 51.6 ± 15.2 years, median time of injury was 10 (3.8, 22.5) hours, and the main categories of injury was car accident (89, 53%). The mortality of severe TBI was 33.8%, which is consistent with others’ reports. The mortality rate of moderate TBI in our study was 5.4% (Table 1).
To investigate the diagnostic power of PLR in TBI patients, we divided all patients into four groups according to the interquartile range of their PLR level:
PLR group1 (< 128.0), PLR group2 (128.0–207.6), PLR group3 (207.6–330.7), PLR group4 (> 330.7). As shown in in Table 2, from PLR group1 to PLR group4, gradual increase of lymphocyte and decrease of platelet as well as PLR were observed. All the results were statistically significant.
PLR level and clinical outcomes
By the end of the study, 47 (27.6%) patients had died and 123 (72.4%) patients were survived. Compared with the other three groups, group 4 had a shorter median survival time (15, 1.3–28.7), and there was a statistical difference among the four groups (Log-Rank p < 0.001) (Fig. 2).
Association between PLR level and mortality
We applied multivariate Cox proportional hazards regression model to study the association of PLR level with mortality (Table 3). Variables including age, gender, history of diabetes, time of injury, GCS score, Rotterdam CT score, blood transfusion and PLR groups were considered in this model. The results showed that patients with different Rotterdam CT score (HR = 1.571, 95%CI 1.232–2.002, p < 0.001) or different PLR levels (HR = 1.523, 95%CI 1.110–2.090, p = 0.009) had significant different mortality rates. Importantly, neither platelet nor lymphocyte number alone was as powerful as PLR to predict the mortality of TBI patients (Table 1).
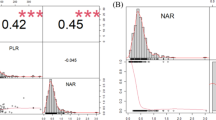
To confirm the diagnostic power of PLR level, we also did ROC curve analysis for short-term mortality. The results showed that the AUC of Rotterdam CT score and PLR groups were 0.729 (95%CI 0.638-0.821, p<0.001) and 0.711 (95%CI 0.618-0.803 p<0.001), respectively (Fig. 3). The cut-off value was determined to yield largest Youden index. The level of PLR group was 3.5 with sensitivity of 51.1% and specificity of 85.4%, while the cut-off value of Rotterdam CT score was 3.5, with sensitivity of 59.6% and specificity of 82.1%.
All these results suggest that, like the well-known marker Rotterdam CT score, PLR level is an independent risk factor, with comparable diagnostic power, for short-term mortality in moderate to severe TBI patients.
Discussion
More than 50 million people worldwide suffer from TBI each year, and half of the whole population on earth will experience at least one TBI in their lifetime. However, the treatment and prediction of traumatic brain injury are still big challenges.
The Rotterdam CT score is a method created in 2005 to assess structural brain injury. Compared to the Marshall CT score, Rotterdam CT score added traumatic subarachnoid hemorrhage and intraventricular hemorrhage, simplified the score calculation system, and reduces the assessment difference between emergency department and NICU21. Studies have shown that the Rotterdam CT score can predict the mortality of adults and children TBI patients22,23,24, which is consistent with the results in our study.
At present, the biomarker research related to the prognosis of TBI mostly focuses on the combined detection of cerebrospinal fluid and/or blood. Markers such as S-100β, NSE, GFAP, UCH-L1, NF, Tau, and MBP are increased to different degrees in blood and cerebrospinal fluid after TBI, suggesting neuronal and astrocyte damage11. The detection of high S-100β in blood and cerebrospinal fluid contribute to identify progressive intracranial hemorrhage after TBI, which is associated with mortality and poor prognosis15.
Neuroinflammation is one of the important mechanisms of secondary injury in traumatic brain injury. Studies have shown that a large number of cytokines (TNFα, IL-1β, IL-6, -10, -18 etc.) and chemotaxis (CCL2, 5 and 20, CXCL1, 9 and 10 etc.) can be detected in the cerebrospinal fluid after TBI, which exacerbate oxidative stress and cause persistent neurological damage. For example, IL-18 is associated with disability and cognitive impairment in post-injury patients25. However, the detection of these markers has a high requirement for technology and equipment. Furthermore, the samples of cerebrospinal fluid are obtained through lumbar puncture or ventricular drainage. These repeated operations are invasive and not widely accepted by patients and their family members, resulting in limited clinical application and universalness. The aim of this study was to find a convenient and easy-to-follow biomarker for predicting short-term mortality in TBI.
PLR is the ratio of platelet to lymphocyte count, which can be obtained by routine laboratory calculation, and is easy to monitor. Studies have shown that PLR is associated with systemic non-specific inflammation and can predict poor prognosis of variety diseases, especially tumors, heart failure, etc.19,20,26. Different cut-off levels of PLR have been reported in patients with NSCLC, colorectal cancer and gastric cancer, varying from 150, 160 to 235 27,28,29. Despite the actual value, high pretreatment PLR suggests poor overall survival and progression-free survival in cancer patients, which is a reverse trend for TBI patients in our study. High PLR is also related to all-cause mortality in peritoneal dialysis patients, which has been reported recently30.
To our knowledge, this is the first research studying the relationship of PLR level with short-term mortality in TBI patients with moderate to severe TBI. Low PLR is mainly achieved by decreasing platelet count and increasing lymphocyte count. The primary injury mechanism of TBI leads to the rupture of capillaries and vessels and the destruction of the blood–brain barrier (BBB), triggering the interaction between platelets and endothelial cells or subendothelial matrix. This results in platelet adhesion-activation, and the formation of platelet embolism at the injury site for hemostasis. The balance between coagulation and anticoagulation is broken in moderate to severe TBI patients, leading to platelet overactivation and the number decreases at the early stage of injury. The spontaneous aggregation and subsequent excessive consumption induce secondary platelet depletion and increase bleeding risk. Studies had shown the increasing risk of intracranial hemorrhage progression when the platelet was less than 175 × 109/L31, and nine-fold higher mortality when the number is below 100 × 109/L32.
Huang et al. reported the effect of different gender on PLR ratio to predict cardiovascular mortality of peritoneal dialysis (PD) patients. For female PD patients, high PLR ratio was related to worse prognosis due to higher level of estrogen and low levels of serum iron33. Our study did not find the same effect of gender in our TBI patients. Other confounding variables about PLR incudes alcohol level, smoking and so on. More importantly, in our study, we used both adjusted and non-adjusted Cox regression model as well as ROC curve to test the predicting power of PLR. All results showed that PLR is independent and powerful, which means PLR may not be significantly affected by these confounding variables at least in the case of predicting short-term mortality in patients with moderate to severe TBI.
Lymphocytes are classified into T lymphocytes, B lymphocytes and NK cells according to their origin, morphological structure, surface markers and immune function. They are important cellular components of the body's immune response. Animal experiments have shown that focal cortical contusion and subcortical neuronal damage occur within minutes after trauma, accompanied by a rapid local neuroinflammatory response34,35. This contributes to immune cells (macrophages, granulocytes, dendritic cells, NK cells etc.) accumulation in the injured area and surrounding tissues. The immune cell-derived cytokines (IL-1β, IL-18, TNFα, etc.) and chemokines (CCL2, CCL22, CCL17, etc.) further recruit immune cells (neutrophils, lymphocytes, lymphocytes, etc.) to the site of injury and coordinate subsequent activities36,37. This kind of immune response generates a self-perpetuating pro-inflammatory environment that damages the brain parenchyma. Peripheral inflammation also affects the outcome of TBI38. Peripheral immune cells rapidly expand and are activated after TBI with remarkable extravasation from spleen. These immune cells contribute to both innate and adaptive immune responses after TBI, with some infiltrating the central nervous system38,39. Once in the damaged brain area, T cells are activated by antigens on macrophages, dendritic cells, and microglia. The steady increase in T cell number and composition suggests a shift from innate non-specific immune to adaptive immune40,41. Decreased PLR level suggests early coagulation imbalance and neuroinflammation hyperactivity, which is independently associated with short-term mortality.
The limitations of our study are: firstly, this study is a single-center study. Secondly, this study is a retrospective cohort study. Although we use the multivariate regression model to reduce the interference, it is still unable to fully guarantee the complete balance and comparability of each group. Finally, further randomized controlled trials are needed to determine the underlying mechanism of the association between PLR and short-term mortality.
Conclusion
PLR level is an independent biomarker with great diagnostic power for short-term mortality in patients with moderate to severe brain injury.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to further study but are available from the corresponding author on reasonable request.
Abbreviations
- TBI:
-
Traumatic brain injury
- GCS:
-
Glasgow Come Score
- PLR:
-
Platelets-to-lymphocyte ratio
- NICU:
-
Neuro-Intensive Care Unit
- AIS:
-
Abbreviate Injury Score
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- BBB:
-
Blood–brain barrier
- PD:
-
Peritoneal dialysis
References
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053), 1545–1602 (2016).
Collaborators, G. B. D. N. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18(5), 459–480 (2019).
Thompson, H. J. et al. Lateral fluid percussion brain injury: A 15-year review and evaluation. J. Neurotrauma 22(1), 42–75 (2005).
Bramlett, H. M. & Dietrich, W. D. Progressive damage after brain and spinal cord injury: Pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 (2007).
Marklund, N., Bakshi, A., Castelbuono, D. J., Conte, V. & McIntosh, T. K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 12(13), 1645–1680 (2006).
Maas, A. I. R. et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16(12), 987–1048 (2017).
Xiong, Y., Mahmood, A. & Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 21(3), 137–151 (2018).
Bigler, E. D. Distinguished Neuropsychologist Award Lecture 1999. The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Arch. Clin. Neuropsychol. 16(2), 95–131 (2001).
LoBue, C. et al. Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology 32(4), 401–409 (2018).
Unden, J., Ingebrigtsen, T., Romner, B. & Scandinavian Neurotrauma, C. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med. 11, 50 (2013).
Adrian, H., Marten, K., Salla, N. & Lasse, V. Biomarkers of traumatic brain injury: Temporal changes in body fluids. eNeuro. 3(6). https://doi.org/10.1523/ENEURO.0294-16.2016 (2016).
Thelin, E. P. et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: A systematic review. Front. Neurol. 8, 300 (2017).
Wang, K. K. et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 18(2), 165–180 (2018).
Mercier, E. et al. Prognostic value of S-100beta protein for prediction of post-concussion symptoms after a mild traumatic brain injury: Systematic review and meta-analysis. J. Neurotrauma 35(4), 609–622 (2018).
Mercier, E. et al. Predictive value of S-100beta protein for prognosis in patients with moderate and severe traumatic brain injury: Systematic review and meta-analysis. BMJ 346, f1757 (2013).
Mercier, E. et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: A systematic review. Brain Inj. 32(1), 29–40 (2018).
Bogoslovsky, T. et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid beta up to 90 days after traumatic brain injury. J. Neurotrauma 34(1), 66–73 (2017).
Li, B. et al. Platelet-to-lymphocyte ratio in advanced cancer: Review and meta-analysis. Clin. Chim. Acta 483, 48–56 (2018).
Liu, J. et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 33(8), e22964 (2019).
Stojkovic Lalosevic, M. et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis. Markers 2019, 6036979 (2019).
Elkbuli, A. et al. Utility of the Marshall & Rotterdam classification scores in predicting outcomes in trauma patients. J. Surg Res. 264, 194–198 (2021).
Bahloul, M. et al. Prognosis of traumatic head injury in South Tunisia: A multivariate analysis of 437 cases. J. Trauma 57(2), 255–261 (2004).
Asim, M. et al. Rotterdam and marshall scores for prediction of in-hospital mortality in patients with traumatic brain injury: An observational study. Brain Inj. 35(7), 803–811 (2021).
Deepika, A., Prabhuraj, A. R., Saikia, A. & Shukla, D. Comparison of predictability of Marshall and Rotterdam CT scan scoring system in determining early mortality after traumatic brain injury. Acta Neurochir. (Wien) 157(11), 2033–2038 (2015).
Ciaramella, A. et al. Increased levels of serum IL-18 are associated with the long-term outcome of severe traumatic brain injury. NeuroImmunoModulation 21(1), 8–12 (2014).
Chen, T. & Yang, M. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int. Immunopharmacol. 78, 106063 (2020).
Sanchez-Lara, K. et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: A prospective study. Nutr. Cancer 64(4), 526–534 (2012).
Wang, F. et al. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol. Lett. 10(6), 3411–3418 (2015).
Kim, J. H. et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J. Gastroenterol. 23(3), 505–515 (2017).
Liu, S. et al. Platelet-to-lymphocyte ratio is associated with the mortality in peritoneal dialysis patients. Iran. J. Kidney Dis. 15(3), 206–212 (2021).
Joseph, B. et al. Acquired coagulopathy of traumatic brain injury defined by routine laboratory tests: which laboratory values matter?. J. Trauma Acute Care Surg. 76(1), 121–125 (2014).
Schnuriger, B. et al. The impact of platelets on the progression of traumatic intracranial hemorrhage. J. Trauma 68(4), 881–885 (2010).
Sheng, H. et al. Sexual effect of platelet-to-lymphocyte ratio in predicting cardiovascular mortality of peritoneal dialysis patients. Mediators Inflamm. 2022, 8760615 (2022).
Trahanas, D. M., Cuda, C. M., Perlman, H. & Schwulst, S. J. Differential activation of infiltrating monocyte-derived cells after mild and severe traumatic brain injury. Shock 43(3), 255–260 (2015).
Rhodes, J. Peripheral immune cells in the pathology of traumatic brain injury?. Curr. Opin. Crit. Care 17(2), 122–130 (2011).
Ono, S. J. et al. Chemokines: Roles in leukocyte development, trafficking, and effector function. J. Allergy Clin. Immunol. 111(6), 1185–1199 (2003) (quiz 1200).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25(12), 677–686 (2004).
Tobin, R. P. et al. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol. Commun. 2, 143 (2014).
Schwartz, M. & Deczkowska, A. Neurological disease as a failure of brain-immune crosstalk: The multiple faces of neuroinflammation. Trends Immunol. 37(10), 668–679 (2016).
Ni, K. & O’Neill, H. C. The role of dendritic cells in T cell activation. Immunol. Cell Biol. 75(3), 223–230 (1997).
Pozzi, L. A., Maciaszek, J. W. & Rock, K. L. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J. Immunol. 175(4), 2071–2081 (2005).
Author information
Authors and Affiliations
Contributions
W.L. was responsible for the study design, execution, coordination, oversight of data management, data interpretation, and manuscript writing and editing. W.D. was responsible for manuscript reviewing and editing. All authors have read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, W., Deng, W. Platelet-to-lymphocyte ratio predicts short-term mortality in patients with moderate to severe traumatic brain injury. Sci Rep 12, 13976 (2022). https://doi.org/10.1038/s41598-022-18242-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-18242-4
This article is cited by
-
Predictive value of peripheral blood inflammatory markers for epilepsy occurrence in traumatic brain injury patients
Acta Epileptologica (2025)
-
Neutrophil-albumin ratio serves as a superior prognostic biomarker for traumatic brain injury
Scientific Reports (2024)
-
Development and validation of a nomogram for predicting mortality in patients with acute severe traumatic brain injury: A retrospective analysis
Neurological Sciences (2024)