Abstract
Mesothelioma lies one of the most malignant tumors, in which the identification of the corresponding biomarkers is extremely critical. This study aims to investigate the prognostic value of enhancer homolog 2 (EZH2) mRNA expression in mesothelioma patients accompanied with its immune infiltration analysis. Gene expression, clinical information and enrichment analysis were obtained based on the Cancer Genome Atlas (TCGA), the immune infiltration analysis and bioinformatics analysis were performed. Clinical information and gene expression were obtained from 86 patients with mesothelioma based on TCGA database. Survival analysis, GSEA enrichment analysis, and immune infiltration analysis of EZH2 expression were carried out using R (version 3.6.3) (statistical analysis and visualization). The correlation of EZH2 expression with immune cell infiltration in mesothelioma was analyzed according to the TIMER database (Fig. https://cistrome.shinyapps.io/timer/). A univariate and multivariate analysis of general data obtained from the TCGA database was performed, involving age, gender, stage, pathological type, and whether they had received radiotherapy, the results indicated the association of high expression of EZH2 with poor prognosis in mesothelioma patients, with the worse prognosis in the High group (HR = 2.75, 95% CI 1.68–4.52, P < 0.010). Moreover, ROC curves showed that EZH2 expression predicted 1-year survival with an AUC of 0.740, 2-year survival with an AUC of 0.756, and 3-year survival with an AUC of 0.692, suggesting a robust predictive effect of EZH2 expression on prognosis. KEGG pathway analysis indicated five pathways showing the strongest positive correlation with EZH2 expression: cell cycle, DNA replication, Cell adhesion molecules cams, Primary immuno deficiency, Tsate transduction, and five pathways showing the strongest negative correlation with EZH2 expression: Glycolysis gluconeogenesis, Drug metabolism, cytochrome P450, retinol metabolism, fatty acid metabolism ribosome. We investigated the correlation between EZH2 expression and the level of immune infiltration in mesothelioma tissues. The results indicated that EZH2 expression played a critical role in immune infiltration, of which the high expression was correlated with the reduced number of NK cells, Mast cells, and Th17 cells. Moreover, mesothelioma patients with high EZH2 expression differ from those with low EZH2 expression in their tumor immune microenvironment. EZH2, as a new prognostic biomarker for mesothelioma, contributes to elucidating how changes in the immune environment promote the development of mesothelioma. Further analysis, EZH2 may serve as a biological test to predict the prognosis of mesothelioma.
Similar content being viewed by others
Introduction
Mesothelioma is described as a malignant tumor arising from the mesothelial surface of the pleural cavity, peritoneal cavity, testicular tunica vaginalis, or pericardium, of which 80% of cases originate from the pleura1. The disease as a rare malignancy possess a poor prognosis and overall survival (OS) of approximately 9–17 months after diagnosis1, particularly in patients with pleural mesothelioma where the effect is not obvious due to its low incidence in mesothelioma despite some related studies, chemotherapy, immunization, and local radiotherapy. The identification of molecular biomarkers predictive of prognosis is limited by the small number of mesothelioma patients who have been involved in clinical trials as well as the heterogeneity of the study population. Therefore, it is significant to identify the therapeutic targets for this malignancy.
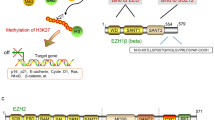
EZH2 and Embryonic Ectoderm Development (EED) encoding polycomb repression complex-2 (PRC-2) have been demonstrated to be overexpressed in MPM lines, occupying about 85% of Mesothelioma in comparison with normal pleura, which is related to the reduced survival rate of patients2. EZH2 plays roles in manipulating gene expression to inhibit the differentiation of stem cells and progenitor cells, of which the abnormal activity is considered to be the driving factor of carcinogenesis. The studies of EZH2 mRNA are in full swing recently, with its mechanism demonstrated to be associated with silencing epigenetic genes3. Studies have indicated that the abnormal expression of EZH2 is associated with poor prognosis in various malignancies such as lung4. EZH2 manipulates gene expression to prevent the differentiation of stem and progenitor cells, of which the abnormalities in activity are considered to be drivers of carcinogenesis. While the monotherapy as an EZH2 inhibitor showed robust antitumor activity in the treatment of anti-mesothelioma, with good safety/tolerability5. Macrophages can directly deliver cytotoxicity to mesothelioma cells, without phagocytosis6. Tumor-associated macrophages (TAM) serve as the drivers of Mesothelioma growth, progression, and drug resistance to Tazemetostat. The therapeutic effect of EZH2 pharmacological inhibition can be enhanced by evaluating the TAM consumption strategies7. The robust safety and tolerance of tazemetostat were initially demonstrated by a multicenter, open-label, phase 2 study of EZH2 inhibitor tazemetostat in patients with recurrent or refractory BAP1-inactivated malignant pleural mesothelioma. In comparison to traditional second-line cytotoxic drugs, involving gemcitabine, vinorelbine and even immunotherapy: nivolumab plus ipiliminumab can achieve the elevated response rate and progression-free survival (PFS)8. The inactivation of BAP1 induces the upregulation of BAP1 and the dependence on methyltransferase EZH2, which trimethylates lysine 27 on histone H3 (H3K27me3) as one of the chromatin modified PRC-29,10. The lack of BAP1 will induce mesothelioma cells sensitive to EZH2 drug inhibition9.
However, the studies on the correlation of EZH2 with mesothelioma are still lacked, remaining the role in mesothelioma unknown. In this paper, the expression of EZH2 was statistically analyzed for the prognosis of mesothelioma depending on the clinical data from the TCGA database, and the correlation between EZH2 and immune infiltration in patients with mesothelioma was statistically analyzed, so as to further elaborate the potential mechanism of EZH2’s immune impact in this disease (tumor microenvironment) and seek the relevant impact mechanism. Our work revealed that the increased expression of EZH2 was linked to poor OS among Mesothelioma patients. GSEA pathways indicated that Cell cycle, DNA replication, Primary immuno deficiency, Metabolism were all involved in the association with EZH2 expression. Moreover, a connection was identified between EZH2 and tumor-infiltrating immune cells. The presented work here provides a detailed analysis of the role of EZH2 in Mesothelioma development and indicates mesothelioma prognosis, which contributes to the understanding of the underlying mechanisms of Mesothelioma.
Materials and methods
Evidence from TCGA database
The analyses are based on TCGA (https://portal.gdc.cancer.gov), which was obtained from the MESO (mesothelioma) project with immune system infiltration, gene expression (HTSeq-FPKM), and related clinical information11. Samples missing or lacking sufficient data in terms of age, TNM stage, OS time, and distant and lymph node metastasis were excluded from the study. RNA-Seq and clinical data were retained for further study. Our study conformed to the publication guidelines provided by TCGA.
Survival analysis
Based on the clinical information obtained from the TCGA database, the correlation of the EZH2 expression (high and low expression in median) with mesothelioma patient survival was analyzed using the software: R (version 3.6.3) (statistical analysis and visualization) R package: survminer package [0.4.9 version] (for visualization), survival package [3.2-10 version] (for statistical analysis of survival data); survival curves were plotted. Statistical criteria were: P < 0.05.
GSEA enrichment analysis
We performed GSEA on RNA-Seq data obtained from TCGA12. The number of permutations was set to 1,000. KEGG pathways were analyzed to investigate the possible biological functions of EZH213,14,15. The statistical criteria were: false discovery rate (FDR) < 0.05 and P-value < 0.05.
Immune infiltrate analysis
Related modules were utilized to analyze the potential relationship between EZH2 expression and mesothelioma immune infiltration by TIMER (Fig. https://cistrome.shinyapps.io/timer/). We examined the EZH2 expression in mesothelioma tissues and its relationship with the abundance of immune infiltrates by gene modules, involving CD4 T+ cells, dendritic cells, B cells, CD8+ T cells, neutrophils, and macrophages. The relationship between gene expression levels and immune infiltration was also plotted.
Statistical analysis
Statistical analysis was performed using R (version 3.6.3) (Statistical Analysis and Visualization). For each level in the measurement data meeting, the chi-square test was adopted in the conditions that the theoretical frequency is greater than 5 and the total sample size is greater than 40. Otherwise, Fisher’s exact test shall be used. In the enumeration data meeting the normal distribution, the chi-square test was selected, otherwise the Wilcoxon rank sum test was selected. Log-rank test, Cox regression analysis, and Survival curve were performed for high and low EZH2 mRNA expression according to the survival time in the database. The results at P < 0.05 were considered statistically significant.
Ethics approval and consent to participate
The data for this study come from the TCGA database and do not require ethical approval.
Results
Correlation between EZH2 expressions and clinicopathologic features of mesothelioma
Based on, we divided the 86 patients downloaded from the TCGA database into high expression group and low expression group according to the median EZH2, covering 43 patients with high expression and 43 patients with low expression, with Patients’ pathologic clinico-characteristics listed in Table 1.
Survival outcomes and analysis variables
The results of the survival analysis of EZH2 expression group indicated that the survival time was 26.3 (23–32.7) months in the low expression group, and 13.7 (9.9–17.9) months in the high expression group. Statistical tests were performed on the data of the two groups: the results of the log-rank test suggested the statistically significant difference in the survival time distribution of group groups (P < 0.001), which was consistently indicated by the results of Cox regression (P < 0.001). The high expression group had a worse prognosis (HR = 2.75, 95% CI 1.68–4.52, P < 0.001)(Fig. 1A).
Cox analysis was chosen to explore the relationship between EZH2 expression and OS as well as other multivariate characteristics in mesothelioma patients. Both univariate and multivariate analyses indicated the association of high expression of EZH2 with poor prognosis in mesothelioma patients (Table 2). Univariate correlation analysis showed significant association of EZH2 expression with OS (P < 0.001), and the pathological type (HR = 5.96, 95% CI 0.55–64.67, P = 0.020) and EZH2 expression (HR = 2.35, 95% CI 1.62–3.42, P < 0.001) were significantly associated with OS in mesothelioma patients. The multivariate Cox regression forest plot was drawn according to the results of multivariate analysis, from which it could be concluded that EZH2 expression acted as an independent prognostic factor for the survival of mesothelioma patients (HR = 2.63, 95% CI 1.02–6.79, P = 0.046) (Fig. 1B). The distribution of EZH2 expression, survival of mesothelioma patients, and EZH2 expression are depicted in Fig. 1C. The expression of EZH2 provided a robust predictive effect on prognosis as ROC curves showed the AUC of 0.740 in EZH2 expression predicting 1-year survival, 0.756 in 2-year survival, and 0.692 in 3-year survival (Fig. 1D).
GSEA enrichment analysis
The GSEA software was utilized to perform KEGG pathway analysis to investigate the potential biological functions of EZH2. The most highly enriched signaling pathways were selected according to the normalized enrichment fraction (NES). As shown in Table 3, five pathways exhibited the strongest positive correlation with EZH2 expression: cell cycle pathway, DNA replication pathway, cell adhesion molecule pathway, primary immunodeficiency pathway, and taste conduction pathway; and five with the strongest negative correlation with EZH2 expression: glycolytic gluconeogenesis pathway, drug metabolism cytochrome P450 pathway, retinol metabolism pathway, fatty acid metabolism pathway, and ribosome pathway (Fig. 2). These results suggested the essential significance of pathways regulating cell cycle control and fatty acid metabolism, glycolysis, and gluconeogenesis in mesothelioma patients, which are closely associated with EZH2 expression.
Immune infiltration
Independent tumor-infiltrating lymphocytes play crucial roles in the prediction of OS and lymph node metastasis. Therefore, we analyzed the correlation of EZH2 expression with the level of immune infiltration in mesothelioma tissues using TIMER. As depicted in Fig. 3A, the positive correlation was indicated of EZH2 expression with the levels of B cells (p-value = 3.40 × 10−3), CD4 + T cells (p-value = 5.31 × 10−2), and Dendritic cells (p-value = 1.32 × 10−4), indicating a key role of EZH2 expression in immune infiltration. Furthermore, we also sought to determine whether there lies a difference in the tumor immune microenvironment between mesothelioma patients with high or low EZH2 levels. The 86 tumor specimens were divided into two groups, 43 in the high expression group and 43 in the low expression group. The R package was adopted: GSVA package [version 1.34.0]16 to download the gene expression profiles of the samples to determine the levels of 24 immune cells, in which the GSEA algorithm applied to 24 immune cell subtypes contributed to assessing the differences in expression levels in the two groups. In Natural Killer (NK) cells, High was lower than Low, with statistically significant difference (P = 0.023); in Mast cells, High was lower than Low, with statistically significant difference (P = 0.040); in T helper (TH) cells, High was higher than Low, with statistically significant difference (P = 0.021); in Th17 cells, High was lower than Low, with statistically significant difference (P = 0.005); in Th2 cells, High was higher than Low, with statistically significant difference (P < 0.001) (Fig. 3).
Discussion
Malignant mesothelioma is a rare primary cancer with survival of less than 1 year1. A trimodality treatment approach in combination of surgery, chemotherapy, and sequential radiation therapy (RT) represents the mainstream of current mesothelioma treatment options17. While the survival still remains unimproved, urgently requiring the prognostic biomarkers to guide clinical decision-making. The TCGA database can provide a large amount of data from mesothelioma patients, through which new potential prognostic biomarkers can be searched for.
EZH2 as the catalytic subunit of polycomb repressive complex 2 (PRC2) represses target genes by H3K27me318, of which the highly activated mutations frequently locate in the early stages of cancer, suggesting a role for activated PRC2 cancer drivers. Excessive EZH2 expression alters histone modifications, leading to an aberrant epigenetic landscape that may contribute to cancer progression19. Based on this, we carried out the correlation between EZH2 mRNA levels and the prognosis of mesothelioma patients and immune infiltration analysis depending on the TCGA database.
We performed a univariate and multivariate analysis of the collated general data obtained from the TCGA database, covering age, gender, stage, pathological type, and whether they had received radiotherapy, demonstrating the association of high expression of EZH2 with poor prognosis in mesothelioma patients. The results of the survival analysis showed that the survival time was 26.3 (23–32.7) months in low expression group, while 13.7 (9.9–17.9) months in high expression group was, indicating that the EZH2 expression acted as an independent prognostic factor for the survival of mesothelioma patients (HR = 2.63, 95% CI 1.02–6.79, P = 0.046) (Fig. 1B). As the ROC curves showed in Fig. 1A, EZH2 expression predicted 1-year survival with an AUC of 0.740, 2-year survival with an AUC of 0.756, and 3-year survival with an AUC of 0.692 (Fig. 1D), indicating a good predictive effect of EZH2 expression on prognosis. To further investigate the mechanism of EZH2 action, we performed the KEGG pathway analysis by GSEA software to explore the potential biological function of EZH2. KEGG pathway analysis reported five pathways lying the strongest positive correlation with EZH2 expression: cell cycle, DNA replication, Cell adhesion molecule, primary immunodeficiency, and taste conduction; and five pathways lying the strongest negative correlation with EZH2 expression: glycolytic gluconeogenesis pathway, drug metabolism cytochrome P450 pathway, retinol metabolism pathway, fatty acid metabolism pathway, and ribosome pathway (Fig. 2). These results suggest the essential roles of pathways regulating cell cycle control and fatty acid metabolism, glycolysis, and gluconeogenesis in mesothelioma patients, which are closely associated with EZH2 expression.
The complex interplay between tumors and their microenvironment remains to be elucidated. Recently, the immune infiltrating components have been found to vary at every tumor stage, where the specific cells significantly impact on survival. The tumors progress is accompanied by the increased density of TH cells and NK cells, while the decreased density of most T cells. B cells serve as a key role in the core immune network and are associated with prolonged survival, while play a dual role in promoting tumor recurrence and progression with high expression in advanced patients, thus exhibiting a dual role in tumor development. Our results indicated that EZH2 was closely related to the survival of mesothelioma patients. In order to explore the relationship between EZH2 and tumor immune microenvironment, we analyzed the correlation of EZH2 expression in mesothelioma tissues with immune infiltration level using TIMER, demonstrating the crucial role of EZH2 expression in immune infiltration. In addition, we also determined whether existing a difference in the tumor immune microenvironment between mesothelioma patients with high or low EZH2 levels. Among them, in NK cells, the High expression group was lower than the low expression group (P = 0.023). In Mast cells, the high expression group was lower than the low expression group (P = 0.040). In TH cells, the high expression group was higher than the low expression group (P = 0.021). In Th17 cells, the high expression group was lower than the low expression group (P = 0.005). In Th2 cells, the high expression group was higher than the low expression group (P < 0.001).
There appears increasing evidence that EZH2 can not only inhibit tumor genes but also play roles in manipulating collective immune homeostasis as well as immune-related cells, especially in the development, differentiation, and function of T cells20. EZH2 has the certain regulatory effects on T cell differentiation and epigenetic inheritance of Treg function. The pharmacological inhibition of EZH2 in human T cells using CPI-1205 resulted in phenotypic and functional alterations in Tregs, enhancing the cytotoxic activity of Teffs. The regulation of EZH2 expression in T cells could alleviate the antitumor response elicited by anti-CTLA-4 treatment21. EZH2 inhibition was demonstrated to restore the cytotoxic response of CD8 T cells in patients with systemic lupus erythematosus (SLE), inhibiting the incidence of infection and thus reducing death resulting from infection22. Shane et al. showed that the phosphorylation status of EZH2 determines its property to maintain anti-tumor immunity in CD8+ T memory precursor cells, and Akt-mediated EZH2 phosphorylation lies a key target for enhancing anti-tumor immunotherapy strategies23. Combined with the recent data published in Mesothelioma Immunotherapy deserves our attention (https://www.mesothelioma.com/treatment/immunotherapy/), we learned that immunotherapy helps to improve the survival time of mesothelioma patients and maximize the efficacy of first-line treatments, such as chemotherapy and radiotherapy, but how to seeking optimal options in mesothelioma treatment remains a focus of future research, and modulation of the immune microenvironment may be one of these options.
In addition, EZH2 has also been demonstrated associated with tumor resistance and its inhibitors can overcome resistance to immunotherapy24,25. EZH2 as a major driving force in cancer cell immunoediting mediates immune escape by down-regulating immune recognition and activation, up-regulating immune checkpoints, thus generating an immunosuppressive tumor microenvironment26. The knockdown or inhibition of EZH2 upregulated the MET expression and phosphorylation, modifying cell proliferation and EGFR-TKI resistance in vitro. The inhibition of MET or PI3K/AKT elevated EZH2 levels and restored sensitivity to EGFR-TKIs. These findings suggest that the “MET-AKT-EZH2” feedback loop manipulates EGFR-TKI resistance27. EZH2 was also highly expressed in the lung cancer with positive KRAS expression, exhibiting a positive correlation, as well as with the expression of BRAF, especially in lung squamous cell carcinoma. High expression of EZH2 possibly possess a synergy with KRAS and BRAF mutations4.
There exists a correlation between EZH2 and the level of H3K27me3 modification, in which EZH2 as a subunit of PRC2 trimethylates H3K27 in the nucleus. PRC1 binds to PRC2-modified H3K27me3 and ubiquitinated histone H2A at lysine 119, facilitating the chromatin compaction and transcriptional silencing of downstream genes, as well as playing roles in the tumor28. Malignant mesothelioma as a highly invasive cancer, is generally diagnosed at an advanced stage. Therefore, highly sensitive and specific markers are highly required for early diagnosis. The deletion of BAP1 and high expression of EZH2 were both observed in malignant mesothelioma, which were merely observed in benign lesions. The combination of the two signals can serve as a high sensitive and specific a marker contributing to differentiating epithelioid/biphasic malignant mesothelioma from benign mesothelial lesions. Survival analysis indicated the missed BAP1, while not high expression of EZH2, which indicated a better prognosis. This combination elevated the diagnostic accuracy. Epigenetic regulation is adopted as a new method for cancer treatment, among which the inhibition of EZH2 can provide targeted epigenetic regulation. The relation of the abnormal expression of EZH2 with poor prognosis has been demonstrated in a variety of malignant tumors. EZH2 regulates gene expression to prevent the differentiation of stem cells and progenitor cells, of which the abnormal activity is considered to act as the driving factor of carcinogenesis. Currently, the research on epigenetics involving EZH2, DNA methyl transferases (DNMTs), as well as histone methyl transferases (HMTs) has absorbed increasing attention, with their corresponding inhibitors displaying great value in cancer treatment. Studies have indicated that the dual inhibition of DNMTs and EZH2 can eliminate the inherent and acquire drug resistance of myeloma cells to IMiDs in a brain-independent way29, which will perhaps play a more contributing role in the treatment of malignant mesothelioma, which is worthy of further study and exploration30.
EZH2 can manipulate the metabolic activity of tumor cells through epigenetic regulation, which in turn affects tumor progression31. The famous Warburg Effect indicates that the glucose metabolism can be remodeled by cancer cells even under hypoxia32. According to our results, the expression of EZH2 is closely linked to the glycolytic gluconeogenesis pathway. Brookes et al. found that the up-regulation of EZH2 can elevate the intracellular deoxyglucose levels and induce a slight increase in mitochondrial oxidative capacity. However, cells overexpressing EZH2 may also obtain energy mainly rely on enhanced glycolysis as source. EZH2 can promote tumorigenesis and malignant progression by activating glycolysis through the EAF2-HIF1α signaling axis33. Accumulating evidence demonstrates that EZH2 as the catalytic subunit of PRC2 also directly or indirectly plays a key role in the metabolic process of cancer cells31. Recent studies have indicated the significantly downregulated EZH2 expression in response to glucose deprivation in glucose-sensitive colorectal cancer cell lines in which the EZH2 knockout cells are more resistant to glucose deprivation. EZH2 deficiency upregulates glutaminase (GLS) expression and promotes glutamate production, leading to the increased intracellular glutathione (GSH) synthesis and glucose deprivation-induced reactive oxygen species (ROS)-mediated cell death is ultimately attenuated34.
Conclusion
High expression of EZH2 predicts a poor prognosis in mesothelioma or can serve as a prognostic indicator and target gene in mesothelioma. The mechanism may be linked to immune infiltration and metabolism. The increasing attention to the expression of EZH2 and the immune microenvironment in mesothelioma may significantly contribute to enhancing prognosis, which is worthy of further exploration.
Data availability
The datasets used and/or analyzed during the current study are available from the TCGA. Our study conformed to the publication guidelines provided by TCGA.
Abbreviations
- EZH2:
-
The prognostic value of enhancer homolog 2
- TCGA:
-
The Cancer Genome Atlas
- OS:
-
Overall survival
- DSS:
-
Disease free survival
- PFI:
-
Progression-free interval
- NES:
-
Normalized Enrichment Score
- PRC2:
-
Polycomb repressive complex 2
References
Neumann, V., Rütten, A., Scharmach, M., Müller, K.-M. & Fischer, M. Factors influencing long-term survival in mesothelioma patients–results of the German mesothelioma register. Int. Arch. Occup. Environ. Health 77, 191–199 (2004).
Kemp, C. D. et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 18, 77–90 (2012).
Gan, L. et al. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 6, 10 (2018).
Fan, K. et al. Prognostic value of EZH2 in non-small-cell lung cancers: A meta-analysis and bioinformatics analysis. BioMed. Res. Int. 2020, 2380124 (2020).
Zauderer, M. G. et al. Phase 2, multicenter study of the EZH2 inhibitor tazemetostat as monotherapy in adults with relapsed or refractory (R/R) malignant mesothelioma (MM) with BAP1 inactivation. J. Clin. Oncol. 36, 8515–8515 (2018).
Hamaidia, M. et al. Inhibition of EZH2 methyltransferase decreases immunoediting of mesothelioma cells by autologous macrophages through a PD-1-dependent mechanism. JCI Insight 4, 128474 (2019).
Mola, S. et al. Inhibition of the histone methyltransferase EZH2 enhances protumor monocyte recruitment in human mesothelioma spheroids. Int. J. Mol. Sci. 22, 4391 (2021).
Zauderer, M. G. et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: A multicentre, open-label, phase 2 study. Lancet Oncol. 23, 758–767 (2022).
LaFave, L. M. et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 21, 1344–1349 (2015).
Pinton, G. et al. CDKN2A determines mesothelioma cell fate to EZH2 inhibition. Front. Oncol. 11, 678447 (2021).
Wang, Z., Jensen, M. A. & Zenklusen, J. C. A practical guide to the cancer genome atlas (TCGA). Methods Mol. Biol. Clifton NJ 1418, 111–141 (2016).
Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 102, 15545–15550 (2005).
Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M. & Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545–D551 (2021).
Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 14, 7 (2013). Doi: 10.1186/1471-2105-14-7.
Brims, F. Epidemiology and clinical aspects of malignant pleural mesothelioma. Cancers 13, 4194 (2021).
Hanaki, S. & Shimada, M. Targeting EZH2 as cancer therapy. J. Biochem. (Tokyo) 170, 1–4 (2021).
Bödör, C. et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 122, 3165–3168 (2013).
Huang, J. et al. Easy or not-the advances of EZH2 in regulating T cell development, differentiation, and activation in antitumor immunity. Front. Immunol. 12, 741302 (2021).
Goswami, S. et al. Modulation of EZH2 expression in T cells improves efficacy of anti-CTLA-4 therapy. J. Clin. Invest. 128, 3813–3818 (2018).
Katsuyama, E. et al. The CD38/NAD/SIRTUIN1/EZH2 axis mitigates cytotoxic CD8 T cell function and identifies patients with SLE prone to infections. Cell Rep. 30, 112-123.e4 (2020).
He, S. et al. Ezh2 phosphorylation state determines its capacity to maintain CD8+ T memory precursors for antitumor immunity. Nat. Commun. 8, 2125 (2017).
Kim, H.-J., Cantor, H. & Cosmopoulos, K. Overcoming immune checkpoint blockade resistance via EZH2 inhibition. Trends Immunol. 41, 948–963 (2020).
Rugo, H. S. et al. The promise for histone methyltransferase inhibitors for epigenetic therapy in clinical oncology: A narrative review. Adv. Ther. 37, 3059–3082 (2020).
Kang, N. et al. EZH2 inhibition: A promising strategy to prevent cancer immune editing. Epigenomics 12, 1457–1476 (2020).
Quan, C. et al. Loss of histone lysine methyltransferase EZH2 confers resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Cancer Lett. 495, 41–52 (2020).
Cao, R. & Zhang, Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14, 155–164 (2004).
Verma, S. K. Recent progress in the discovery of epigenetic inhibitors for the treatment of cancer. Methods Mol. Biol. Clifton NJ 1238, 677–688 (2015).
Dimopoulos, K. et al. Dual inhibition of DNMTs and EZH2 can overcome both intrinsic and acquired resistance of myeloma cells to IMiDs in a cereblon-independent manner. Mol. Oncol. 12, 180–195 (2018).
Zhang, T., Gong, Y., Meng, H., Li, C. & Xue, L. Symphony of epigenetic and metabolic regulation-interaction between the histone methyltransferase EZH2 and metabolism of tumor. Clin. Epigenet. 12, 72 (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011).
Pang, B. et al. EZH2 promotes metabolic reprogramming in glioblastomas through epigenetic repression of EAF2-HIF1α signaling. Oncotarget 7, 45134–45143 (2016).
Liu, Y. et al. Tumor-suppressive function of EZH2 is through inhibiting glutaminase. Cell Death Dis. 12, 975 (2021).
Acknowledgements
Thanks to the staff of the department of Department of Radiation Oncology, Hebei Province Cangzhou Hospital of Integrated Traditional and Western Medicine for their help in data collecting for publication.
Author information
Authors and Affiliations
Contributions
K.F. and C.Z. conceived the manuscript and analyzed the data. K.F. and C.Z. wrote the manuscript and contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fan, K., Zhang, Cl., Zhang, Bh. et al. Analysis of the correlation between Zeste enhancer homolog 2 (EZH2) mRNA expression and the prognosis of mesothelioma patients and immune infiltration. Sci Rep 12, 16583 (2022). https://doi.org/10.1038/s41598-022-21005-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-21005-w
This article is cited by
-
The Role of H3K27me3-Mediated Th17 Differentiation in Ankylosing Spondylitis
Inflammation (2024)