Abstract
Recent studies have shown a correlation between high-density lipoprotein cholesterol (HDL-C) and bone mineral density (BMD) in adults, but their relationship is unclear in adolescents. This study aimed to explore whether a correlation existed between them among adolescents aged 12–19. Data analyzed in our study was fetched from the National Health and Nutrition Examination Survey (NHANES) database 2011–2018. The relationship between HDL-C level and total BMD value was analyzed by multivariate logistic regression models, fitted smoothing curves, and generalized additive models. 3770 participants participated in this analysis. After adjusting for all relevant covariates involved in this study, we found a negative correlation between HDL-C levels and total bone density in male adolescents.Furthermore, the stratified analysis showed that all covariables-adjusted models retained the negative correlation excepting female, black, or Mexican American subgroups. An inverted U-shaped curve represented the correlation of HDL-C and total BMD among adolescents aged 16 to 19, and the turning point was 1.06 mmol/L. After adjusting for all relevant covariates involved in this study, the study found a negative correlation between HDL-C levels and total BMD in male adolescents aged 12 to 19, particularly among those of races other than Black and Mexican. There was a saturation effect between HDL-C level and total BMD in 16–19-year-old adolescents. The turning point was 1.06 mmol/L. Therefore, HDL-C might be a biomarker to detect bone health and further perform a more detailed examination.
Similar content being viewed by others
Introduction
Osteoporosis is a common skeleton disease characterized by decreased bone mass, bone fragility, and increased fracture risk1. It is noteworthy that osteoporosis affects not only elderly subjects or postmenopausal women, but more attention has been paid to adolescents and children2. In adolescence, which is a progressive ontogenesis period, skeleton microarchitecture and mineralization make a qualitative change, and 40% of the peak bone mass is accumulated in this window3. Making an exact assessment of adolescents’ skeletal health benefits their current and future quality of life. World Health Organization proposed bone mineral density (BMD) value tested using dual X-ray absorptiometry (DXA) is the gold standard to diagnose osteoporosis4. Identifying associated and potential detrimental factors for bone health is significant for preventing and detecting osteoporosis.
Serum lipids, which consist of many substances, play a crucial role in physiopathology and are strongly associated with various metabolic diseases. High-density lipoproteins-cholesterol (HDL-C) is considered beneficial cholesterol5. Higher HDL-C levels meant better cardiovascular health in the past6. However, some researchers have proposed different perspectives about HDL-C. Hamer et al. indicated that the high HDL-C also increased mortality in a large population-based sample7.
Numerous kinds of research revealed that lipids level was closely related to BMD value8,9,10. Some studies showed a negative correlation between HDL-C and adult BMD11,12. Despite Ibrahim Duran et al. demonstrated muscle mass inversely correlated with HDL-C level among adolescents13, no research clarified and defined the relationship between HDL-C level and BMD value in adolescents. Therefore, exploring the correlation of HDL-C concentration and BMD value would be beneficial for estimating the risk of osteoporosis, which could effectively promote the prevention and treatment of osteoporosis.
This study aimed to clarify the relationship between HDL-C and total BMD in adolescents aged 12–19. Population-based subjects were extracted from the National Health and Nutrition Examination Survey (NHANES) to provide credible evidence for this issue.
Materials and methods
Patient collection and data extraction
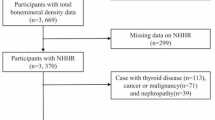
NHANES database is the largest population-based national survey in the world and is organized by the National Center for Health Statistics (NCHS) in the US. This survey is conducted every 2 years to evaluate the nutritional and health status of a representative of the noninstitutionalized US population. A complex stratified and multistage sampling design was applied to extract the representative population in the NHANES database. More details of the database are available at http://www.cdc.gov/nchs/nhanes/. The current study consisted of four data cycles (2011–2018). In this study, there were 39,156 participants enrolled from NHANES 2011–2018 database. After limiting to adolescents aged 12 to 19, the participants narrowed to 5215. Subjects with missing total BMD data (n = 1033) or HDL-C data (n = 412) were excluded. Finally, the data of 3770 cases were retained for analysis (Fig. 1). All NHANES protocols were ratified by the ethics review board of NCHS14.
Covariates
Our study's dependent and independent variables were HDL-C and BMD, respectively. The Roche Modular P chemistry analyzer detected the HDL-C concentration or the Roche modular P and Roche Cobas 6000 chemistry analyzers in the 2011–2018 cycle. The BMD value of all cases in this study was tested by using dual-energy X-ray absorptiometry (DXA). Hologic Discovery Model A densitometer (Hologic, Inc., Bedford, Massachusetts) and Apex version 3.2 software were used to detect and analyze BMD. The subject was scanned, and their BMD was calculated using a whole-body scan. According to the relative regulations and guidelines of WHO, the mean BMD value of a young adult was a reference value.
Furthermore, demographic characteristics in the NHANES database were regarded as potential confounders, which were analyzed in this study. These covariates included categorical variables (age, gender, race) and continuous variables (ratio of family income to poverty and various physiological and biochemical indexes including total calcium (Tca), serum phosphorus (P), total protein (TP), total cholesterol (TC), alkaline phosphatase (ALP), serum uric acid (SUA), triglyceride (TG), glycohemoglobin (HbA1c), urinary albumin creatinine ratio (ACR), serum creatinine (Scr), and blood urea nitrogen (BUN).
Statistical analysis
Based on the complex, multistage, stratified probability sampling design, sample weights were used in all statistical analyses through software R and Empower Stats. The study utilized weighted multivariate linear regression models to assess the correlation between HDL-C level and BMD value. Covariates were regulated as possible effect modulators. Therefore, we established three models for statistical inference in the subgroup analyses. Model 1 was adjusted with no covariates. Three covariates (age, gender, and race) were adjusted in model 2. In model 3, the ratio of family income to poverty, Tca, P, TP, ALP, TC, TG, HbA1c, Scr, ACR, SUA, and BUN were further adjusted. If the association of HDL-C with BMD was nonlinear, smooth curve fittings were applied to address the nonlinearity in these three models. Percentage and means ± standard deviation represented the categorical and continuous variables, respectively. Categorical variables adopted weighted chi-square tests, and continuous variables adopted weighted linear regression models to evaluate differences between groups.
Ethics approval and consent to participate
Participants in NHANES must sign an informed consent form, the data is now publicly available, and the National Center for Health Statistics Ethics Review Board evaluates and authorizes it. Converting data into a format that can be analyzed is already feasible. The research methodology will be based on all statistics. All research will conform to applicable laws and standards when following the research's data usage guidelines.
Results
Characteristics of the study participants
Table 1 describes the weighted sociodemographic and medical characteristics of 3770 eligible participants. The variables, including Tca, P, ALP, TC, TG, Scr, SUA,TP, HDL-C, ACR, BUN,and total BMD, were significantly different among different gender groups.
Associations between HDL-C and total BMD
The multivariate regression analysis to reveal the association of total BMD with HDL-C was presented in Table 2. An inversive association existed between HDL-C level and total BMD value in model 1 [− 0.047 (− 0.059, − 0.035)] with no variables adjusted, model 2 [− 0.038 (− 0.048, − 0.028)] with age, gender and race were adjusted, and model 3 [− 0.025 (− 0.036, − 0.014)] with all potential covariables were adjusted. Furthermore, there were three stratified analysis (stratified by age, gender, or race) that indicated the correlation was not significant in female adolescents [− 0.015 (− 0.029, 0.000)], black subjects [− 0.005 (− 0.028, 0.017)] and Mexican American subjects [− 0.018 (− 0.044, 0.007)]. The current study adopted smooth curve fittings to present the nonlinear correlation of HDL-C and BMD (Fig. 2). Moreover, the results of the stratified analysis are visualized with generalized additive models in Fig. 3. In age-stratified analysis, among adolescents aged 16 to 19, an inverted U-shaped curve represented the correlation of HDL-C and total BMD (Fig. 3a).
When stratified according to gender and age (Table 3), we found that after adjusting all variables, there was a negative correlation between HDL-C and total BMD in male adolescents of all ages but not in female adolescents. When stratified according to gender and race, we found that after adjusting all variables, there was a negative correlation between HDL-C and total BMD between white men and people of other races. Additionally, Table 4 of this study reveals a saturation effect analysis between HDL-C and total BMD. The results indicate a saturation effect was observed in adolescents aged 16 to 19. The turning point of direct HDL-C was the same in males and females (1.06 mmol/L). However, we did not find this effect in 12–16 year-old teenagers.
Discussion
We explored the relationship between HDL-C and BMD in adolescents. A higher quality of skeleton in adolescence protects against osteoporosis later in life15. Multivariate logistic regression analysis indicated an inversive correlation between HDL-C concentration with total BMD value. HDL-C was well known as beneficial cholesterol, promoting reversed cholesterol transportation and decreasing the incidence of various chronic diseases, including cardiovascular disease and endocrine disorders16,17. However, more and more researchers suggested the protective effect of HDL-C in some diseases was overestimated7,18. Previous research has confirmed that the correlation of HDL-C with muscle mass was also negative among adolescents13,19. Therefore, HDL-C level might be a potential biomarker for completing the evaluation of skeleton health in adolescents and improving quality of life in the future.
The relationship between HDL-C level and BMD value had been widely studied, but a unanimous agreement was not reached. Yuchen Tang et al. showed that HDL-C levels were inversely correlated with BMD value and might predict bone loss and osteoporosis20. Qi Zhang et al. explored the relationship of serum cholesterols (HDL-C, LDL-C, and TC) with lumber BMD. These three cholesterols indicated an inversive association with BMD in Chinese postmenopausal participants12. Peng Niu et al. confirmed the inverse correlation of HDL-C level and BMD through their analysis of the MIDUS II study21. However, Irene Zolfaroli et al. revealed that HDL-C level, but not TC and LDL-C, was positively relevant to the femoral neck and lumbar BMD22. Song-Seng Loke et al. suggested a positive association existed in Taiwanese older women, which was a reverse correlation in men. This finding reminded the importantce of gender23. Furthermore, Lian-Hua Cui et al. recruited 730 rural pre- or postmenopausal women and revealed no significant connection between HDL-C and BMD24. The reasons for controversial issues in previous studies may include small sample sizes, insufficient scientific data collection process, different populations analyzed, and differences in adjusted covariates.These controversial conclusions prompted us to develop more exploration, and the current study was more plausible. Firstly, 4 cycles of data were extracted from the NHANES database, which provided a larger sample. Second, the study considered gender, which might be a possible impact. Adolescents, not only females, were included in the study. Third, more potential variables that influence total BMD were adjusted. We found a negative association between HDL-C and BMD in a specific population of adolescents. This result enriches the research field more detailed and comprehensive to provide a more substantial reference for clinical application.
In this study, after adjusting all variables, the female subgroup did not have an association between HDL-C and BMD. Previous research had also suggested that gender hormones were possibly influencing factors in HDL-C's and BMD's correlation25,26,27. Ke Xu et al. investigated the relationship between gender hormones with BMD value. They demonstrated that the relationship between sex hormones such as testosterone, estradiol, or sex hormone-binding globulin levels and BMD were opposite in the boy and girl subgroups28. In summary, sex hormones might be the crucial cause of this association. In addition, a saturation analysis showed an inverted U-shaped curve representing the correlation between HDL-C and total BMD among adolescents aged 16 to 19, stratified by gender and age. The turning point was 1.06 mmol/L for both male and female adolescents. This variation tendency could be explained by a dramatic change in metabolic status, environmental risk factors, and other variables.
Limitation and strengths
Recognizing the correlation between HDL-C and BMD could help physicians better evaluate bone health status.The advantages of this study are the relatively large sample size, the use of data from public databases which ensures scientific data collection, and gender and age stratified analysis. however, there were some limitations in this study. (i) All of the included participants were American. The inversive association might be inconsistent in other countries. (ii) Even though research had revealed that the sex hormones were influencing factors of the correlation, the NHANES databases did not contain detailed information about these factors. (iii) A cross-sectional design cannot confirm the cause-effect connection. Researchers should conduct more prospective studies to solve the causality.
Conclusion
This study indicated a negative association between HDL-C and total BMD values among male adolescents. Therefore, adolescents with higher HDL-C concentrations were needed to monitor the value of BMD and prevent bone loss in the future.
Data availability
Data from the survey is publicly available online at http://www.cdc.gov/nchs/nhanes/ for data users worldwide.
References
Clynes, M. A. et al. The epidemiology of osteoporosis. Br. Med. Bull. 133(1), 105–117. https://doi.org/10.1093/bmb/ldaa005 (2020).
Makitie, O. Causes, mechanisms and management of paediatric osteoporosis. Nat. Rev. Rheumatol. 9(8), 465–475. https://doi.org/10.1038/nrrheum.2013.45 (2013).
Weaver, C. M., Peacock, M. & Johnston, C. C. Jr. Adolescent nutrition in the prevention of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 84(6), 1839–1843. https://doi.org/10.1210/jc.84.6.1839 (1999).
Kline, G. A., Lix, L. M. & Leslie, W. D. Patient outcomes in the years after a DXA-BMD treatment monitoring test: Improved medication adherence in some, but too little too late. J. Bone Miner. Res. 36(8), 1425–1431. https://doi.org/10.1002/jbmr.4333 (2021).
Chiesa, S. T. & Charakida, M. High-density lipoprotein function and dysfunction in health and disease. Cardiovasc. Drugs Ther. 33(2), 207–219. https://doi.org/10.1007/s10557-018-06846-w (2019).
Chang, T. I., Streja, E. & Moradi, H. Could high-density lipoprotein cholesterol predict increased cardiovascular risk?. Curr. Opin. Endocrinol. Diabetes Obes. 24(2), 140–147. https://doi.org/10.1097/med.0000000000000318 (2017).
Hamer, M., O’Donovan, G. & Stamatakis, E. High-density lipoprotein cholesterol and mortality: Too much of a good thing?. Arterioscler. Thromb. Vasc. Biol. 38(3), 669–672. https://doi.org/10.1161/atvbaha.117.310587 (2018).
Tian, L. & Yu, X. Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated (review). Mol. Med. Rep. 12(1), 783–794. https://doi.org/10.3892/mmr.2015.3472 (2015).
Zheng, J. et al. The effect of plasma lipids and lipid-lowering interventions on bone mineral density: A Mendelian randomization study. J. Bone Miner. Res. 35(7), 1224–1235. https://doi.org/10.1002/jbmr.3989 (2020).
Li, G. H. Y. et al. Positive effects of low LDL-C and statins on bone mineral density: An integrated epidemiological observation analysis and Mendelian randomization study. Int. J. Epidemiol. 49(4), 1221–1235. https://doi.org/10.1093/ije/dyz145 (2020).
Maghbooli, Z. et al. Negative correlation of high-density lipoprotein-cholesterol and bone mineral density in postmenopausal Iranian women with vitamin D deficiency. Menopause 25(4), 458–464. https://doi.org/10.1097/gme.0000000000001082 (2018).
Zhang, Q. et al. Association between bone mineral density and lipid profile in Chinese women. Clin. Interv. Aging 15, 1649–1664. https://doi.org/10.2147/cia.S266722 (2020).
Duran, I. et al. Inverse association of high-density lipoprotein cholesterol concentration with muscle mass in children. Child. Obes. 15(7), 476–484. https://doi.org/10.1089/chi.2019.0122 (2019).
Zipf, G. et al. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. Ser. 1. Prog. Collect. Proced. 56, 1–37 (2013).
Tamoliene, V., Remmel, L., Gruodyte-Raciene, R. & Jurimae, J. Relationships of bone mineral variables with body composition, blood hormones and training volume in adolescent female athletes with different loading patterns. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph18126571 (2021).
Stadler, J. T. & Marsche, G. Obesity-related changes in high-density lipoprotein metabolism and function. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21238985 (2020).
Xie, R., Huang, X., Liu, Q. & Liu, M. Positive association between high-density lipoprotein cholesterol and bone mineral density in US adults: The NHANES 2011–2018. J. Orthop. Surg. Res. https://doi.org/10.1186/s13018-022-02986-w (2022).
Madsen, C. M., Varbo, A. & Nordestgaard, B. G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality inmen and women: Two prospective cohort studies. Eur. Heart J. 38(32), 2478–2486. https://doi.org/10.1093/eurheartj/ehx163 (2017).
Pietrobelli, A., Lee, R. C., Capristo, E., Deckelbaum, R. J. & Heymsfield, S. B. An independent, inverse association of high-density-lipoprotein-cholesterol concentration with nonadipose body mass. Am. J. Clin. Nutr. 69(4), 614–620 (1999).
Tang, Y., Wang, S., Yi, Q., Xia, Y. & Geng, B. High-density lipoprotein cholesterol is negatively correlated with bone mineral density and has potential predictive value for bone loss. Lipids Health Dis. https://doi.org/10.1186/s12944-021-01497-7 (2021).
Niu, P. et al. Association between HDL-C and bone mineral density: An cross-sectional analysis. Int. J. Gen. Med. 14, 8863–8872. https://doi.org/10.2147/ijgm.S334972 (2021).
Zolfaroli, I. et al. Positive association of high-density lipoprotein cholesterol with lumbar and femoral neck bone mineral density in postmenopausal women. Maturitas 147, 41–46. https://doi.org/10.1016/j.maturitas.2021.03.001 (2021).
Loke, S.-S., Chang, H.-W. & Li, W.-C. Association between metabolic syndrome and bone mineral density in a Taiwanese elderly population. J. Bone Miner. Metab. 36(2), 200–208. https://doi.org/10.1007/s00774-017-0826-7 (2018).
Cui, L. H. et al. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos. Int. 16(12), 1975–1981. https://doi.org/10.1007/s00198-005-1977-2 (2005).
Mohamad, N.-V., Soelaiman, I.-N. & Chin, K.-Y. A concise review of testosterone and bone health. Clin. Interv. Aging 11, 1317–1324. https://doi.org/10.2147/cia.S115472 (2016).
Khosla, S., Oursler, M. J. & Monroe, D. G. Estrogen and the skeleton. Trends Endocrinol. Metab. 23(11), 576–581. https://doi.org/10.1016/j.tem.2012.03.008 (2012).
Jirapinyo, M., Theppisai, U., Manonai, J., Suchartwatnachai, C. & Jorgensen, L. N. Effect of combined oral estrogen/progestogen preparation (Kliogest((R))) on bone mineral density, plasma lipids and postmenopausal symptoms in HRT-naive Thai women. Acta Obstet. Gynecol. Scand. 82(9), 857–866. https://doi.org/10.1034/j.1600-0412.2003.00185.x (2003).
Xu, K., Fu, Y., Cao, B. & Zhao, M. Association of sex hormones and sex hormone-binding globulin levels with bone mineral density in adolescents aged 12–19 years. Front. Endocrinol. https://doi.org/10.3389/fendo.2022.891217 (2022).
Acknowledgements
Support from colleagues and friends from Shenzhen Traditional Chinese Medicine Hospital is gratefully acknowledged.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82104759); The Natural Science Foundation of Guangdong Provincial (No. 2019A1515110108).
Author information
Authors and Affiliations
Contributions
G-X.W. and J-T.L. designed this study, analyzed, wrote, and revised the manuscript, H-L.L., D-L.L., S-F.C., H-X.Z., Z-B.F., and W.X. supervised and reviewed the manuscript. All authors reviewed and approved the final manuscript, which had not been previously published. The work was not under consideration for publication elsewhere.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, GX., Li, JT., Liu, DL. et al. The correlation between high-density lipoprotein cholesterol and bone mineral density in adolescents: a cross-sectional study. Sci Rep 13, 5792 (2023). https://doi.org/10.1038/s41598-023-32885-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-32885-x
This article is cited by
-
Adiposity-lipid-glycemic clusters as potential warning signals of bone mass reduction in Asia’s largest urban communities – based bone health assessment via ultrasound
Lipids in Health and Disease (2025)
-
Non-linear association of atherogenic index of plasma with bone mineral density a cross-sectional study
Lipids in Health and Disease (2024)
-
The association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of osteoporosis among U.S. adults: analysis of NHANES data
Lipids in Health and Disease (2024)
-
Non-linear association of the platelet/high-density lipoprotein cholesterol ratio with bone mineral density a cross-sectional study
Lipids in Health and Disease (2024)
-
Association between triglyceride-glucose index and bone mineral density in US adults: a cross sectional study
Journal of Orthopaedic Surgery and Research (2023)