Abstract
As a enrichment plant, ramie can be used for the phytoremediation of cadmium (Cd)-contaminated soil. However, it is worth exploring the role of plant growth regulators and foliar fertilizers in the process of plant growth and development and Cd adsorption. By measuring the agronomic traits, Cd content of aboveground and underground ramie, calculating the Cd transfer coefficient (TF) and Cd bioconcentration factors (BCF), and the correlation between various indicators. This study examined the effects of plant growth regulators and foliar fertilizers on ramie’s capacity for Cd accumulation and transportation. Plant growth regulators and foliar fertilizers increased the Cd content of the aboveground ramie, reduced the Cd content of the underground ramie, and increased the TF. Among them, GA-1 increased the Cd content of the aboveground ramie to 3 times more than that of the control and reduced the Cd content of the underground ramie by 54.76%. Salicylic acid (SA) increased the Cd content of the aboveground ramie to three times more than that of the control. The combination of GA and foliar fertilizer reduced the Cd content of the aboveground and underground ramie and the TF and BCF of the underground ramie. After the hormones were sprayed, the TF of ramie had a significant positive correlation with the Cd content of the aboveground ramie; the BCF of the aboveground ramie had a significant positive correlation with the Cd content and TF of the aboveground ramie. The results indicate that Brassinolide (BR), gibberellin (GA), ethephon (ETH), polyamines (PAs), and salicylic acid (SA) have different effects on the enrichment and transport of Cd in ramie. This study provided an effective method to improve the capacity for ramie to adsorb heavy metals during cultivation.
Similar content being viewed by others
Introduction
With the advances in urbanization and industrialization, heavy metal pollution in China has become a serious problem. The pollution of water and soil poses a threat to the ecosystem, food security, agriculture, and sustainable development1,2,3. According to the Bulletin of the National Survey of Soil Pollution, 16.1% of the country’s soil contamination exceeded the legal limit, with heavy metal pollution contributing the most (82%)4. Cadmium (Cd) is a nonbiological essential heavy metal often combined with other heavy metals and one of the most toxic components of heavy metal pollution5. Cd-contaminated soil not only causes serious economic losses to agricultural production in China but also entails risks to human health. Therefore, research should be conducted on the prevention and control of Cd pollution in farmlands.
The phytoremediation of heavy metal-contaminated soils has received considerable attention6. The process involves using plants to remove pollutants from the environment7,8. Phytoremediation is a method of remediation that considers both ecological and economic effects and is a green technology developed for its strong potential to remove environmental pollution. Ramie (Boehmeria nivea [L.] Gaudich.) is the genus Urticaceae ramie's perennial bast fiber plant. It is commonly known as "China grass" and is widely cultivated for at least 5000 years in southern China9. Its fast growth, high fecundity, and high biological yield10 make up for the deficiencies of other hyperaccumulators, such as mustard plants11 and sunflowers12. Research has demonstrated that the bioavailability of Cd and lead in soil can be decreased through rhizosphere fixation and plant absorption and that soil can be stabilized by ramie and modifiers13. Although ramie is commonly used for its fiber, its products do not enter the food chain, and it is not associated with any health risk. Researchers have also modified varieties of ramie at the genetic level to improve its tolerance and ability to accumulate heavy metals8. Moreover, ramie is a permanent crop that provides ecological benefits to cultivation measures, and the cost of restoration can be recovered by ending continuous cropping. Therefore, ramie, the ideal phytoremediation material for Cd-contaminated soil, has great potential for use in the control of Cd pollution.
To obtain high removal efficiencies, lots of regulators including chelating agents and plant growth regulators have been used to improve the bioavailability of metals in soil and shoot biomass, respectively14,15. Plant growth regulators play a crucial role in the regulation of plant growth and development and in the response to external stresses16. The main Plant growth regulator are auxin, gibberellin (GA), cytokinin, abscisic acid, ethylene (ETH), and Brassinolide (BR) as well as some recently identified plant regulator, including polyamines (PAs) and salicylic acid (SA)17. GA has been proven to enhance the resistance of plants to heavy metal stress and to promote the accumulation of heavy metals18. Masood found that 10 mol L−1 GA can reverse the adverse effects of Cd on brassica. The 10−6 mol L−1 GA3 treatment increased Cd accumulation by 289% and the bioaccumulation coefficient by 128% in parthenium10. ETH is mainly used as a ripening agent in practical applications, but several studies have demonstrated that ETH plays a vital role in Cd stress. The tolerance of drupe to Cd can be increased by maintaining an appropriate level of ETH and a low ETH sensitivity through an antioxidant defense mechanism19. SA can reduce the accumulation of Cd in the aboveground part of rice20. SA can enable plants to resist abiotic stresses, such as ultraviolet radiation, low temperatures, heat shock, water deficit, salt injury, and heavy metals, and plays a role in the cross-protection response of plants to abiotic stresses21,22. SA can also increase mineral nutrition in plant organs and regulate the photosynthesis system, improving overall crop quality23,24. BR has been reported to promote plant growth, improve photosynthesis, and reduce heavy metal toxicity in plants25,26. PAs are compounds containing two or more amino groups. The raw materials used in its synthesis are ornithine and arginine. PAs play a crucial role in promoting the absorption of inorganic ions by roots, which improves resistance to stress and osmotic stress27. Binding PAs may play an essential role in resistance to Cu2+ stress28. Therefore, Plant growth regulators can improve the resistance of plants to heavy metals and promote the enrichment of heavy metals. Plant growth regulators can improve shoot biomass and enhance their accumulation capacity for heavy metals in aboveground plant parts. However, no effect of Plant growth regulators has been observed on the enrichment and transport of heavy metals in ramie.
Foliar fertilizer is a key source of nutrient elements for plant growth and development. Foliar fertilizer can also improve plants’ resistance to stress, promote plant growth and development, and increase yield. Potassium and phosphorous are the key elements for plant growth. Potassium activates many types of enzymes, which can enhance photosynthesis and the synthesis and metabolism of carbohydrates29. The use of potassium fertilizer during production can increase the yield and stress resistance of crops. Phosphorus is a key component of nucleic acid, nucleoproteins, phospholipids, and enzymes. The use of phosphorus fertilizer during production can enhance the crops’ resistance against drought and cold30. However, few studies have investigated the effects of fertilizers on the growth and ability of ramie to accumulate and transport Cd.
Plant growth regulators and foliar fertilizer can stimulate absorption of available nutrients that may be driven by the electrochemical gradient generated by the electrogenic H+-pump31. However, their activity depends on the concentration of their use, the environmental factors that affect their absorption, and the physiological state of the plant15. The effects of Plant growth regulators alone or in combination with Foliar fertilizer on the phytoextraction efficiency of ramie were unclear. In this study, In this study, two field experiment were conducted, one was using GA, ETH, SA, PAs, and BR foliar spray of ramie and another was using GA with KH2PO4 or KNO3 mixed foliar spray of ramie aimed to aimed to (1) investigate the treatment influence on Cd contents, translocation and accumulation in plant; and (2) estimate the treatment effects on the agronomic traits of ramie. These results will be helpful to compare the effects of different plant growth regulators and GA in combination with KH2PO4 or KNO3 as potential amendments for enhancing Cd phytoextraction by ramie.
Materials and methods
Plant materials and soil sample
Ramie is an asexual perennial plant propagated by using cuttings of lateral branches of approximately 15 cm in length. Ramie for Experiment A and Experiment B were arranged in two completely randomized plots with three replicates. Each plot contained six plants, planted in rows spaced 0.5 m apart, with a distance of 0.4 m between plants within rows. Experiment A: Ramie was planted in a Cd-polluted farmland in Hunan Agricultural University’s training base, Changsha City, Hunan Province, China. Experiment B: Ramie was planted in a Cd-polluted farmland in Liu yang City, Hunan Province, China. The lateral branches of ramie variety 171 were cut and propagated in April 2017. The plants were planted in the field in June 2017 and were mowed in December 2017. The ramie variety 171 was provided by the Ramie Research Institute of Hunan Agricultural University (Changsha, China).
Surface soil samples (0–20 cm) were taken from the test site, Then, the soil samples were air-dried and sieved through a 2-mm nylon screen to remove any debris before testing. Six air-dried soil samples were randomly taken to determine the physical–chemical properties (GB15618-2018). The soil type was red soil in both test sites, pH = 5.73 (Experiment A)and 5.78 (Experiment B), the average Cd content in soil was 3.27 mg kg−1 (Experiment A)and 3.43 mg kg−1(Experiment B), soil organic matter = 29.25 g kg−1 (Experiment A) and 21.54 g kg−1 (Experiment B), total nitrogen = 1.56 g kg−1 (Experiment A) and 1.13 g kg−1 (Experiment B), total phosphorus = 0.51 g kg−1 (Experiment A) and 0.43 g kg−1 (Experiment B), total potassium = 14.71 g kg−1 (Experiment A) and 11.56 g kg−1 (Experiment B).
Field experiment
Experiment A and Experiment B were conducted at vigorous growing period. In experiment A, five plant growth regulators in different concentrations were sprayed into the positive and negative sides of ramie leaves in the corresponding plots. (Table 1) In experiment B, GA, KNO3 and KH2PO4 are compounded in different concentrations was sprayed onto the positive and negative sides of ramie leaves in the corresponding plots (Table 2). This procedure was repeated five times, each spraying amount is about 100 ml per plant, once every 15 days, from April 19, 2018. To reduce the effect of direct sunlight and to prevent the agents from being washed away by the rain, the agents were administered on sunny mornings when the sun is not too strong.
The ramie grew in the soil for 125 days after transplantation and then harvested and divided into various parts for further processing. Before ramie was harvested, the agronomic traits of the ramie under each treatment were examined during the mature stage. Plant height was measured from the root neck to the upper most part of the stalk. The stem diameter (SD) and bark thickness (BT) were measured in the middle of stem using a Vernier caliper (ST22302, SG tools, Hangzhou, China). The area of the leaves was calculated by measuring their length and width with a straightedge. Plant biomass was measured by weighing both stems and leaves. Ramet number was measured through manual calculation. The tissues were carefully washed with tap water and double-distilled water to ensure no dust or other undesirable materials remained on the surface of the samples. The tissues were dried in an oven at 60 ± 5 °C for 4 days to ensure the constant weight. The weight of the samples was then measured, and the samples were crushed into powder for the Cd analysis.
Take the soil in the rhizosphere of ramie, and then the soil samples were air dried, crushed gently, and passed through a 2-mm sieve prior to the Cd analysis for calculation of index.
Determination of Cd concentration
The dried plant materials and soil samples were ground into powder and sieved, and then digested in mixed acid with a HNO3-HClO4 solution32 and HCl-HNO3-HF-HClO4 solution33, respectively. The Cd concentration in the digested solution were determined using a graphite furnace atomic absorption spectrometer after a digestion (PinAAcle 900T AAS, Perkin Elmer Instruments Co., Ltd, Waltham, MA, USA). The linear fitting of the results of the samples measurements was 0.998 and the fitting degree of the equation was tested by chi square. Certified soil (GBW07405) and rice (GBW10045) reference materials were used for quality control, and the recovery rate of Cd was 89–102%.
Statistical analysis
Comparison of Ramie field performance with different treatments
Field performance among different treatments were compared using ANOVA (analysis of variance) in SAS 9.4 software (SAS Institute, Cary, NC, United States). Plant data with different treatments were considered independent variables. The mean of each trait was tested at the p < 0.05 level and p < 0.01 level using Duncan’s multiple range test. (The following is the same) Evaluation of the overall field performance is a multi-criteria decision-making process that involves many factors.
In this study, a Membership function (MF) value and synthetic membership function (SMF) value were used to comprehensively express overall field performance34. The MF value of each field performance trait was calculated based on the following formula:
where yi(k) represents the MF value of the k th field performance trait, xi(k) denotes the field-recorded value of the kth field performance, and max x(k) and min x(k) represent the largest and smallest value of xi(k), respectively.
The SMF value of each treatment was calculated based on the following formula:
Comparison of Cd related indexes of Ramie with different treatments
The accumulation and absorption of Cd in Ramie with different treatments can be shown by many indexes35. The (BCF) value of each treatment was calculated based on the following formula:
where yBCF (k) the Cd bioconcentration factor value (BCF) of the k th treatments, x part of plant (k) denotes the Cd concentration value of the k th, and x soil (k) represent the Cd concentration value of the k th soil.
The Cd transfer coefficient (TF) value of each treatment was calculated based on the following formula:
where yTF(k) represents the Cd transfer coefficient value (TF) of the k th treatments, x aboveground denotes the Cd concentration value of the k th aboveground, and x underground (k) represent the Cd concentration value of the k th soil.
The Enrichment quantity value of each treatment was calculated based on the following formula:
where yEQ (k) represents the enrichment quantity value of the k th treatments, x biomass(k) denotes the biomass of the k th treatments, and x Cd content (aboveground + underground) (k) represent the k th Cd content of the sum of aboveground and underground.
Correlation between Cd related indexes and agronomic traits of ramie in different treatments
Correlation analysis (CA analysis) was used to evaluate the relationship between the growth and development of ramie and the accumulation and absorption of Cd. Correlations between the ramie’s overall agronomic traits and the Cd related indexes were performed using the CORR procedure in SAS 9.4 software. Pearson’s correlation coefficients and their significance were used to assess the strength of the correlations.
All assays were made in triplicate. Graphs were drawn using GraphPad Prism 7.0(GraphPad Software, San Diego, CA, USA).
Legal statement
Experimental and field studies of plants (whether cultivated or wild) used in this study, including the collection of plant material, comply with relevant institutional, national and international guidelines and legislation.
Results
Analysis of agronomic traits and enrichment quantity after plant growth regulator treatment and mixture of GA and foliar fertilizers
Effects of plant growth regulators on agronomic traits and Cd enrichment
The effects of plant growth regulator on the agronomic traits were evident. Plant growth varied in accordance with variety and concentration of plant growth regulators. Plant height, stem diameter, skin thickness, and leaf area were the main parameters influencing biomass. As shown in Table 3, GA-3 and PAs-3 treatments significantly increased plant height. For all treatments, plant height significantly decreased after plants were sprayed with ETH. All hormone treatments except the ETH treatments caused significant increases in biomass accumulation compared with the control 1(CK-1) treatment. The effect of plant growth regulators treatments on the leaf area, stem diameter, and skin thickness of ramie was negligible; the ETH-3 treatment was the only treatment to significantly reduce these measures in comparison with control.
Cd enrichment is the overall capacity of ramie to adsorb Cd. Treatment with PAs achieved the most noticeable effect on Cd enrichment; Cd enrichment after treatment with all three concentrations of PAs was significantly higher than after CK-1 treatment. Cd enrichment after treatment with SA-1 and SA-3 was significantly higher than that after CK-1 treatment. The BR-1 and GA-2 treatments also increased Cd enrichment. However, Cd enrichment after the ETH-1, ETH-2, ETH-3 treatments was lower than that after CK-1 treatment.
Effects of GA and foliar fertilizer mixture on agronomic traits and Cd enrichment
The GP and GN treatments positively affected the agronomic traits and Cd enrichment of ramie (Table 4). Plant height and biomass of ramie under GP-2, GP-3 and GN-2, GN-3 treatments were generally significantly higher than those under CK-2, but GP-1 and GN-1 treatments were significantly lower. Among the GW treatments, Cd enrichment after the GP-3 and GN-3 treatments was significantly higher than that after CK-2 treatment. The GP-1, GP-2, GP-3 and GN-1, GN-2, GN-3 reduced the biomass and Cd enrichment of ramie more than GW-1, GW-2, GW-3.
Effects of plant growth regulators on Cd content, Cd TF, and Cd BCF of ramie
Effects of plant growth regulators on Cd content of ramie
Compared with the control, different plant growth regulators had different effects on Cd content in the aboveground parts of ramie. The Cd content of ramie aboveground varied depending on the type and concentration of plant growth regulators (Fig. 1a). The BR, GA, SA, ETH, and PAs plant growth regulator treatments increased the Cd content of the aboveground ramie. According to the results, the Cd content after treatments with various concentrations of plant growth regulators, except the BR-2,BR-3,GA-3, SA-2, and SA-3 treatments, was considerably higher than that of the control group. The GA-1 and SA-1 treatments exerted the strongest effect; Cd content after these treatments was 3 times higher than that after CK-1 treatment. The Cd content of the aboveground ramie in the GA-1, GA-2, GA-3 group and PAs-1, PAs-2, PAs-3 group decreased as the concentration of GA and PAs increased. The Cd content of the aboveground ramie in the ETH-1, ETH-2, ETH-3 group exhibited the opposite effect. The Cd content of the aboveground ramie in the SA-1, SA-2, SA-3 group was similar to that of the BR-1, BR-2, BR-3 group; as the concentration of SA and BR increased, the Cd content of the aboveground ramie decreased at first and then increased.
The plant growth regulators affected the Cd content of both the aboveground and the underground parts of ramie. The Cd content of the underground ramie after all treatments was generally lower than that of the control group (Fig. 1b), especially the Cd content of the groups treated with BR-2 and GA-1, which was 59.45% and 54.76% lower than that of the control, respectively. Similarly, the Cd content of the groups treated with GA-3, PAs-1, and PAs-2 was significantly lower than that of the control group. The Cd content of the underground ramie treated with BR decreased when the concentration of BR increased, which was contrary to the trend for the GA and SA treatments. The Cd content of the underground ramie after the ETH and PAs treatments did not change significantly.
Effects of plant growth regulators on Cd TF of ramie
Cd TF refers to the ratio of Cd content of the aboveground part of ramie to that of the underground part. TF is an index used to evaluate the transportation of Cd from underground to aboveground parts of plants. Figure 2 shows that the Cd TF of ramie treated with lant growth regulators significantly increased. The TFs after the ETH-1, ETH-2, ETH-3 group treatment increased with an increasing concentration of ETH. By contrast, the TFs after the GA-1, GA-2, GA-3 group and PA-1, PA-2, PA-3 group treatment decreased as the concentration of the plant growth regulators increased. The TFs after the SA-1, SA-2, SA-3 group treatment decreased at first and then increased with the concentration of SA. The GA-1 treatment was the most effective and significantly stronger than CK-1, which yielded a TF greater than 2.
Effects of plant growth regulators on Cd BCF of ramie
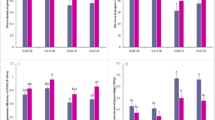
A mount of heavy metals a plant absorbs and enriches from soil can be used as an indicator of the plant’s enrichment ability. The BCF of Cd is the ratio of the element content in a certain part of the plant to the corresponding element content in the soil. To a certain extent, the BCF of Cd reflects the degree of difficulty for an element to migrate through the soil–plant system and indicates Cd enrichment in plants. The inter-root soil Cd content and above-ground part Cd content were detected, and the BFC was calculated (Table S1). As shown in Fig. 3a, following GA-1, GA-2, GA-3, PA-1, PA-2, PA-3, and SA-1, SA-2, SA-3 treatments, Cd BCF decreased as GA, PAs, and SA concentrations rose. The Cd BCF treatment with GA-1, GA-2, PAs-1, and SA-1 were noticeably greater than the control group. Following SA-3 treatment, the aboveground ramie group's Cd BCF was significantly lower than that of the control group. The Cd BCF of aboveground ramie in the ETH-1, ETH-2, and ETH-3 groups grew initially before declining; the Cd BCF of aboveground ramie in the ETH-2 group was substantially greater than that in the CK-1 group.
(a) Cd bioconcentration factors in aboveground ramie sprayed by plant growth regulators; (b) Cd bioconcentration factors in underground ramie sprayed by plant growth regulators; Bars marked with different letters are significantly different among treatments (P < 0.01). Values are means ± SD (n = 3). Duncan’s multiple range test.
The Cd BCF of the underground ramie after all plant growth regulator treatments was generally significantly lower than that after CK-1 treatment. The Cd BCF of the underground ramie after the GA-1 and SA-1, GA-3 and SA-3, PAs-2, and PAs-3 treatments were significantly lower than that after CK-1 treatment and did not markedly change after the GA-2, SA-3 and PAs-1 treatments. The changes in the Cd BCF of the underground ramie upon increasing concentrations of BR and ETH were similar to those in the aboveground Cd BCF (Fig. 3b).
Effects of GA and foliar fertilizer mixture on Cd content, Cd TF, and Cd BCF of ramie
Effects of GA and foliar fertilizer mixture on Cd content of ramie
GA significantly increased the Cd content, TF, and BCF of the aboveground ramie (Figs. 1, 2, 3). Because fertilizers composed of nitrogen and potassium are known to promote the growth and development of ramie, this study examined whether a mixture of nitrogen foliar fertilizer, potassium fertilizer, and GA could enhance the ability of ramie to absorb and enrich Cd. Figure 4a shows that treatment with a mixture of GA mixed and foliar fertilizers did not significantly affect the Cd content of the aboveground ramie compared with CK-2 treatment, except the GN-1 treatment, which reduced the Cd content by 59.88%. The combination of GA and foliar fertilizer significantly reduced the Cd content of the aboveground ramie in comparison with GA alone.
(a) Cd content in aboveground ramie by spraying mixed plant growth regulator and fertilizers; (b) Cd content in underground ramie by spraying mixed plant growth regulators and fertilizers; Bars marked with different letters are significantly different among treatments (P < 0.01). Values are means ± SD (n = 3). Duncan’s multiple range test.
The combination of GA and foliar fertilizer did not significantly affect the Cd content of the underground ramie. The Cd content of the underground ramie after the GN-1, GP-3 and GN-3 treatments was slightly higher than that after CK-2 treatment (Fig. 4b).
Effects of GA and foliar fertilizer mixture on Cd TF of ramie
We discovered that unlike GA alone, the mixture of GA and fertilizers reduced the Cd TF of ramie. However, most compound treatments did not significantly affect the Cd TF in comparison with CK-2 treatment; only the GN-1 treatment significantly affected the Cd TF. The Cd TF after the GN-1 treatment was half that after CK-2 treatment (Fig. 5).
Effects of GA and foliar fertilizer mixture on Cd BCF of ramie
After treatment with Gw-1, Gw-2, and Gw-3, the enrichment coefficient of Cd in the aboveground part of ramie was much higher than that of the control group, as shown in Fig. 6a and Table S1. There were no statistically significant differences between the other therapies and CK. However, as shown in Fig. 6b, the Cd enrichment coefficient of ramie roots treated with Gw-1, Gw-2, and Gw-3 was much lower than that of the control group. Furthermore, the Cd enrichment coefficient of subterranean ramie in the GP-2 treatment group was marginally lower than in the control group. There was no statistically significant difference between the other therapies and CK.
(a) Cd bioconcentration factors in aboveground ramie by mixed plant growth regulator and fertilizers; (b) Cd bioconcentration factors in underground ramie by spraying mixed plant growth regulator and fertilizers; Bars marked with different letters are significantly different among treatments (P < 0.01). Values are means ± SD (n = 3). Duncan’s multiple range test.
The Cd BCF of the aboveground ramie after the GP-1, GP-2, GP-3 group treatment and GN-1, GN-2, GN-3 group treatment were generally lower than that after the CK-2 and GW-1, GW-2, GW-3 group treatment, and the interaction between GP-1, GP-2, GP-3 group treatment and GN-1, GN-2, GN-3 group treatment made the Cd BCF of the aboveground ramie significantly lower than did the GW-1, GW-2, GW-3 group treatment with the same concentration of GA (Fig. 6a).
The Cd BCF of the underground ramie after the GP-1, GP-2, GP-3 group treatment and GN-1, GN-2, GN-3 group treatment were generally higher than that after the GW-1, GW-2, GW-3 group treatment, but were not significantly different from that after CK-2 treatment, except for the GP-3 treatment (Fig. 6b). The Cd BCF of the underground ramie after the GP-2 treatment was not significantly different from that after the GW-2 treatment. Therefore, treatment with GA significantly reduces the Cd BCF of the underground part of ramie, but the mixture of GA of foliar fertilizer can negate this effect.
Correlation analysis of traits
Effects of plant growth regulators on correlation
Figure 7 presents a significant correlation among various indicators after plant growth regulators treatment. For example, plant height was significantly correlated with leaf area and biomass. Leaf area and biomass were positively correlated with plant height, with correlation coefficients of 0.74 and 0.85 respectively. A significant positive correlation was observed between leaf area and biomass, with a correlation coefficient of 0.77. Aboveground Cd content was significantly correlated with Cd TF and aboveground Cd BCF. Cd TF and aboveground Cd BCF were positively correlated, with correlation coefficients of 0.82 and 0.84. A significant negative correlation was observed between soil Cd content, aboveground Cd BCF, and underground Cd BCF, with correlation coefficients of − 0.74 and − 0.79, respectively. The correlation coefficient of the significant positive correlation between Cd TF and aboveground Cd BCF was 0.73. The correlation coefficient of the significant positive correlation between aboveground Cd BCF and underground Cd BCF was 0.63. Cd enrichment had a significant positive correlation with plant height (0.72), leaf area (0.68), and biomass (0.88).
Correlation index of relevant indicators by plant growth regulators treatments. The shades of the colors and the corresponding numbers represent correlations. The darker the blue, the greater the negative correlation, and the darker the gray, the greater the positive correlation. Pearson’s correlation coefficients were used.
Effects of GA and foliar fertilizers mixture on correlation
Biomass and Cd enrichments had a significantly positive correlation after treatment with GA and foliar fertilizer mixtures (Fig. 8). Aboveground Cd content was positively correlated with Cd TF (0.86) and aboveground Cd BCF (0.92) and negatively correlated with underground Cd BCF (− 0.85) and underground Cd content (− 0.71). A significant negative correlation was observed between soil Cd content and underground Cd content, with a correlation coefficient of − 0.68. Cd TF was significantly and positively correlated with aboveground Cd BCF and negatively correlated with underground Cd BCF, with correlation coefficients of 0.95 and − 0.86, respectively. Cd enrichment had a significant positive correlation with stem diameter (0.66), leaf area (0.71), and biomass (0.90).
Correlation index of relevant indicators by mixed plant growth regulator and fertilizers. The shades of the colors and the corresponding numbers represent correlations. The darker the blue, the greater the negative correlation, and the darker the gray, the greater the positive correlation. Pearson’s correlation coefficients were used.
Discussion
Treating Cd soil pollution is an urgent task, and the phytoremediation technology-based approach can achieve superior results both economically and ecologically36. Ramie is a strong candidate and can promote the green revolution. Planting ramie can not only promote the development of the textile industry but also prevent soil pollution from entering the food chain, which would positively affect human health37. Investigation of the physiological and molecular mechanisms of Cd in ramie is crucial to regulating the amount of Cd moving from soil to plants and repairing soil. For the short-distance transport of Cd through the roots, phytochelatin secretion and vacuolar partition via ion channels and transporters are the key elements in the absorption, transport, and accumulation of Cd; for long-distance transport, the loading and unloading of phloem is a crucial element in the transport and accumulation of Cd to ramie plants. The transport and accumulation of Cd plants also causes physiological responses to Cd stress in ramie plants, which can manifest as changes in plant growth regulators levels, photosynthesis, water absorption, and mineral element absorption38,39. This study examined the effects of several plant growth regulators and the combination of fertilizers and GA on ramie. The increases in the Cd TF of ramie after the hormone treatments may be explained by the following: plant growth regulators caused the increases in the number of physiological and biochemical molecules absorbing and carrying Cd and enhanced the Cd resistance of ramie, resulting in the Cd enrichment of the aboveground part of ramie (Table 3).
GA, ETH, SA, BR, and PAs play a crucial role in alleviating abiotic stress and regulating the growth and development of plants40,41. According to results of this study, SA, BR, and PA played an essential role in the transportation of Cd in both the aboveground and underground parts of ramie, and the effects of BR and PA were dependent on concentration and ramie part (Table 3 and Fig. 2). GA is a plant growth stimulation hormone that regulates several physiological and biochemical processes, promotes growth and development, affects morphogenesis, and plays an essential role in the response to both biotic and abiotic stresses in plants42,43. Studies have demonstrated that plant growth regulators such as SA, GA, and indole-3-acetic acid (IAA) can alleviate abiotic stresses44,45,46,47. For example, the use of GA on leaves can reduce the uptake of nickel by mung bean plants, increase biomass, and promote growth. The importance of GA under abiotic stress has been well documented. This study confirmed that GA can promote the growth of ramie and improve its ability to absorb and enrich Cd (Table 3). ETH can inhibit the growth of plants, and the results in "Analysis of agronomic traits and enrichment quantity after plant growth regulator treatment and mixture of GA and foliar fertilizers" section indicated that ETH caused a decrease in biomass and Cd enrichment (Table 3). Studies on other plants have demonstrated that ETH can promote leaf abscission and plant maturation and inhibit apical dominance but that spraying at certain stages may produce the opposite effects48. The height of ramie decreased after ETH was applied, but the tillering and leaf area of ramie did not change significantly. ETH reduces the main components of biomass, thereby leading to a decrease of biomass. The enrichment of Cd in the plants decreased in accordance with changes in biomass (Table 3). BR are able to coordinate phytomorphogenesis, germination of seeds, cell division and elongation, flowering, vascular differentiation, formation of stomata, male fertility, and plant senescence49. BR, can increase the antioxidant enzyme content of plants and promote the secretion of heavy metal binding proteins, such as glutathione reductase (GR) and glutathione sulfotransferase (GST). This means that spraying plants with BR can increase their absorption of heavy metal ions50. In this experiment, spraying ramie plants with BR significantly increased their above-ground biomass and Cd enrichment (Table 3). Interestingly, the effect of low concentrations of BR was better than that of high concentrations. The effect of BR on Cd TF and BCF was minimal (Figs. 2, 3), suggesting that the Cd enrichment was mainly due to the increased plant biomass. SA participates in the coordination of plant growth and development, ripening, and responses to abiotic stresses51. Cd stress was found to increase the levels of free SA in the roots. This suggests that SA can reduce Cd toxicity by affecting Cd detoxification mechanisms, rather than activating antioxidant defense systems52. Additionally, SA can bind to JA and ethylene to increase plant transport. In this experiment, the low concentration of SA increased the biomass of ramie and significantly increased its Cd enrichment, aboveground Cd content, TF, and aboveground BCF (Table 3, Figs. 2, 3). It was hypothesized that SA increased the translocation capacity of ramie. Therefore, spraying ramie with SA may increase the above-ground cadmium accumulation in ramie. Polyamines (Pas) are nitrogen compounds present in all living cells. It participate in different cellular processes ranging from growth promotion and cell division to inhibition of ethylene production and senescence53. Many papers report changes in Pas levels in relation to heavy metal stress54. This experiment showed that polyamines significantly increased the height of ramie plants, the biomass, aboveground Cd content, and Cd enrichment (Table 3, Figs. 2, 3). It was hypothesized that polyamines could enhance the above-ground cadmium enrichment of ramie by increasing the plant height and biomass.
N, P, and K are vital nutrients for plant growth. The addition of fertilizer to the cultivation process benefits the growth and development of crops55. The results of this study demonstrated that GA alone or in combination with KNO3 or KH2PO4 positively influenced the agronomic traits of ramie (Table 4), indicating that the mixture of GA and fertilizer with N or P exhibited the same effects on the growth and development of ramie as in previous studies56. In addition, treatment with GP (including GP-1, GP-2, GP-3) and GN (including GN-1, GN-2, GN-3) promoted the enrichment of Cd in ramie (Fig. 6). This may be related to the physiological process ramie undergoes during heavy metal stress. A key regulator of plant growth and development, the functional site of GA at the cellular, tissue, and organ level of ramie is unknown. The site of application for GA on ramie can be a subject for future research. P+, K+, and Cd+ share the same transport pathway in plants. Although treatment with fertilizer promoted the growth and development of the plants, their Cd+ absorption and transport capacity decreased. Phosphates can increase the ionic strength of Cd adsorption57. Therefore, the decrease caused by GN (including GN-1, GN-2, GN-3) was more apparent than that caused by GP, especially in aboveground Cd content and aboveground BCF (Fig. 6). The BCF after the GP (including GP-1, GP-2, GP-3) and GN (including GN-1, GN-2, GN-3) treatments was lower than that after the GW-1, GW-2, GW-3 group treatment, which may be related to the chelation of root exudates (Fig. 6).
Plants’ response to Cd stress is a complex physiological process involving ion transporters58. Studies on yeast59, Arabidopsis60, and rice61 have demonstrated that adenosine triphosphate–binding cassette transporters, heavy metal–associated transporters, and natural resistance–associated macrophage protein transporters are involved in the response to Cd stress. The results of the study revealed common phenomena among the hormone treatments: the aboveground Cd content (Figs. 1 and 4) and Cd BCF increased (Figs. 3, 6); the underground Cd content and Cd BCF decreased (Figs. 1, 3, 4, 6); and the TF increased significantly after all hormone treatments (Fig. 2). Traditionally a textile crop, ramie has high cellulose, hemicellulose, and lignin content in the phloem, which establishes the conditions for Cd accumulation. The pulp inside ramie contains a large number of vessels that can transport Cd+. Hormones promote the growth of the aboveground part of ramie, the transport of Cd, and the accumulation of Cd in the underground part of the plant. Liu62 discovered that high concentrations of endogenous ETH delayed the formation of an ectoblast barrier and promoted the accumulation of Cd in the root ectoblasts. Studies by Neumann63 have demonstrated that ETH-mediated responses usually have high genotypic variability and may partially share common pathways under certain nutritional constraints. Although the biomass and Cd enrichment of ramie decreased after the ETH treatment, the Cd content and BCF of the aboveground ramie increased under high TFs (Table3, Fig. 2).
Phytoremediation of heavy metal-contaminated soil is a long-term process. Scientists face the challenge of how to overcome the problem of low biomass in cadmium-enriched plants, while high biomass plants are not tolerant to cadmium. Ramie, a crop with high cadmium tolerance, poses the question of how to improve its aboveground cadmium enrichment ability while maintaining its economic yield. Here, we propose using hormone treatment or hormone mixed with other leaf fertilizers to increase the cadmium concentration in the aboveground part of ramie, thus speeding up the process of phytoremediation. Our experiment showed that GA3 has a good effect. However, the use of GA3 will also reduce the fiber finesse of ramie64, which we want to avoid. In order to achieve sustainable development, ramie restoration of polluted land must make every effort to increase the accumulation of cadmium in the aboveground while ensuring economic benefits. The next step in the study of ramie remediation of heavy metal-contaminated soil can be explored through the mixed use of different hormones, foliar fertilizers, and soil fertilizers.
Conclusions
This study concludes that spraying GA3, BR, SA, and PA on ramie leaves can increase the biomass and cadmium enrichment. This is mainly due to an increase in the cadmium TF and aboveground BCF. The use of ETH can also improve the TF and aboveground BCF, but it significantly reduces the biomass of ramie, leading to a decrease in cadmium enrichment. GA3 alone is more effective than the combination of GA3 and foliar fertilizer. However, the interaction between GA3 + KNO3 and GA3 + KH2PO4 can inhibit the absorption, transportation, and accumulation of cadmium. Therefore, planting ramie on polluted land and spraying plant hormones such as GA3, BR, SA, and PA can improve its ability to absorb and accumulate cadmium. Considering the price of drugs, it is recommended to use GA3 as the preferred hormone for improving cadmium remediation efficiency in crop ramie.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Cai, L. M., Wang, Q. S., Wen, H. H., Luo, J. & Wang, S. Heavy metals in agricultural soils from a typical township in Guangdong Province, China: Occurrences and spatial distribution. Ecotoxicol. Environ. Saf. 168, 184–191. https://doi.org/10.1016/j.ecoenv.2018.10.092 (2019).
Huang, D. L. et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta 245, 863–873. https://doi.org/10.1007/s00425-017-2664-1 (2017).
Zeng, G. M. et al. Precipitation, adsorption and rhizosphere effect: The mechanisms for phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 339, 354–367. https://doi.org/10.1016/j.jhazmat.2017.05.038 (2017).
Wu, Y. et al. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 181, 106261. https://doi.org/10.1016/j.resconrec.2022.106261 (2022).
Pande, A. et al. Heavy metal toxicity in plants and the potential NO-releasing novel techniques as the impending mitigation alternatives. Front. Plant Sci. https://doi.org/10.3389/fpls.2022.1019647 (2022).
Huang, C. et al. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 243, 294–303. https://doi.org/10.1016/j.biortech.2017.06.124 (2017).
Yang, Y. & Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. Int. 27, 4905–4916. https://doi.org/10.1007/s11356-019-07256-7 (2020).
Zhu, H. H. et al. Phytohormones-induced senescence efficiently promotes the transport of cadmium from roots into shoots of plants: A novel strategy for strengthening of phytoremediation. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2020.122080 (2020).
Tang, H. et al. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud) under cadmium stress. Environ. Sci. Pollut. R 22, 9999–10008. https://doi.org/10.1007/s11356-015-4187-2 (2015).
Ali, N. & Hadi, F. Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ. Sci. Pollut. Res. Int. 22, 13305–13318. https://doi.org/10.1007/s11356-015-4595-3 (2015).
Du, J. et al. Screening of Chinese mustard (Brassica juncea L.) cultivars for the phytoremediation of Cd and Zn based on the plant physiological mechanisms. Environ. Pollut. https://doi.org/10.1016/J.Envpol.2020.114213 (2020).
Zamani, S., Naderi, M. R., Soleymani, A. & Nasiri, B. M. Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol. Environ. Saf. https://doi.org/10.1016/J.Ecoenv.2019.110017 (2020).
Lan, M. M., Liu, C., Liu, S. J., Qiu, R. L. & Tang, Y. T. Phytostabilization of Cd and Pb in highly polluted farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph17051661 (2020).
Hasan, M. M. et al. Assisting phytoremediation of heavy metals using chemical amendments. Plants https://doi.org/10.3390/plants8090295 (2019).
Rostami, S. & Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 220, 818–827. https://doi.org/10.1016/j.chemosphere.2018.12.203 (2019).
Bunsick, M., McCullough, R., McCourt, P. & Lumba, S. Plant hormone signaling: Is upside down right side up?. Curr. Opin. Plant Biol. https://doi.org/10.1016/j.pbi.2021.102070 (2021).
Masood, A. et al. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. PPB/Societe francaise de physiologie vegetale 104, 1–10. https://doi.org/10.1016/j.plaphy.2016.03.017 (2016).
Wang, B., Wei, H., Xue, Z. & Zhang, W. H. Gibberellins regulate iron deficiency-response by influencing iron transport and translocation in rice seedlings (Oryza sativa). Ann. Bot. 119, 945–956. https://doi.org/10.1093/aob/mcw250 (2017).
Wang, Y. et al. Effects of ethylene biosynthesis and signaling on oxidative stress and antioxidant defense system in Nelumbo nucifera G. under cadmium exposure. Environ. Sci. Pollut. Res. Int. 27, 40156–40170. https://doi.org/10.1007/s11356-020-09918-3 (2020).
Wang, F. J. et al. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol. Environ. Saf. https://doi.org/10.1016/J.Ecoenv.2020.111198 (2021).
Madany, M. M. Y. et al. Salicylic acid confers resistance against broomrape in tomato through modulation of C and N metabolism. Plant Physiol. Biochem. PPB/Societe francaise de physiologie vegetale 147, 322–335. https://doi.org/10.1016/j.plaphy.2019.12.028 (2020).
Yadav, T. et al. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet- wheat. Saudi J. Biol. Sci. 27, 2010–2017. https://doi.org/10.1016/j.sjbs.2020.06.030 (2020).
Kou, M. Z. et al. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an epichloe endophyte. J. Fungi https://doi.org/10.3390/Jof7080633 (2021).
Sharma, A. et al. The role of salicylic acid in plants exposed to heavy metals. Molecules https://doi.org/10.3390/molecules25030540 (2020).
Guo, J. K. et al. Effects of salicylic acid, Epi-brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxicol. Environ. Saf. 157, 491–496. https://doi.org/10.1016/j.ecoenv.2018.04.010 (2018).
Li, B. et al. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 43, 2469–2480. https://doi.org/10.1007/s00726-012-1327-6 (2012).
Pal, M. et al. Role of polyamines in plant growth regulation of Rht wheat mutants. Plant Physiol. Biochem. PPB/Societe francaise de physiologie vegetale 137, 189–202. https://doi.org/10.1016/j.plaphy.2019.02.013 (2019).
Chen, L. H., Wang, L., Chen, F. G., Korpelainen, H. & Li, C. Y. The effects of exogenous putrescine on sex-specific responses of Populus cathayana to copper stress. Ecotoxicol. Environ. Saf. 97, 94–102. https://doi.org/10.1016/j.ecoenv.2013.07.009 (2013).
Zhao, W. Q., Dong, H. R., Zhou, Z. G., Wang, Y. H. & Hu, W. Potassium (K) application alleviates the negative effect of drought on cotton fiber strength by sustaining higher sucrose content and carbohydrates conversion rate. Plant Physiol. Biochem. 157, 105–113. https://doi.org/10.1016/j.plaphy.2020.10.014 (2020).
Atafar, Z. et al. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 160, 83–89. https://doi.org/10.1007/s10661-008-0659-x (2010).
Claussen, D. W. HELIDAC therapy (bismuth subsalicylate/metronidazole/tetracycline hydrochloride). Gastroenterol. Nurs. 20, 188–189. https://doi.org/10.1097/00001610-199709000-00009 (1997).
Tang, H. et al. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. Int. 22, 9999–10008. https://doi.org/10.1007/s11356-015-4187-2 (2015).
Yin, Z. R. et al. Inter-annual reduction in rice Cd and its eco-environmental controls in 6-year biannual mineral amendment in subtropical double-rice cropping ecosystems. Environ. Pollut. https://doi.org/10.1016/j.envpol.2021.118566 (2022).
Jin, D. et al. Evaluation of cotton (Gossypium hirsutum L.) leaf abscission sensitivity triggered by thidiazuron through membership function value. Plants https://doi.org/10.3390/plants10010049 (2020).
Wei, J. L., Lai, H. Y. & Chen, Z. S. Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotoxicol. Environ. Saf. 84, 173–178. https://doi.org/10.1016/j.ecoenv.2012.07.004 (2012).
Wang, J. C. et al. Influence of Cd toxicity on subcellular distribution, chemical forms, and physiological responses of cell wall components towards short-term Cd stress in Solanum nigrum. Environ. Sci. Pollut. R 28, 13955–13969. https://doi.org/10.1007/s11356-020-11505-5 (2021).
Yaseen, M., Aziz, M. Z., Jafar, A. A., Naveed, M. & Saleem, M. Use of textile waste water along with liquid NPK fertilizer for production of wheat on saline sodic soils. Int. J. Phytorem. 18, 502–508. https://doi.org/10.1080/15226514.2015.1109596 (2016).
Wang, R. et al. Characteristics of cadmium enrichment and pollution evaluation of a soil-crop system in a typical karst area. Huan jing ke xue = Huanjing kexue/[bian ji, Zhongguo ke xue yuan huan jing ke xue wei yuan hui “Huan jing ke xue” bian ji wei yuan hui.] 42, 941–951. https://doi.org/10.13227/j.hjkx.202008085 (2021).
Yoneyama, T., Ishikawa, S. & Fujimaki, S. Route and regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: Metal transporters, metal speciation, grain cd reduction and Zn and Fe biofortification. Int. J. Mol. Sci. 16, 19111–19129. https://doi.org/10.3390/ijms160819111 (2015).
Alcazar, R., Bueno, M. & Tiburcio, A. F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells https://doi.org/10.3390/cells9112373 (2020).
Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 60, 406–416. https://doi.org/10.1007/s00244-010-9551-0 (2011).
Shu, K., Zhou, W., Chen, F., Luo, X. & Yang, W. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front. Plant Sci. 9, 416. https://doi.org/10.3389/fpls.2018.00416 (2018).
Spence, C. & Bais, H. Role of plant growth regulators as chemical signals in plant-microbe interactions: A double edged sword. Curr. Opin. Plant Biol. 27, 52–58. https://doi.org/10.1016/j.pbi.2015.05.028 (2015).
Hussain, I. et al. Foliar applied acetylsalicylic acid induced growth and key-biochemical changes in chickpea (Cicer arietinum L.) under drought stress. Dose-Response 18, 1559325820956801. https://doi.org/10.1177/1559325820956801 (2020).
Jia, H. L. et al. Exogenous salicylic acid regulates cell wall polysaccharides synthesis and pectin methylation to reduce Cd accumulation of tomato. Ecotoxicol. Environ. Saf. https://doi.org/10.1016/J.Ecoenv.2020.111550 (2021).
Saleem, M., Asghar, H. N., Khan, M. Y. & Zahir, Z. A. Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ. Sci. Pollut. Res. Int. 22, 10610–10617. https://doi.org/10.1007/s11356-015-4275-3 (2015).
Zhang, P. et al. Species-specific toxicity of ceria nanoparticles to Lactuca plants. Nanotoxicology 9, 1–8. https://doi.org/10.3109/17435390.2013.855829 (2015).
Schubert, R., Grunewald, S., von Sivers, L. & Hause, B. Effects of jasmonate on ethylene function during the development of tomato stamens. Plants https://doi.org/10.3390/plants8080277 (2019).
Kalbfuss, N. et al. A role for brassinosteroid signalling in decision-making processes in the Arabidopsis seedling. PLoS Genet. 18, e1010541. https://doi.org/10.1371/journal.pgen.1010541 (2022).
Cao, S., Xu, Q., Cao, Y., Qian, K. & Kuai, B. Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol. Plant. 123, 57–66 (2010).
Rivas-SanVicente, M. & Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 62, 3321–3338. https://doi.org/10.1093/jxb/err031 (2011).
Li, Q. et al. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 172, 317–325. https://doi.org/10.1016/j.ecoenv.2019.01.078 (2019).
Kebert, M. et al. Metal- and organ-specific response to heavy metal-induced stress mediated by antioxidant enzymes’ activities, polyamines, and plant hormones levels in Populus deltoides. Plants https://doi.org/10.3390/plants11233246 (2022).
Tajti, J., Janda, T., Majlath, I., Szalai, G. & Pal, M. Comparative study on the effects of putrescine and spermidine pre-treatment on cadmium stress in wheat. Ecotoxicol. Environ. Saf. 148, 546–554. https://doi.org/10.1016/j.ecoenv.2017.10.068 (2018).
Xia, N. et al. Effects of nitrogen addition on soil methane uptake in global forest biomes. Environ. Pollut. 264, 114751. https://doi.org/10.1016/j.envpol.2020.114751 (2020).
Liu, T. et al. Morphological and physiological changes of ramie (Boehmeria nivea L. Gaud) in response to drought stress and GA(3) treatment. Russ. J. Plant Physiol. 60, 749–755. https://doi.org/10.1134/S102144371306006x (2013).
Yan, Y., Zhou, Y. Q. & Liang, C. H. Evaluation of phosphate fertilizers for the immobilization of Cd in contaminated soils. PLoS ONE 10, e0124022. https://doi.org/10.1371/journal.pone.0124022 (2015).
Lu, Q. et al. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. Int. 27, 8719–8731. https://doi.org/10.1007/s11356-019-07512-w (2020).
Mesquita, V. A., Silva, C. F. & Soares, E. V. Toxicity induced by a metal mixture (Cd, Pb and Zn) in the yeast Pichia kudriavzevii: The role of oxidative stress. Curr. Microbiol. 72, 545–550. https://doi.org/10.1007/s00284-016-0987-y (2016).
Zheng, X., Chen, L. & Li, X. F. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Bot. Stud. https://doi.org/10.1186/s40529-018-0238-6 (2018).
Pan, B. G. et al. Regulating effects of silicon on Cd-accumulation and stress-resistant responding in rice seedling. Ying yong sheng tai xue bao = The journal of applied ecology/Zhongguo sheng tai xue xue hui, Zhongguo ke xue yuan Shenyang ying yong sheng tai yan jiu suo zhu ban 32, 1096–1104. https://doi.org/10.13287/j.1001-9332.202103.005 (2021).
Liu, Y. et al. Ethylene-mediated apoplastic barriers development involved in cadmium accumulation in root of hyperaccumulator Sedum alfredii. J. Hazard. Mater. 403, 123729. https://doi.org/10.1016/j.jhazmat.2020.123729 (2021).
Neumann, G. The role of ethylene in plant adaptations for phosphate acquisition in soils—A review. Front. Plant Sci. 6, 1224. https://doi.org/10.3389/fpls.2015.01224 (2015).
Ullah, S. et al. Interactive effect of gibberellic acid and NPK fertilizer combinations on ramie yield and bast fibre quality. Sci. Rep. 7, 10647. https://doi.org/10.1038/s41598-017-09584-5 (2017).
Funding
This research was funded by Key R & D Program of Hunan Province, grant number 2022NK2017.
Author information
Authors and Affiliations
Contributions
Conceptualization, W.P. and Y.H.; methodology, H.X; software, W.P. and Y.H.; validation, W.P.; investigation, X.Z.; resources, Y.J.; data curation, J.L. and Y.Z.; writing-original draft preparation, W.P.; writing—review and editing, W.P., S.H. and Y.H.; visualization, W.P.; supervision, H.X.; project administration, H.X.; funding acquisition, H.X. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, W., He, Y., He, S. et al. Exogenous plant growth regulator and foliar fertilizers for phytoextraction of cadmium with Boehmeria nivea [L.] Gaudich from contaminated field soil. Sci Rep 13, 11019 (2023). https://doi.org/10.1038/s41598-023-37971-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-37971-8