Abstract
Bee propolis is one of the most common natural extracts and has gained significant interest in biomedicine due to its high content of phenolic acids and flavonoids, which are responsible for the antioxidant activity of natural products. The present study report that the propolis extract (PE) was produced by ethanol in the surrounding environment. The obtained PE was added at different concentrations to cellulose nanofiber (CNF)/poly(vinyl alcohol) (PVA), and subjected to freezing thawing and freeze drying methods to develop porous bioactive matrices. Scanning electron microscope (SEM) observations displayed that the prepared samples had an interconnected porous structure with pore sizes in the range of 10–100 μm. The high performance liquid chromatography (HPLC) results of PE showed around 18 polyphenol compounds, with the highest amounts of hesperetin (183.7 µg/mL), chlorogenic acid (96.9 µg/mL) and caffeic acid (90.2 µg/mL). The antibacterial activity results indicated that both PE and PE-functionalized hydrogels exhibited a potential antimicrobial effects against Escherichia coli, Salmonella typhimurium, Streptococcus mutans, and Candida albicans. The in vitro test cell culture experiments indicated that the cells on the PE-functionalized hydrogels had the greatest viability, adhesion, and spreading of cells. Altogether, these data highlight the interesting effect of propolis bio-functionalization to enhance the biological features of CNF/PVA hydrogel as a functional matrix for biomedical applications.
Similar content being viewed by others
Introduction
The most pronounced application of three-dimensional (3D) tissue-like biocompatible materials is to direct tissue regeneration or healing after damage. This relies on the ability of these materials to optimize the physiological microenvironment by using biochemical, biophysical cues, and sometimes mechanical stimulation to enhance cell function1,2. Indeed, bioactive materials play multiple prominent roles in triggering cell proliferation and differentiation, as well as minimizing inflammation response that can delay the healing process3,4. Hydrogels are smart biomaterials that can be applied for the healing of a variety of tissues, such as skin, cartilage, bone, and blood vessels5. They can provide an optimal (3D) structure similar to native extracellular matrix (ECM) and allow diffusion of gases, nutrients, and waste through the elastic crosslinked networks6. Over the past decades, a variety of polymeric materials of either natural or synthetic origin have been used to develop functional hydrogels. Fiber-reinforced hydrogels are a class of composite hydrogels in which the gel networks are usually reinforced with fiber structures to improve the mechanical performance and also constrain the swelling behavior7,8,9.
Cellulose is the most abundant naturally derived polymer on earth, it’s the main component of plant cell walls and a few animal cells10. It is a linear homopolysaccharide consisting of β-d-anhydroglucopyranose units, linked by β (1→4) ether bonds (glycosidic links). The formed cellulose chains are linked by hydrogen bonds to form fibrils that consist of amorphous and crystalline regions. CNF signifies a specific class of nanocelluloses that composed of amorphous and highly ordered domains alternately associated and are typically obtained through the mechanical disintegration of cellulose fibrils11,12. As a result, CNF has been emerging nano-size biomaterials that display highly strength, surface area, and tunable surface chemistry, allowing for controlled interactions with polymers, nanoparticles, small molecules, and biological materials. For example, CNF was embedded in an alginate and polyvinyl alcohol solution to form a stable hydrogel that promotes in situ mineralization of calcium phosphates13. Also, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized CNF, which has carboxyl groups, was grafted by soy protein hydrolysate via amidation of carboxylic groups. The grafted CNF assisted the hydroxyapatite mineralization from twice-simulated body fluid to form a new bioactive material14.
One of the hydrophilic polymers used to produce biocompatible synthetic hydrogels is PVA, a semi-crystalline polymer. PVA hydrogel can be physically cross-linked to produce porous biocompatible materials with microstructure and deformation properties comparable to biological membranes, making them ideal for biomedical applications15,16. Physical crosslinking of PVA can be performed by repeated freezing and thawing cycles. In this method, the prepared PVA solution is frozen, which allows ice crystals to form pushing away the PVA chains and packing them together in confined spaces. Crystallization of the polymer happens in these regions. Then, through the thawing process, the ice crystals melt, forming porous cryogels with crystals working as crosslinking junctions17. Although traditional hydrogels possess excellent properties such as safety, biocompatibility, and higher hydrophilicity, they have limited biological performance. Today, the combination of hydrogel with therapeutic agents (i.e., drugs, natural extracts, functional proteins, or genes) represents an effective method to offer more favorable environments for the tissue regeneration process and to avoid the rapid metabolism of therapeutic agents18.
Propolis is a natural byproduct of beekeeping that has been used for wound healing since ancient times. More than 300 compounds are present in propolis, and the colour varies with the regions vegetation and the plant source. Depending on the regional vegetation, it can be yellowish-green or dark brown19. As well as securing cracks in hives, propolis strengthens honeycomb cells and protects hives from microbial invasions20. The main polyphenolic components of propolis are flavonoids and phenolic acid esters, followed by aromatic acids, lignans, and terpenoids21. Usually, bee wax makes up 30% of the mixture, pollen makes up 5%, and resins and vegetable balsams make up 50%21. In addition to balsamic, resinous, and gummous substances, propolis also contains pollen, saliva, and wax, and is derived from the exudates and sprouts of plants by honey bees22.
The antimicrobial properties of propolis are attributed to flavonoids, which are effective against Gram-positive and Gram-negative bacteria23. Many biological properties are associated with propolis, including antibacterial, antifungal, antiviral, anti-inflammatory, anticancer, and antitumor properties24,25. Propolis-based materials have been applied as wound healing materials. For example, propolis as a cream is used for enhancement of dermal tissue healing and reduce the wound inflammation better than silver sulfadiazine26. It promotes the proliferation, activation, and growth capacity of skin cells, with no toxicity or allergic reactions.
Recently, functional hydrogels have attracted huge attention for tissue engineering and regeneration applications due to their hydrophilicity, flexibility, and inherent adhesive potential to biological tissues. These distinctive characteristics play a critical role in increasing the residence time of a given therapeutic ingredient in direct contact with its biological target. Therefore, the present study aims to prepare a 3D hydrogel from PVA and CNF using the repeated freezing and thawing cycles, as a safe physically cross-linked hydrogel for effective combination of PE without undesired side interactions with cross-linkers that are involved in chemically cross-linked hydrogels. The microstructure features of PE-loaded hydrogels were analyzed through Fourier transform infrared spectroscopy (FT-IR), SEM, and X-ray diffraction (XRD). In addition, in vitro test cell viability and attachment to human normal fibroblast cells (HFB4) were determined. Finally, antibacterial and anti-inflammation potentials were also investigated, as summarized in Fig. 1.
Results and discussions
Polyphenol compounds determination of PE
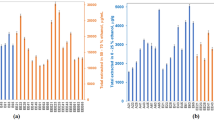
The chemical composition of PE may vary depending on the honey bee’s food source. Therefore, it is important to illustrate the chemical composition of PE to emphasise the presence or absence of certain compounds. As shown in Table 1 and Fig. 2, the HPLC detection method was able to identify 18 polyphenol compounds in PE, which play extremely important roles in its biological activity. The polyphenol compounds with highest content illustrated in PE were hesperetin (183.73 µg/mL), chlorogenic acid (96.92 µg/mL), caffeic acid (90.28 µg/mL), daidzein (67.89 µg/mL) and apigenin (66.59 µg/mL). While the rutin and vanillin had low concentrations of 0.8 and 0.7 µg/mL, respectively. On the other hand, the only catechin not observed in the PE when compared with the standard. Geographical origin and extraction solvent of propolis are the main factors that affect the availability and concentrations of polyphenol compounds, as illustrated in Table 2.
Characterizations of CNF
For the selective oxidation of primary hydroxyl groups in cellulose (Fig. 3A), TEMPO-mediated oxidation is a suitable method for C-6 oxidation to generate a reactive carboxylic groups. Figure 3B shows the FT-IR spectrum of CNF, which exhibits the characteristic peaks for cellulose chains35. A new band, however, was seen at 1739 cm−1 that was attributed to the stretching of carbonyl groups (C=O), from the TEMPO oxidation process. The prepared CNF was confirmed by the XRD pattern reported in Fig. 3C. It shows two main diffraction peaks at 16.4° (110) and 23.1° (200) which are characteristic of native cellulose. Also, the crystallinity percentage was estimated according to the Segal method and recorded ∼ 73%36. The internal structure of CNF was also examined using transmission electron microscope (TEM) analysis. Figure 3D showed CNF with a micrometric length and a diameter varying from 4 to 20 nm, calculated via image analysis. Moreover, the nanofibers exhibited a uniform structure due to the homogeneous formation of carboxylate groups on the surface of the fibers.
Microstructure features of the prepared fiber-reinforced hydrogels
Indeed, polymer hydrogels are fabricated by physical or chemical cross-linking, or even both. Physically cross-linked hydrogels are obtained through weak interactions, while chemical covalent bonding is formed when hydrogels are cross-linked chemically. Among them, PVA hydrogels can be simply formed by the freeze-thawing technique. In this study, PE-loaded hydrogels based on CNF and PVA were prepared by simple physical crosslinking after successive runs of freezing and thawing followed by freeze drying to produce porous 3D structures. Figure 4A–C illustrates SEM images of the surfaces of the CNF/PVA, CNF/PVA/PE1 and CNF/PVA/PE2 fiber reinforced hydrogels, along with the cross section images for the same hydrogel sample. Figure 4D shows the cross-section images for the same hydrogels. Images of cross section and surface confirm the formation of 3D rough interconnected porous structures with irregular pores varying in size from 10: 100 µm. This microporous structure is very important for cell recruitment because it facilitates the transport of oxygen and nutrients. The introduction of PE into the hydrogel results in a decrease in pore size and surface roughness. This decrease in pore size suggests the presence of PE on the surface of the hydrogel which promotes its fast release when implanted in vivo. This fast release of antibacterial agents such as PE is important to stop the bacterial invasion.
Figure 5 illustrates the FT-IR spectra of PE and PE-loaded PVA/CNF hydrogel samples. The IR spectrum of the PE sample, Fig. 5A, shows a broad band centered at 3410 cm−1, which is typically attributed to the O–H stretching vibration of the phenol compounds37. The two bands at 2920 and 2840 cm−1 are related to C–H asymmetric and symmetric stretching of hydrocarbon compounds, respectively38. While the bands at 1720 and 1460 cm−1 are assigned to the carbonyl C=O stretching vibration of flavonoid and lipid content. Furthermore, the bands at 1370 and 1271 cm−1 are due to scissor vibrations of C–H groups and C–O–H stretching vibrations, respectively39. Finally, the bands at 1165, 1040, and 870 cm−1 are related to the stretching vibration of alkenes C=C bond, the aromatic ether C–O–C bond and the C–H wagging vibration of phenolic compounds40.
On the other hand, the FT-IR spectrum of CNF/PVA hydrogel shows a broad band at 3294 cm−1, which is due to the O–H stretching vibration of hydroxyl group of both PVA and CNF, as shown in Fig. 5B. The bands at 2905, 1708 and 1420 cm−1 belong to C–H stretching, C=O stretching, and CH2 bending vibrations, respectively. The other bands at 1040 and 870 cm−1 are related to the C–O and CH2 stretching vibrations41. The FT-IR spectra of CNF/PVA/PE1 and CNF/PVA/PE2 fiber reinforced hydrogels are shown in Fig. 5C and D, respectively. The spectra of both samples exhibit peaks related to CNF/PVA hydrogel, with some modifications. For instance, the O–H stretching bands were shifted to 3335 and 3351 cm−1 for the CNF/PVA/PE1 and CNF/PVA/PE2 samples, respectively. Moreover, the C=O stretching bands were observed at 1739 cm−1 for CNF/PVA/PE1 sample and at 1725 cm−1 for the CNF/PVA/PE2 sample. Together, these shifts can be attributed to the interaction between the CNF/PVA polymer matrix and propolis41.
Water uptake
The tendency to water uptake or swelling behavior of CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels is illustrated in Fig. 6. The ratios of water uptake for all samples are high and increased gradually with time. This may refer to the high porous structure of the samples, which allows water to diffuse easily through the hydrogel. As shown in Fig. 6, the samples loaded with PE (CNF/PVA/PE1 and CNF/PVA/PE2) exhibit lower water uptake ratios than those of neat (CNF/PVA). For example, at a time interval of 12 h, the ratio of water uptake for CNF/PVA is about 907%, while for CNF/PVA/PE1 and CNF/PVA/PE2 are 648% and 590%, respectively. Therefore, the presence of PE in the CNF/PVA/PE1 and CNF/PVA/PE2 fiber reinforced hydrogels diminishes their ability to water uptake which may be attributed to the hydrophobic nature of PE and the decrease in pore size as indicated from SEM observations (Fig. 4)40.
Assessment of antimicrobial activity
Propolis is a resinous material extracted from the buds and flowers of plants and used as a protective agent against microbial contamination42. Polyphenols are the major chemical components of PE, and they are responsible for its antimicrobial activity43. The antimicrobial action of PE and CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels was explored on pathogens for humans using different approaches. The antimicrobial activity of PE is presented in Table 3 and Fig. 7. Although, PE exhibited antimicrobial potential against all of the studied microorganisms, it has no influence on S. typhimurium as reported by Atik et al.44. Control CNF/PVA hydrogel has no effect against the studied microorganisms. The results exhibit a significantly higher antimicrobial activity against Gram-positive (S. mutans), with no significant difference between Gram-negative (E. coli) and C. albicans. Moreover, the MIC was also determined through the investigation of antimicrobial activity at different concentrations. The MIC of PE for E. coli was 0.012 mg/mL, 0.05 mg/mL for S. mutans, and 0.025 mg/mL for C. albicans. On the other hand, the CNF/PVA fiber reinforced hydrogels with PE at different concentrations exhibit antimicrobial action against the strains used, with the highest antimicrobial activity being observed in S. typhimurium, followed by S. mutans, and other microbes showing no significant differences. The difference in antimicrobial behavior of the PE could be due to the different cell walls between the Gram-positive and negative bacteria45. The activity of PE as antimicrobial agents is related to the high percent of hesperetin (183.7 µg/mL), chlorogenic acid (96.9 µg/mL) and caffeic acid (90.2 µg/mL) as the main polyphenol compounds of PE. As illustrated in Table 1, hesperetin, chlorogenic acid, and caffeic acid were reported as three compounds from PE with high concentrations. Hesperetin was obtained from the enzymatic hydrolysis of hesperidin and evaluated for antimicrobial activity against Gram-positive and Gram-negative bacteria, the results revealed that hesperetin more effectively inhibited the model microbes than hesperidin and hesperidin glucoside 46. Yen et al.47. found that chlorogenic acid was one of the main components of the water extract of green coffee beans under high pressure and demonstrated antibacterial activity against both Gram-positive (Staphylococcus aureus and Listeria innocua) and Gram-negative (Escherichia coli and Salmonella enterica). Caffeic acid and its combination with antibiotic phytochemicals were evaluated against Staphylococcus aureus, and the results showed diverse effects against Staphylococcus aureus, with the MIC varying from 256 to 1024 µg/mL48. Most of the tested polyphenol compounds were not effective against the model microbes used when tested as a single compound, but when combined with other compounds, the activity of the antimicrobials increased. According to Ahmed et al.49 gallic acid at 200 and 400 µg/mL was effective against S. aureus and S. pyogenes, but not against Gram-negative bacteria. On the other hand, the antisolvent extracted from olive mill wastewater combined with gallic acid was tested using low minimal inhibitory concentrations, which revealed that 50/100–100/100 µg/mL caused complete growth inhibition of all the bacteria used, owing to the synergistic effects of phenolic compounds against the model bacteria used. Other study report that polyphenol compounds extracted from grape pomace combined with representatives of different classes of antibiotics such as β-lactam, quinolone, fluoroquinolone, tetracycline, and amphenicol act in synergy in all S. aureus and E. coli strains tested with fractional inhibitory concentration index (FICI) values varying from 0.031 to 0.155. The MIC was reduced 4 to 75 times due to the synergistic effects of phenolic compounds with different antibiotics50. Previous studies promoted the composite membrane by propolis to improve their biological assessments, for example polylactic acid/essential oil51, poly-ε-caprolactone52, bacterial cellulose/ZnO-NPs53, chitosan/Ag-NPs54, and polyurethane/ nanolignin55. We can summarize the main bioactive polyphenol compounds from PE as having antimicrobial activity in Table 4.
Cell viability
HFB4 cells were incubated with PE for 24 h at 37 °C in medium to develop a complete monolayer sheet. Different concentrations of PEs were applied up to concentrations of 1000 µg/mL. We observed that PE at concentrations greater than 125 µg/mL significantly reduced cell viability according to MTT (Fig. 8). Concentrations up to 125 µg/mL did not affect cell viability. Furthermore, light microscopy was used to confirm the cellular proliferation upon treatment of HFB4 cells with different doses of PEs. A decrease of cell number upon increasing PE concentration was clearly seen as compared to the control (Fig. 8). These results indicate that concentrations of PEs beyond the limit of 125 µg/mL may induce harmful effects in HFB4 cells. Previous studies demonstrated that supplementation with natural extracts may increase reactive species production and oxidative stress in vitro and in vivo tests, leading to oxidative damage and cell death73,74.
Viability of HFB4 cells treated with different concentrations (31.25–1000 μg/mL) of PE for 24 h. Data were expressed as the mean percentage of the formazan formation in untreated cells (control). Data were obtained from three independent experiments (six replicates for each experiment). (*) means p less than 0.05 and **p more than 0.05 compared to the control.
Figure 9 shows the viability of HFB4 cells as determined by the MTT assay when treated with different samples of hydrogels. The viability of cells treated with samples containing PE (CNF/PVA/PE1 and CNF/PVA/PE2) exceeds than CNF/PVA sample without PE. This reflects that the presence of PE enhances the viability of HFB4 cells.
SEM images of the spreading and adhesion of HFB4 cells treated with CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels are shown in Fig. 9. As seen, all seeded cells have a rounded shape, which may be caused by the dehydration during the fixation process. The spreading of cells on samples loaded with propolis (CNF/PVA/PE1 and CNF/PVA/PE2) is higher compared to a control sample without PE (CNF/PVA). Also, the spreading and attachment of cells in the sample with a higher content of PE (CNF/PVA/PE2) is greater than in the sample with a lower content of PE (CNF/PVA/PE1). Therefore, the presence of propolis enhances cell growth and adhesion. Overall, the in vitro tests performed in this work illustrate the importance of the prepared propolis-reinforced hydrogel as a cytocompatible material promoting cell adhesion and spreading in a 3D environment.
In vitro anti-inflammatory tests (hemolysis inhibition)
The stabilization of erythrocyte membranes is an established assay to investigate the anti-inflammatory effect of implanted biomaterials. Inflammation occurs due to release of different enzymes from decomposed lysosomal vesicles75. Therefore, stabilization of erythrocyte membrane prevents leakage of these enzymes, as well as incidence of inflammation75. Hemolysis assay is very critical assay especially for materials that will be in contact with the blood. Table 5 explains the results of hemolysis inhibition for PE, samples of CNF/PVA hydrogel and those loaded with propolis (CNF/PVA/PE1 and CNF/PVA/PE2). The indomethacin drug was used as the slandered (positive control) and it has the highest protection (100 ± 2.362%) at a concentration of 200 µg/mL against hypotonicity induced lysis of RBCs. As shown in Table 2, all samples protect human erythrocytes in a concentration dependent manner. The protective effect of CNF/PVA/PE2 sample and PE is almost the same at different concentrations. The lowest protective effect is recorded for the CNF/PVA sample (without PE). From these findings, it’s clear that, the PE loaded hydrogels are promising as anti-inflammatory agents.
Conclusion
Propolis is a natural byproduct with high value for diverse biological applications. The HPLC analyses of PE revealed about 18 polyphenol compounds, with the highest concentration being hesperetin, followed by chlorogenic acid and caffeic acid. CNF was prepared by oxidation-defibrillation of cellulose obtained from bleached sugarcane bagasse, and used to prepare sustainable and porous CNF/PVA loaded with various PE concentrations. The prepared scaffolds exhibited a high porous structure with pore sizes up to 100 µm. The obtained PE-loaded CNF/PVA hydrogels have a highly antimicrobial activity against E. coli, S. mutans bacteria and then C. albicans. The cellular responses also showed that the cells on the PE-functionalized CNF/PVA hydrogels revealed an optimal microenvironment for the attachment and proliferation of HFB4 cells. Remarkably, our results suggested that PE-functionalization of CNF/PVA not only enhanced the antimicrobial features of PE-loaded CNF/PVA hydrogels, but also resulted in significant anti-inflammation potential. Thus, in vivo experiments are highly recommended prior to clinical application to underline the potency of PE-loaded CNF/PVA hydrogels in wound healing.
Materials and methods
Materials
Bleached bagasse pulp was obtained from Qena Company of Paper Industry, Egypt. TEMPO was purchased from Merck. Sodium bromide was purchased from Sigma-Aldrich. The raw propolis material used in this work was collected from a local beekeeper (Mansoura, Dakahlia, Egypt) in July 2022. Polyvinyl alcohol—PVA (MW85,000–124,000 g/mol, 99.1% hydrolized) was purchased from Sigma-Aldrich. Sodium hydroxide, distilled water, and ethanol were also employed in this research.
Preparation of PE
PE was collected according to Bozkuş et al.31 with little modifications. In brief, about 10 g of raw material was dissolved in 100 mL of 70% ethanol and then placed in an incubator at 37 °C for 14 days in a dark place. After filtration using Whatman filter paper (No. 1), the mixture was centrifuged at 5000 rpm for 15 min and then oven dried at 40 °C for 5 days until constant weight. The PE obtained was stored in the refrigerator until used.
Evaluation of polyphenol compounds
The polyphenol (phenolic and flavonoids) compounds of PE were analyzed by HPLC (Agilent 1260 series, USA), according to Kong et al., with slight modifications76. Chromatographic conditions: the chromatographic separation was carried out on an Eclips C18 column (4.6 × 250 mm, 5.0 µm). The mobile phase comprised (A) water and (B) 0.05% trifluoroacetic acid in acetonitrile at a flow rate of 1 mL/min. The solvent gradient was: 0 min 82% A, 0–5 min 80% A, 5–8 min 60% A, 8–12 min 60% A, 12–15 min 82% A, 15–16 min 82% A, and 16–20 min 82% A. The multi-wavelength detector was monitored at 280 nm. The injecting volume was 5 µl for each of the sample solutions. The column temperature was maintained at 40 °C.
Preparation of CNF
Bleached bagasse was used for the preparation of TEMPO-oxidized CNF by using the method described by Salama et al.35. In brief, 20 g of bleached bagasse pulp were dispersed in distilled water with TEMPO (0.8 g) and sodium bromide (8 g). Following this, 300 mL of sodium hypochlorite solution (15%) was continuously added and the pH was adjusted to 10 using NaOH (3 mol/L) solution. At the end of the reaction, the pH was reduced to 7.0 and the product was centrifuged several times at 10,000 rpm. Dialysis using deionized water was performed to purify the product.
Preparation of CNF/PVA hydrogel reinforced by PE
The CNF/PVA porous hydrogel was prepared using the freeze-thawing and freeze drying methods according to Müller et al.77. The polymeric weight ratio was adjusted to 50:50 (CNF:PVA). In brief, 30 mL of PVA solution with a concentration of 10 wt% was prepared in distilled water using magnetic stirring at 95 °C. After 4 h, 100 mL of CNF solution (3 wt%) was added to PVA solution and underwent mechanical agitation for 10 min at 5000 rpm. Then, the CNF/PVA mixture was poured into a 10 cm round mould and freezen at − 40 °C for 3 h, followed by thawing at room temperature for 3 h to allow PVA crosslinking. The obtained hydrogels were subjected to four freeze-thawing cycles and subsequently freeze-dried at − 80 °C. To produce PE loaded CNF/PVA hydrogels, different proportions of the PE (0.1 and 0.2 g) were dissolved in 2 mL of ethanol and then added to 22 mL of the CNF/PVA suspension solution mixture. The mixtures were kept on a magnetic stirrer at 40 °C for 1 h to assure complete dissolution of PE. The solutions were homogenized using mechanical agitation for 10 min at 5000 rpm before being poured into round mold and subjected to freeze-thawing cycle and finally lyophilized at − 80 °C, as mentioned above. The prepared samples were named CNF/PVA for control (without PE), CNF/PVA/PE1 for sample with 0.1 g PE and CNF/PVA/PE2 for sample with 0.2 g PE.
Characterizations of composite hydrogel
Structure and morphological analysis
The morphology of the prepared CNF/PVA hydrogel reinforced with different concentrations of PE was examined using field emission scanning electron microscopy (FE-SEM) (JSM 6360LV, JEOL/Noran). The estimation of pore size was determined from different SEM images using the angle tool of ImageJ with Java 1.8.0 software (National Institute of Health (NIH), USA)78. The FT-IR (model FT/IR-6100 type A) was used to investigate the chemical functional groups of the samples. The spectra were recorded at wave numbers in the range of 400–4000 cm−1. The XRD pattern was recorded with an empyrean powder diffractometer (Cu Kα, 0.154 nm) between 5 and 70° 2θ with a step size of 0.01°/s to examine the phase formed in the prepared CNF. Ultra High-Resolution Transmission Electron Microscope (JEOL-2010) was applied to examine the internal structure of CNF.
Water uptake ratios
The evaluation of prepared hydrogel for water uptake was carried out according to Eskandarinia et al.79 with slight changes. Prepared fiber reinforced hydrogels (CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2) were cut into 1 cm × 1 cm, accurately weighed (Wi), and immersed in 10 mL of distilled water in sealed vials at 37 °C. At scheduled time intervals (1, 2, 3, 4, 5, 6, 8, and 12 h), samples were withdrawn from the vials, blotted with a tissue paper to remove the surface water and weighed (Ww). The water uptake (swelling) ratio of the samples is specified according to the following equation:
Biological applications of PE and composite hydrogel
Antimicrobial activity
Microorganism and growth conditions
The antimicrobial activity of the PE, CNF/PVA, CNF/PVA/PE1 and CNF/PVA/PE2 fiber reinforced hydrogels was assessed against four selected pathogens, including Gram-negative bacteria [Escherichia coli ATCC 25922 (E. coli) and Salmonella typhimurium ATCC 14028 (S. typhimurium)], Gram-positive bacteria [Streptococcus mutans ATCC 25175 (S. mutans)], and yeast [Candida albicans ATCC 10231 (C. albicans)]. All microbes were provided by American Type Culture Collection (ATCC). The microbial strains were cultivated in a test tube containing 5 mL of Mueller Hinton broth composed of (%): 0.2 beef extract, 1.75 acid hydrolysate of casein, and 0.15 starch, under staring cultivation (200 rpm) for 1 day at 37 °C. The antimicrobial activity of the propolis and different hydrogel samples were carried out through the agar well and disk diffusion approach, respectively.
Agar well diffusion technique
The antimicrobial action of the PE was evaluated qualitatively by the agar well diffusion technique according to Roy and Rhim80, with little modification. Shortly, the microbial suspension (108 CFU/mL) with approximately equal concentration or density with 0.5 McFarland standards was spread on the Petri dish surface containing Mueller Hinton agar medium (2%). The minimum inhibitory concentration (MIC) is the minimum concentration of the PE that shows a low inhibition zone against the microbial target. For MIC determination, the propolis at different concentrations of 0.1, 0.05, 0.025, 0.012, and 0.006 mg/mL (labelled as 1, 2, 3, 4 and 5, respectively) was dug in the media wells (4 mm in diameter) by a sterile glassy borer and loaded with 65 μL of tested propolis. The negative control was performed by loading the center wells with DMSO.
Disk diffusion technique
The antimicrobial activity of the prepared CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels was carried out through the disk diffusion approach according to Yahia et al.81, with minor modifications. In brief, 100 µL of serially diluted pathogens were separately distributed on the Petri dishes of Mueller Hinton agar medium along with disks (5 mm in diameter) of the composite membranes loaded with different concentrations of propolis. The fiber reinforced hydrogel without propolis was used as a control.
Incubation conditions and assay
All the plates were left at 4 °C for 120 min. to complete the diffusion of the tested sample as well as inhibit the model microbes, subsequently incubated at 37 °C for 1 day, where the antibacterial activity was assessed by measuring the developed inhibition-zone diameter (including the well or membrane disk) after the incubation period. To ensure the aseptic conditions, all membranes were sterilized for 30 min under UV light prior to application. All experiments were conducted in triplicate, and the mean results were represented.
Cytotoxicity assay
Cytotoxicity assay of CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels and also PE against HFB4 cells was performed using the MTT (3-4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay82,83. The prepared hydrogels were rinsed with media three times and then plated in the bottom of a 24 well tissue culture plate. The 24 well plate was inoculated with 1 × 105 cells/mL (1000 µL/well) and incubated at 37 °C for 24 h to develop a complete monolayer sheet. Cells were observed for sheet formation and cytotoxicity effects. The HFB4 cells were checked for any physical signs of toxicity, e.g., complete or partial loss of the monolayer, rounding, shrinkage, or cell granulation. MTT solution was prepared (5 mg/mL in PBS) (BioBasic Canada Inc). 100 µL MTT solution was appended to each well. Put on a shaking table, 150 rpm for 5 min, to thoroughly blend the MTT into the media. Incubate (37 °C, 5% CO2) for 4 h to allow the MTT to be metabolized. After that, 200 μL of DMSO was mixed with the culture cells to solubilize the formazan crystals, which were obtained via the reduction of MTT in living cells. Subsequently, the mean absorbance of each well at 570 nm was calculated using a spectrophotometer. Three parallel experiments were performed. The absorbance should be directly correlated with cell quantity82,83. For PE, the 24 well tissue culture plate was inoculated with 1 × 105 cells/mL (100 µL/well) and incubated at 37 °C for 24 h to develop a complete monolayer sheet. Growth medium was decanted from 24 well microtiter plates after a confluent sheet of cells was formed. Then the cell monolayer was washed twice with wash media. A twofold dilution of the tested sample was made in RPMI medium with 2% serum (maintenance medium). 0.1 mL of each dilution was tested in different wells, leaving 3 wells as controls, receiving only maintenance medium. Then the same steps were performed as mentioned above.
Cell adhesion
HFB4 cells were grown in 24-well plates containing CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels84. After 24 h incubation, the cells were fixed with 5% (V/V) glutaraldehyde and then dehydrated gradually with serial solutions of ethanol (70–100% for 20 min each). After that, the samples sputtered with gold for SEM observations.
Anti-inflammatory assay
The preparation of erythrocyte suspension was done according to Anosike et al.75. In brief, about 3 mL of fresh whole blood gathered from healthy volunteers was placed in heparinized tubes and then centrifuged at 3000 rpm for 10 min. A volume of normal saline equal to that of the supernatant was utilized to dissolve the red blood pellets. The volume of the obtained dissolved red blood pellets was measured and returned as a 40% v/v suspension with an isotonic buffer solution (10 mM sodium phosphate buffer, pH 7.4). The buffer solution was formed from 0.2 g of NaH2PO4, 1.15 g of Na2HPO4 and 9 g of NaCl in 1 L of distilled water. The returned red blood cells (resuspended supernatant) were used as such.
Hypotonicity induced hemolysis
Equal weights of CNF/PVA, CNF/PVA/PE1, and CNF/PVA/PE2 fiber reinforced hydrogels were ground carefully and suspended in distilled water (hypotonic solution). 5 mL of the hypotonic solution of graded doses of the samples (100, 200, 400, 600, 800, and 1000 µg/mL) were placed into duplicate pairs (per dose) of the centrifuge tubes. 5 mL of isotonic solution of graded doses of the samples (100–1000 µg/mL) were also placed into duplicate pairs (per dose) of the centrifuge tubes. The same previous steps were occurred for PE resin, but without grinding. A tube containing 5 mL of the vehicle (distilled water) and another tube containing 5 mL of 200 µg/mL of indomethacin were used as the control tubes, respectively. A suspension (0.1 mL) of erythrocytes was added to each of the tubes and mixed gently. The mixtures were incubated for 1 h at room temperature (37 °C), and afterwards, they were centrifuged for 3 min at 1300 rpm. The absorbance (Ab) of hemoglobin within the supernatant was estimated at 540 nm using a Spectronic (Milton Roy) spectrophotometer. To calculate the hemolysis percentage, the hemolysis formed in the presence of distilled water was assumed to be 100%. The percent inhibition of hemolysis by the extract was calculated thus:
where Ab1 = absorbance of test sample in isotonic solution, Ab2 = absorbance of test sample in hypotonic solution, Ab3 = absorbance of control sample in hypotonic solution75,85.
Statistical analysis
All experiments were done in triplicate, and the results were presented as mean ± standard deviation. Statistical analyses were performed with the one-way ANOVA test, by using Sigma Stat 3.5 software (Dundas Software Ltd, Toronto; Canada). P values ≤ 0.05 were considered statistically significant.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Loh, Q. L. & Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 19, 485–502 (2013).
Salerno, A., Di Maio, E., Iannace, S. & Netti, P. Tailoring the pore structure of PCL scaffolds for tissue engineering prepared via gas foaming of multi-phase blends. J. Porous Mater. 19, 181–188 (2012).
Mazzoni, E. et al. Bioactive materials for soft tissue repair. Front. Bioeng. Biotechnol 9, 613787 (2021).
Yu, Q., Chang, J. & Wu, C. Silicate bioceramics: From soft tissue regeneration to tumor therapy. J. Mater. Chem. B. 7, 5449–5460 (2019).
Mantha, S. et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials 12, 3323 (2019).
Geckil, H., Xu, F., Zhang, X., Moon, S. & Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 5, 469–484 (2010).
Hoffman, A. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 64, 18–23 (2012).
Khan, F. et al. Discovery and evaluation of a functional ternary polymer blend for bone repair: Translation from a microarray to a clinical model. Adv. Funct. Mater. 23, 2850–2862 (2013).
Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 6, 105–121 (2015).
McNamara, J. T., Morgan, J. L. & Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 84, 895–921 (2015).
Habibi, Y., Lucia, L. A. & Rojas, O. J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 110, 3479–3500 (2010).
Azizi Samir, M. A. S., Alloin, F. & Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromol 6, 612–626 (2005).
Abouzeid, R. E., Salama, A., El-Fakharany, E. M. & Guarino, V. Mineralized polyvinyl alcohol/sodium alginate hydrogels incorporating cellulose nanofibrils for bone and wound healing. Molecules 27, 697 (2022).
Salama, A., Abou-Zeid, R. E., Cruz-Maya, I. & Guarino, V. Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr. Polym. 229, 115472 (2020).
Peixoto, L. S. et al. Macromolecular Symposia. 190–199 (Wiley Online Library).
Tolba, E. et al. In situ polyphosphate nanoparticle formation in hybrid poly (vinyl alcohol)/karaya gum hydrogels: A porous scaffold inducing infiltration of mesenchymal stem cells. Adv. Sci. 6, 1801452 (2019).
Coukouma, A. E., Smith, N. L. & Asher, S. A. Removable interpenetrating network enables highly-responsive 2-D photonic crystal hydrogel sensors. Analyst 140, 6517–6521 (2015).
Do, N. H., Truong, Q. T., Le, P. K. & Ha, A. C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 294, 119726 (2022).
Piccinelli, A. L. et al. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography–photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 59, 6484–6491 (2011).
Anjum, S. I. et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 26, 1695–1703 (2019).
Moreno, M. A. et al. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 107, 105973 (2020).
Ghisalberti, E. Propolis: A review. Bee World 60, 59–84 (1979).
Cui, J. et al. Extraction, purification, structural character and biological properties of propolis flavonoids: A review. Fitoterapia 157, 105106 (2021).
Falcão, S. I. et al. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 396, 887–897 (2010).
El-Sheikh, S. M. et al. Chitosan propolis nanocomposite alone or in combination with apramycin: An alternative therapy for multidrug-resistant Salmonella typhimurium in rabbits: In vitro and in vivo study. J. Med. Microbiol. 70, 001412 (2021).
Magro Filho, O. & de Carvalho, A. C. P. Application of propolis to dental sockets and skin wounds. J. Nihon Univ. Sch. Dent. 32, 4–13 (1990).
Zhang, Y. et al. Simultaneous determination of 20 phenolic compounds in propolis by HPLC-UV and HPLC-MS/MS. J. Food Compos. Anal. 115, 104877 (2023).
Pellati, F., Orlandini, G., Pinetti, D. & Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 55, 934–948 (2011).
El Adham, E. K., Hassan, A. I. & ADawoud, M. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 12, 1–22 (2022).
Chang, R., Piló-Veloso, D., Morais, S. A. & Nascimento, E. A. Analysis of a Brazilian green propolis from Baccharis dracunculifolia by HPLC-APCI-MS and GC-MS. Rev. Bras. Farmacogn. 18, 549–556 (2008).
Bozkuş, T. N., Değer, O. & Yaşar, A. Chemical characterization of water and ethanolic extracts of Turkish propolis by HPLC-DAD and GC-MS. J. Liq. Chromatogr. Relat. 44, 77–86 (2021).
Aldana-Mejia, J. A. et al. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 198, 114029 (2021).
Rebiai, A. et al. Determination of some phenolic acids in Algerian propolis. Ovidius Univ. Ann. Chem. 32, 120–124 (2021).
Salas, A. L. et al. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 23, 27–31 (2016).
Salama, A., Mohamed, F. & Hesemann, P. Preparation and dielectric relaxation of a novel ionocellulose derivative. Carbohydr. Polym. Technol. Appl. 2, 100087 (2021).
Segal, L., Creely, J. J., Martin, A. Jr. & Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 29, 786–794 (1959).
De Figueiredo, A. C. et al. Nanostructured electrospun polycaprolactone—propolis mats composed of different morphologies for potential use in wound healing. Molecules 27, 5351 (2022).
Ibrahim, N., Zakaria, A. J., Ismail, Z., Ahmad, Y. & Mohd, K. Application of GCMS and FTIR fingerprinting in discriminating two species of Malaysian stingless bees propolis. Int. J. Eng. Technol. 7, 106–112 (2018).
Svečnjak, L., Marijanović, Z., Okińczyc, P., Marek Kuś, P. & Jerković, I. Mediterranean propolis from the Adriatic Sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants 9, 337 (2020).
Oliveira, R. N. et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 21, 767–779 (2016).
Jayaramudu, T. et al. Electroactive hydrogels made with polyvinyl alcohol/cellulose nanocrystals. Materials 11, 1615 (2018).
Sforcin, J. M. Biological properties and therapeutic applications of propolis. Phytother. Res. 30, 894–905 (2016).
Fernández-Calderón, M. C. et al. Chemical profile and antibacterial activity of a novel Spanish propolis with new polyphenols also found in olive oil and high amounts of flavonoids. Molecules 25, 3318 (2020).
Atik, D. S. et al. Particle morphology and antimicrobial properties of electrosprayed propolis. Food Packag. Shelf Life 33, 100881 (2022).
Vică, M. L. et al. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 41, 101014 (2021).
Choi, S.-S., Lee, S.-H. & Lee, K.-A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 11, 1618 (2022).
Lin, Y.-H., Huang, H.-W. & Wang, C.-Y. Effects of high pressure-assisted extraction on yield, antioxidant, antimicrobial, and anti-diabetic properties of chlorogenic acid and caffeine extracted from green coffee beans. Food Bioprocess. Technol. 15, 1529–1538 (2022).
Kępa, M. et al. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Res. Int. 2018, e7413504 (2018).
Tafesh, A. et al. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid.-Based Complement. Altern. Med. 2011, e431021 (2011).
Sanhueza, L. et al. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 12, e0172273 (2017).
Ardjoum, N. et al. Development of antimicrobial films based on poly (lactic acid) incorporated with Thymus vulgaris essential oil and ethanolic extract of Mediterranean propolis. Int. J. Biol. Macromol. 185, 535–542 (2021).
Mohamadinooripoor, R. et al. Novel elastomeric fibrous composites of poly-ε-caprolactone/propolis and their evaluation for biomedical applications. J. Polym. Res. 29, 1–17 (2022).
Mocanu, A. et al. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 9, 1–10 (2019).
Al-saggaf, M. S. Formulation of insect chitosan stabilized silver nanoparticles with propolis extract as potent antimicrobial and wound healing composites. Int. J. Polym. Sci 2021, 1–9 (2021).
Pahlevanneshan, Z. et al. Polyurethane-nanolignin composite foam coated with propolis as a platform for wound dressing: Synthesis and characterization. Polymers 13, 3191 (2021).
Shehata, M. G., Ahmad, F. T., Badr, A. N., Masry, S. H. & El-Sohaimy, S. A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 65, 209–217 (2020).
Martins, M. L. et al. Cytotoxic and antibacterial effect of a red propolis mouthwash, with or without fluoride, on the growth of a cariogenic biofilm. Arch. Oral Biol. 107, 104512 (2019).
Ferreira, N. S. et al. Flavanone glycosides, triterpenes, volatile compounds and antimicrobial activity of Miconia minutiflora (Bonpl.) DC. (Melastomataceae). Molecules 27, 2005 (2022).
Stojković, D. et al. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 93, 3205–3208 (2013).
Shi, C. et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 197, 100–106 (2016).
Amato, A. et al. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 179, 273–281 (2018).
Saleemi, M. A., Alallam, B., Yong, Y. K. & Lim, V. Synthesis of zinc oxide nanoparticles with bioflavonoid rutin: Characterisation, antioxidant and antimicrobial activities and in vivo cytotoxic effects on Artemia nauplii. Antioxidants 11, 1853 (2022).
Vilela, C. et al. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 73, 120–128 (2017).
Khatkar, A., Nanda, A., Kumar, P. & Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 10, S3804–S3815 (2017).
Polo, L. et al. Antimicrobial activity of commercial calcium phosphate based materials functionalized with vanillin. Acta Biomater. 81, 293–303 (2018).
Pernin, A., Bosc, V., Maillard, M.-N. & Dubois-Brissonnet, F. Ferulic acid and eugenol have different abilities to maintain their inhibitory activity against Listeria monocytogenes in emulsified systems. Front. Microbiol. 10, 137 (2019).
Duda-Madej, A. et al. Antimicrobial O-alkyl derivatives of naringenin and their oximes against multidrug-resistant bacteria. Molecules 25, 3642 (2020).
Buchmann, D. et al. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol 132, 949–963 (2022).
Bustos, P. S. et al. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ. Toxicol. Pharmacol. 48, 253–264 (2016).
Beyki, M. et al. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 54, 310–319 (2014).
Thirukumaran, P., Manoharan, R. K., Parveen, A. S., Atchudan, R. & Kim, S.-C. Sustainability and antimicrobial assessments of apigenin based polybenzoxazine film. Polymer 172, 100–109 (2019).
del Valle, P. et al. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 61, 213–220 (2016).
Ciampi, F. et al. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Diary Sci. 103, 8938–8947 (2020).
Eddebbagh, M. et al. Correlation of the cytotoxic and antioxidant activities of Moroccan pomegranate (Punica granatum) with phenolic and flavonoid contents. J. Pharm. Pharmacol. 68, 511–519 (2016).
Anosike, C. A., Obidoa, O. & Ezeanyika, L. U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 20, 1–7 (2012).
Kong, Y. T. & Kutty, R. V. Physical characterization of propolis encapsulated vitamin E TPGS as nanomedicine. Mater. Today Proc. 48, 807–810 (2022).
Müller, W. E. et al. Fabrication of a new physiological macroporous hybrid biomaterial/bioscaffold material based on polyphosphate and collagen by freeze-extraction. J. Mater. Chem. B. 5, 3823–3835 (2017).
El-Gendi, H., Salama, A., El-Fakharany, E. M. & Saleh, A. K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 302, 120383 (2023).
Eskandarinia, A. et al. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int. J. Biol. Macromol. 149, 467–476 (2020).
Roy, S. & Rhim, J.-W. Starch/agar-based functional films integrated with enoki mushroom-mediated silver nanoparticles for active packaging applications. Food Biosci. 49, 101867 (2022).
Yahia, R. et al. Synthesis and characterization of thermoplastic starch/PVA/cardanol oil composites loaded with in-situ silver nanoparticles. J. Appl. Polym. Sci. 139, 51511 (2022).
Slater, T., Sawyer, B. & Sträuli, U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 77, 383–393 (1963).
Van de Loosdrecht, A., Beelen, R., Ossenkoppele, G., Broekhoven, M. & Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 174, 311–320 (1994).
Sharaf, S. M. et al. Deacetylated cellulose acetate nanofibrous dressing loaded with chitosan/propolis nanoparticles for the effective treatment of burn wounds. Int. J. Biol. Macromol. 193, 2029–2037 (2021).
Shinde, U. et al. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70, 251–257 (1999).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.S., A.M.A.: methodology, investigation, formal analysis, writing original draft. A.Salama, A.Saleh, E.T.: conceptualization, methodology, investigation, formal analysis, writing—review and editing. B.A.E.: investigation, formal analysis, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleh, S., Salama, A., Ali, A.M. et al. Egyptian propolis extract for functionalization of cellulose nanofiber/poly(vinyl alcohol) porous hydrogel along with characterization and biological applications. Sci Rep 13, 7739 (2023). https://doi.org/10.1038/s41598-023-34901-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-34901-6
This article is cited by
-
Bacterial cellulose is a smart scaffold for propolis-loaded polymeric nanoparticles with antimicrobial and antifungal properties
Cellulose (2025)
-
Ex situ functionalization of bacterial cellulose with ethanolic extraction of propolis for enhanced antimicrobial performance
Biomass Conversion and Biorefinery (2025)
-
Citrus aurantium Peel Extract Mediated Green Synthesis of Copper Oxide and Zinc Oxide Nanoparticles: Characterization and Antifungal Efficacy Against Post-Harvest Spoilage of Tomato Fruits by Locally Isolated Alternaria alternata
Journal of Cluster Science (2025)
-
A systematic review on cellulose based hydrogels for anti-microbial applications
Cellulose (2025)
-
Valorization of Sugarcane Bagasse into Cellulose Nanofiber Containing Phosphate Groups: A New Scaffold for in Vitro Calcium Phosphate Mineralization
Chemistry Africa (2025)