Abstract
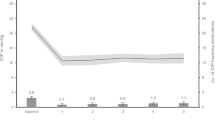
This study aimed to compare the IOP-lowering effectiveness and safety of standalone Preserflo MicroShunt and iStent 1st generation implantation combined with phacoemulsification in Caucasian patients with a 12-month follow-up period. This retrospective study analyzed the medical histories of patients undergoing antiglaucoma surgery at the Department of Ophthalmology, Medical University of Bialystok, between January 2019 and January 2022. The main outcome measures were success rates (complete: proportion of eyes with IOP < 18 mmHg (criterion A) and < 15 mmHg (criterion B) or 20% reduction in IOP without any glaucoma medication; qualified: proportion of eyes achieving IOPs < 18 mmHg and < 15 mmHg or 20% reduction in IOP from baseline with or without medications), mean reduction (%) in IOP, medication burden, number of complications and additional interventions. In both groups, a significant decrease in IOP and medication burden were observed at 6 and 12 months when compared with baseline. At 12 months, qualified surgical success in criterion A was recorded in 67.4% and 85.7% of patients in the Preserfo and iStent groups, respectively (p = 0.045). Complete surgical success in criterion B at 12 months accounted to 61.4% of patients from Prserflo group and 32.7% patients in iStent group (p = 0.04). Surgical failure at 12 months was documented in 30.2% and 6.3% of patients, respectively (p = 0.003). There was a significant difference between groups in %IOP reduction after 12 months. Greater reduction was observed in Preserflo group, MD = − 8.41 CI95 [− 15.88; − 0.95], p = 0.028, (− 33.49% ± 21.59 vs − 25.07% ± 14.15 in iStent group). Both procedures effectively reduced IOP and postoperative use of antiglaucoma medications in glaucoma patients.
Similar content being viewed by others
Introduction
In past decades, a major revolution has occurred in the surgical treatment of glaucoma. From conventional glaucoma filtration surgeries, namely trabeculectomy and glaucoma-draining devices, which are often associated with intra- and postoperative complications and lengthy recovery periods1,2,3,4, safer alternatives such as microinvasive glaucoma surgeries (MIGS) are now available to the patients. The term MIGS was first introduced in 2012 to describe a group of glaucoma surgeries that share five common features, including a high safety profile, minimal tissue trauma, rapid recovery, at least modest efficacy, and a microinvasive (ab-interno) approach5. The first implant that fulfilled these criteria was the first-generation iStent (Glaukos Corporation, Laguna Hills, CA, USA), registered for patients with primary open-angle glaucoma (POAG), pseudoexfoliation glaucoma (PXG), and pigmentary glaucoma (PG), authorized in 2012. The iStent is implanted into the trabecular meshwork, which improve the natural aquas outflow6. The first-generation iStent is an L-shaped device with a pipe-like opening placed in the anterior chamber via ab-interno approach. While the device is small (1 mm in length, 0.33 mm in width, 0.25 mm in length of the pipe), according to mathematical models, its lumen is large enough to reduce IOP effectively7. The iStent inject is different from the first-generation model8,9,10,11.
In 2014, the American Glaucoma Society and the US Food and Drug Administration (FDA) expanded MIGS definition to all procedures performed via ab interno or ab externo approaches that require minimal to no scleral dissection6. This change broaden the classification of MIGS, adding a new category: minimally invasive bleb surgery (MIBS)7,8,9. Preserflo™ MicroShunt (Santen, Osaka, Japan), authorized in Europe in 2019, bypasses the conventional outflow pathway and conduct aqueous humor from the anterior chamber to the subconjunctival and sub-Tenon spaces using a filtering bleb. Similar to the Xen Gel Stent, the construction of the Preserflo™ MicroShunt was derived from the Hagen-Poiseuille equation12. The device has 8.5 mm in length and 1.1 mm in width, with external and internal diameters of 0.70 mm and 0.35 mm, respectively. The small dimensions and permanent location within the scleral pocket enable the device to create a high-filtering bleb posterior to the scleral limbus, which is more comfortable for the patient and less prone to scarring than a bleb obtained after trabeculectomy13. The implant is made of a synthetic poly(styrene-block-isobutylene-block-styrene) (SIBS) polymer14,15, which easily adjusts to the curvature of the eye, reducing the risk of scleral erosion by the implant.
Studies that directly compare MIGS and MIBS are scarce. To the best of our knowledge, only one comparative study has been conducted on trabecular-MIGS (iStent) and filtering procedures (Preserflo Microshunt)16. One potential explanation for this is that MIGS and MIBS have different target populations. The MIGS are typically tailored for patients with mild-to-moderate glaucoma (usually under medical control), low risk of progression, and non-compliance; while MIBS are more suitable for moderate-to-severe glaucoma (usually medically uncontrolled) with a substantial risk of progression. However, there are some indications in which both surgeries can be considered, for instance medically uncontrolled patients with mild-to-moderate disease and medically controlled patients with advanced disease17.
The present study aimed to compare the hypotensive effectiveness and safety of Preserflo and iStent implantation combined with phacoemulsification in Caucasian patients during a 12-month follow-up period.
Methods
This study was based on a retrospective analysis of the medical histories of all consecutive patients who qualified for glaucoma surgeries, either iStent implantation combined with phacoemulsification or Preserflo MicroShunt implantation, at the Department of Ophthalmology, Medical University of Bialystok, and operated between January 2019 and January 2022.
The study was conducted in accordance with the principles of the Declaration of Helsinki. The implantation procedure was approved by the Bioethics Committee of the Medical University of Bialystok (APK.002.581.2021). Informed consent was waived by the Bioethics Committee of the Medical University of Bialystok due to the retrospective character of the study. All patient data were anonymized before statistical analysis of the results.
The qualification criterion for the procedure was the inability to achieve the target intraocular pressure (IOP)16 in patients with either mild or moderate POAG or PXG, despite the implementation of maximally tolerated pharmacotherapy (up to three antiglaucoma medications). In the case of iStent implantation, glaucoma patients who reached the target IOP also qualified as long as they required scheduled cataract surgery. The contraindications for surgical treatment included angle-closure glaucoma (ACG), inflammatory glaucoma, neovascular glaucoma, traumatic glaucoma, angle recession, filtration angle dysgenesis, and posterior adhesions. Additional exclusion criteria were a history of glaucoma surgery and a follow-up period of < 12 months. The implantation was not preceded by a washout period.
The analysis included patient demographics, such as sex and age, along with preoperative characteristics, such as glaucoma type, baseline IOP, best-corrected visual acuity in Snellen decimal (BCVA), number and type of antiglaucoma medications, mean deviation (MD), and pattern standard deviation (PSD) on the preoperative 30–2° Humphrey visual field (VF) test. Glaucoma severity was classified as mild (MD > − 6 dB), moderate (MD − 6 to − 12 dB), or severe (MD < − 12 dB).
Surgical technique
Implantation of the iStent was performed simultaneously with phacoemulsification, as described previously35, with the cataract procedure followed by implantation of the device to the trabecular meshwork within the inferonasal quadrant.
Implantation of the Preserflo MicroShunt was performed as a single procedure using the technique described in our previous study13.
All surgeries were performed under topical anesthesia with additional conjunctival administration of 2% Xylocaine in Preserflo MicroShunt group.
All patients received topical antibiotics postoperatively three times daily for seven days and topical steroids four times daily; the latter was gradually tapered over a 4–5-week period. Antiglaucoma medications were discontinued after the procedure, but when needed, they were prescribed again following EGS rules16.
The analysis included data from control visits before the procedure and at 6 and 12 months thereafter. The main outcome measures were: (1) success rates (complete: no need for repeated glaucoma surgery, and IOP ≤ 18 mmHg (criterion A), ≤ 15 mmHg (criterion B) or 20% reduction from baseline36, and discontinuing all antiglaucoma medications; qualified: no need for repeated glaucoma surgery, IOP ≤ 18 mmHg (criterion A), IOP ≤ 15 mmHg (criterion B) or 20% reduction from baseline, with discontinuation of all antiglaucoma medications or without; (2) mean reduction (%) in IOP, (3) medication burden, (4) number of complications and additional interventions. Surgical failure was defined as the need for another glaucoma surgery at any time after the primary procedure or an IOP > 18 mmHg during two consecutive control visits (at 6 and 12 months) despite administering antiglaucoma medications.
Statistical analysis
Sample size estimation: A minimum of 42 eyes were required to detect a 2.5 mmHg between-group difference in the mean decrease in IOP at twelve months after the procedure, with a significance level and 0.80 power of 0.05, assuming a standard deviation for the IOP decrease in both groups at 4.0 mmHg37.
Analysis was performed using the R package version 4.1.2. Categorical variables are summarized as numbers and percentages, and numerical variables as arithmetic means, standard deviations, medians, and interquartile ranges. The normal distribution of the study variables was verified using the Shapiro–Wilk test and confirmed based on skewness and kurtosis values. The homogeneity of variances was verified using Levene’s test. Between-group comparisons were based on Pearson’s chi-square test, Student’s t-test for independent groups, Welch’s t-test for independent groups, and the Mann–Whitney U-test. The significance of within-group changes over time was verified using t-tests for dependent groups and Wilcoxon’s test. Statistical significance for all analyses was set at α = 0.05.
Results
Baseline characteristics of the study groups
The analysis included two groups of patients: Preserflo group (N = 43) and iStent group (N = 78). Most patients in both groups were women (69.8% and 67.9%, respectively), with no statistically significant between-group differences in sex distribution (p = 0.999). The mean age of patients from the Preserflo and iStent groups was 68.98 ± 10.09 years and 72.48 ± 8.70 years, respectively, with those from the Preserflo group being significantly younger, by 3.5 years on average (MD = − 3.50, CI95 [− 7.00; 0.00], p = 0.049). In addition to age, the groups differed significantly in terms of the number of antiglaucoma medications and baseline IOP, which were higher in Preserflo group (MD = 0.00, CI95 [0.00; 1.00], p = 0.027 and MD = 2.00, CI95 [1.00; 4.00], p = 0.002, respectively, Table 1).
Comparison of parameters recorded at 6 and 12 months in the Preserflo group
The significance of changes over time in the analyzed parameters was analyzed separately within each study group. In the Preserflo group, the number of antiglaucoma medications decreased significantly between baseline and control visits at both 6 and 12 months (MD = − 1.94, CI95 [− 2.40; − 1.49], p < 0.001 and MD = − 1.61, CI95 [− 2.10; − 1.11], p < 0.001, respectively). No statistically significant difference in BCVA was observed when the values obtained at 6 and 12 months were compared with baseline measurements (p = 0.061). A significant decrease in baseline IOP was observed at 6 and 12 months (MD = − 9.54, CI95 [− 11.90; − 7.17], p < 0.001; and MD = − 9.09, CI95 [− 11.26; − 6.92], p < 0.001, respectively; Table 2).
Comparison of baseline parameters and parameters recorded at 6 and 12 months in the iStent group
In the iStent group, the number of antiglaucoma medications at both 6 and 12 months was significantly lower than at baseline (MD = − 1.42, CI95 [− 1.66; − 1.17], p < 0.001 and MD = − 1.29, CI95 [− 1.51; − 1.06], p < 0.001, respectively). The mean BCVA at six months was 0.39 higher than that at baseline (MD = 0.39, CI95 [0.33, 0.45], p < 0.001). The BCVA at 12 months was also significantly higher than that at baseline (MD = 0.40, CI95 [0.34, 0.48], p < 0.001), likely attributable to cataract extraction. Additionally, a significant decrease in IOP was observed at both 6 and 12 months when compared with the baseline value of this parameter (MD = − 6.50, CI95 [− 7.00; − 5.50], p < 0.001 and MD = − 5.50, CI95 [− 6.50; − 4.50], p < 0.001, respectively; Table 3).
Between-group comparison of study parameters
Complete surgical success (≤ 18 mmHg) at 6 months was documented in 63.4% of patients in the Preserflo group and 70.8% from the iStent group. Complete surgical success at 12 months was recorded in 60.5% and 66.7% of the patients in the Preserflo and iStent groups, respectively. No statistically significant differences in the complete surgical success rates were found between the study groups at 6 and 12 months (p = 0.564 and p = 0.655, respectively, Table 4).
Qualified surgical success (≤ 18 mmHg) at 6 months was documented in 73.2% of patients in the Preserflo group and 87.7% from the iStent group; the difference was not statistically significant (p = 0.101). At 12 months, qualified surgical success was recorded in 67.4% and 85.7% of patients in the Preserflo and iStent groups, respectively, with a statistically significant difference (p = 0.045).
Complete surgical success (≤ 15 mmHg) at 12 months accounted to 61.4% of patients from Prserflo group and 32.7% patients in iStent group (p = 0.04).
Qualified surgical success (≤ 15 mmHg) was achieved in 53.5% of patients in the Preserflo group and 35.9% in the iStent group at 12 months. However, this difference was not statistically significant (p = 0.110).
Surgical failure at 12 months was documented in 30.2% (n = 13) of patients in the Preserflo group and 6.3% (n = 4) of patients in the iStent group, with a statistically significant difference (p = 0.003), Table 4.
There was a significant difference between groups in %IOP reduction after 12 months. Greater reduction was observed in Preserflo group, MD = -8.41 CI95 [− 15.88; − 0.95], p = 0.028 (− 33.49% ± 21.59 vs − 25.07% ± 14.15). Statistical difference between groups was not confirmed for IOP reduction after 6 months, Table 5.
Other outcomes measure are shown in Table 6.
Safety
The intraoperative and early and late postoperative complication rates in both groups are presented in Table 7.
Subconjunctival 5-fluorouracil injections were given to 8 patients from the Preserflo group (18.6%). The average dose of 5-FU was 5.0 ± 1.5 mg. In 6 patients from the Preserflo group (14%) the needling was performed. One patient from the Preserflo group was stitched up with additional suture, due to wound leakage. Two patients from the Preserflo group underwent reoperation due to filtering bleb fibrosis. In both cases, a classical trabeculectomy was performed.
No significant intraoperative complications were noted in the iStent group. In the immediate postoperative period microhyphema was observed in 8 eyes, all cases spontaneously resolved within a week of surgery without sequelae. Complications unrelated to iStent implantation occurred in 5 eyes. Four eyes developed posterior capsular opacification and were treated with Nd: YAG capsulotomy. One eye required intravitreal administration of an antiVEGF injections due to onset of exudative form of age-related macular degeneration. Immediately after the surgery, corneal oedema related to an increase in IOP was observed in 3 eyes on postoperative day 1, which resolved within 1 week. Viral corneal inflammation occurred in 1 eye at 6 months postoperative and was treated topically, resolving within 1 month with no visual complications. Four eyes in iStent group required additional glaucoma surgery.
Discussion
The present study compared the efficacy and safety of MIGS and MIBS (implantations of the first-generation iStent combined with phacoemulsification vs Preserflo MicroShunt) in patients with POAG and PXG during a 12-month follow-up.
Both procedures effectively reduced IOP in POAG and PXG patients. iStent group achived better qualified success at 12 months for IOP criteria A < 18 mm Hg, wheather Preserflo group obtained better complete success at 12 months for IOP criteria B < 15 mm Hg. Surgical failure 12 months post op occured more fraquently in Preserflo group in comparison to iStent group (30% vs 6% respectively). There was a significant difference between groups in %IOP reduction after 12 months. Mean percentage reduction of IOP observed in Preserflo group was 33.5% vs 25.1% in iStent group, p = 0.028. The decrease in IOP was associated with a significant reduction in the postoperative use of antiglaucoma medications, down to 0.56 ± 1.08 and 0.46 ± 0.84 at 12 months in the Preserflo and iStent groups, respectively (p > 0.05).
While iStent implantation was not expected to produce the same IOP reduction as filtering surgery, our study revealed a comparable efficacy profile when considering main outcome measures. Additionally, more patients in the Preserflo group achieved an IOP of < 15 mmHg. This was expected because bleb-dependent procedures are known to produce lower IOPs. These findings are consistent with those found in literature, further demonstrating the capability of penetrating technique to achieve low target IOPs in a significant proportion of patients16,17. This is particularly important in patients with severe stages of disease.
Given the higher safety profile of iStent, coupled with a faster return to normal daily life, and comparable efficacy for moderate target IOPs (< 18 mm Hg), when combined with phacoemulsification, it presents a compelling case for its consideration. The number of clinically relevant complications was higher in the Preserflo group, with some adverse events requiring additional surgical intervention or conservative treatment. This positive benefit-to-risk assessment is particularly applicable for patients with mild-to-moderate glaucoma that does not yet warrant the risks of filtering surgery.
While the Preserflo MicroShunt and iStent were designed to normalize IOP through the drainage of aqueous humor from the anterior chamber, they differ in the implantation technique and mechanism of action. The iStent, implanted through the ab interno approach, facilitates the passage of aqueous humor through a natural outflow pathway. Considering the sources of increased resistance within the natural aqueous outflow pathway from the anterior chamber, one should consider not only the external wall of the Schlemm’s canal but also the openings of the collector channels that may play a significant role by changing the size of their entry depending on the IOP level. This hypothesis appears to be supported by the results of studies using more than one microbypass intraoperatively; in those studies, the decrease in IOP was not directly proportional to the number of stents inserted into the Schlemm’s canal. One possible solution could be to optimize the site of microbypass implantation, with the one with the largest number of collector channels being opened16,18,19,20,21,22. In the present study, the iStent was implanted into the nasal quadrant, which contained the highest average number of collector channels. The Preserflo MicroShunt uses the same aqueous outflow pathway as a trabeculectomy, using a filtering bleb to bypass the trabecular meshwork, considered the source of the highest resistance during aqueous outflow.12. The device is implanted using the ab externo approach, and the aqueous humor is drained through the new outflow tract to the suprascleral venous system or through the microcysts within the conjunctiva.
The intensive postsurgical care after Preserflo MicroShunt implantation deserves an attention. In a retrospective study conducted by Fea et al.23 analyzing the efficacy of MicroShunt in POAG and PXG patients, all complications were mild and resolved entirely after treatment. None of the complications posed a risk of vision loss. Postoperative subconjunctival injection of mitomycin C (MMC) or 5-fluorouracil (5-FU) without needling was required for 3 (2.9%) eyes. Needling was required in 19 (18.3%) eyes and revision surgery in 14 (13.5%) eyes. Four eyes (3.9%) required needling, followed by revision surgery. The mean time from surgery to first needling was approximately 30 days, whereas the mean time between surgery and surgical revision was 60 days. More promising results were obtained by Beckers et al.24, who observed transient early postoperative hypotony in 11.1% of cases POAG cases, but no persistent vision-threatening ocular adverse events. A few patients required needling (2.5%), subconjunctival injection of 5-FU (2.5%), or surgical revision (6.2%) to maintain the target IOP after Preserflo MicroShunt implantation Pillunat et al.25 compared Preserflo MicroShunt implantation with trabeculectomy, and found that the frequency of postsurgical interventions in the trabeculectomy group was significantly higher than in the Preserflo group, with a similar efficacy of both methods in reducing IOP. At six months post-procedure, mean IOP values in the Preserflo and trabeculectomy groups were 10.8 mmHg and 10.3 mmHg, respectively. The study groups did not differ significantly in terms of mean postoperative reduction in IOP, peak diurnal IOP, or median diurnal fluctuations.
Regarding the safety outcomes, iStent surgery is in favour in comparison to MicroShunt Preserflo. In a comparative study of iStent implantation combined with phacoemulsification versus phacoemulsification alone conducted by Kozera et al.18, no clinically relevant intraoperative complications were found in any patient subjected to the combined procedure. In the immediate postoperative period, the authors reported microhyphema in the anterior chamber in five eyes from the iStent group and subconjunctival hemorrhage in one eye; in all six cases, the complications resolved spontaneously within a week of the procedure without sequelae. Other studies on the patients with POAG and PXG subjected to combined surgery: phacoemulsification with first-generation iStent implantation18,19 revealed similar satisfactory outcomes. No intraoperative complications were observed. The most common early postoperative complications are microhyphema and Descemet’s membrane folds. An early postoperative increase in IOP occurred in seven (10%) patients; however, the IOP did not exceed 25 mmHg in any of the patients and dropped within a week post-procedure. Hypotony, endophthalmitis, and other serious complications were not observed. During the late postoperative period, two patients presented with secondary cataracts and were treated successfully with a YAG laser; three patients showed a decrease in BCVA due to previously diagnosed age-related macular degeneration (AMD), two experienced iStent rotation, and one required trabeculectomy due to a persistent postoperative increase in IOP up to 26 mmHg.
Another issue is the effect of phacoemulsification alone on the postoperative decrease in IOP, as all eyes in the iStent group in our study underwent a combined procedure. According to different studies, it is averaged at 1.4 mmHg, 1.55 mmHg, 1.88 mmHg, 2.9 mmHg, 3.1 mmHg, and 4.9–5.3 mmHg26,27,28,29,30. Depending on the type of glaucoma, the utmost reduction in IOP occurs in angle-closure glaucoma and PXG glaucoma. Several theories explaining this effect are taken into account. Firstly, after removal of the lens, the position of the ciliary body reccess changes, similar to the treatment with parasympathomimetics20,31,32. Other possible mechanism involves a decrease in aqueous humor production due to the contraction of the lens capsule after phacoemulsification28. In addition, improvement of aqueous humor outflow through the trabecular meshwork and Schlemm’s canal is also considerated31. This mechanism is especially considerated in the case of PXG, since the pseudoexfoliating material is washed out during the procedure from the anterior chamber, especially from the trabecular meshwork, which facilitates the outflow of aqueous humor through the Schlemm’s canal. This hypothesis may also explain the postoperative gradual increase in IOP in this type of glaucoma, which is proportional to the reproduction and accumulation of pseudoexfoliative deposits in the anterior segment of the eye. Another theory suggests an improvement of uveoscleral outflow33, caused by an increased release of prostaglandins during the procedure: PGE-1 increases the outflow of aqueous humor by the conventional route, while PGF-2 by the alternative route34. Analyzing the above-mentioned studies, it becomes evident that the reduction in IOP after surgery has a strong reverse correlation with the depth of the prior chamber before surgery, the width of the filtering angle, and the initial IOP level.
The present study was not free from potential limitations. This was a retrospective study with a relatively small sample size and a short follow-up period of 12 months. Moreover, the iStent and Preserflo groups differed in some preoperative characteristics, with patients in the former being older, presenting with lower baseline IOP, and receiving fewer antiglaucoma medications. Additionally Preserflo was standalone procedure whereas iStent was combined with phacoemulsification. Finally, the implantation procedure in either group was not preceded by a washout period. However, to our knowledge, this is the first study to compare these two devices, and there are no previous reports on the effectiveness of the Preserflo MicroShunt in Caucasian populations.
The landscape of glaucoma surgical armamentarium involves a spectrum of tools, each with unique attributes. However, tailoring the most suitable surgery for a specific patient is not without challenges. The growing popularity of MIGS and the abundance of available techniques warrant research on the subtle differences between various surgical methods. In this context, the article introduces some opinions into the discussion. In summary MIGS (iStent) and MIBS (Preserflo) effectively reduced IOP and the postoperative use of medications in glaucoma patients. However trabeculectomy remains the gold standard for glaucoma treatment, with the possibly to additional regulating IOP through early suture lysis, needling, or administration of antimetabolites. MIGS are less effective than trabeculectomy yet safer, and act synergistically with phacoemulsification, and do not affect the outcome of future conventional glaucoma surgeries. MIBS are equally effective as trabeculectomy or slightly inferior but produce a more predictable hypotensive effect with better morphology of the filtering bleb and less intensive postoperative care. However, the path toward implementing meticulous treatment reccomendations demands further prospective studies with longer follow-up periods. These studies will offer a valuable resource for future research endeavors, ultimately advancing our surgical management of glaucoma. Such studies could help optimize treatment approaches for patients with glaucoma of various severities.
Methods
This study was based on a retrospective analysis of the medical histories of all consecutive patients who qualified for glaucoma surgeries, either iStent implantation combined with phacoemulsification or Preserflo MicroShunt implantation, at the Department of Ophthalmology, Medical University of Bialystok, and operated between January 2019 and January 2022.
The study was conducted in accordance with the principles of the Declaration of Helsinki. The implantation procedure was approved by the Bioethics Committee of the Medical University of Bialystok (APK.002.581.2021). Informed consent was waived by the Bioethics Committee of the Medical University of Bialystok due to the retrospective character of the study. All patient data were anonymized before statistical analysis of the results.
The qualification criterion for the procedure was the inability to achieve the target intraocular pressure (IOP)16 in patients with either mild or moderate POAG or PXG, despite the implementation of maximally tolerated pharmacotherapy (up to three antiglaucoma medications). In the case of iStent implantation, glaucoma patients who reached the target IOP also qualified as long as they required scheduled cataract surgery. The contraindications for surgical treatment included angle-closure glaucoma (ACG), inflammatory glaucoma, neovascular glaucoma, traumatic glaucoma, angle recession, filtration angle dysgenesis, and posterior adhesions. Additional exclusion criteria were a history of glaucoma surgery and a follow-up period of < 12 months. The implantation was not preceded by a washout period.
The analysis included patient demographics, such as sex and age, along with preoperative characteristics, such as glaucoma type, baseline IOP, best-corrected visual acuity in Snellen decimal (BCVA), number and type of antiglaucoma medications, mean deviation (MD), and pattern standard deviation (PSD) on the preoperative 30–2° Humphrey visual field (VF) test. Glaucoma severity was classified as mild (MD > -6 dB), moderate (MD − 6 to − 12 dB), or severe (MD < − 12 dB).
Surgical technique
Implantation of the iStent was performed simultaneously with phacoemulsification, as described previously35, with the cataract procedure followed by implantation of the device to the trabecular meshwork within the inferonasal quadrant.
Implantation of the Preserflo MicroShunt was performed as a single procedure using the technique described in our previous study13.
All surgeries were performed under topical anesthesia with additional conjunctival administration of 2% Xylocaine in Preserflo MicroShunt group.
All patients received topical antibiotics postoperatively three times daily for seven days and topical steroids four times daily; the latter was gradually tapered over a 4–5-week period. Antiglaucoma medications were discontinued after the procedure, but when needed, they were prescribed again following EGS rules16.
The analysis included data from control visits before the procedure and at 6 and 12 months thereafter. The main outcome measures were: (1) success rates (defined as follows complete—no need for repeated glaucoma surgery, and IOP ≤ 18 mmHg (criterion A), ≤ 15 mmHg (criterion B) or 20% reduction from baseline36, and discontinuing all antiglaucoma medications; qualified—no need for repeated glaucoma surgery, IOP ≤ 18 mmHg (criterion A), IOP ≤ 15 mmHg (criterion B) or 20% reduction from baseline, with discontinuation of all antiglaucoma medications or without; (2) mean reduction (%) in IOP, (3) medication burden, (4) number of complications and additional interventions. Surgical failure was defined as the need for another glaucoma surgery at any time after the primary procedure or an IOP > 18 mmHg during two consecutive control visits (at 6 and 12 months) despite administering antiglaucoma medications.
Statistical analysis
Sample size estimation: A minimum of 42 eyes were required to detect a 2.5 mmHg between-group difference in the mean decrease in IOP at twelve months after the procedure, with a significance level and 0.80 power of 0.05, assuming a standard deviation for the IOP decrease in both groups at 4.0 mmHg37.
Analysis was performed using the R package version 4.1.2. Categorical variables are summarized as numbers and percentages, and numerical variables as arithmetic means, standard deviations, medians, and interquartile ranges. The normal distribution of the study variables was verified using the Shapiro–Wilk test and confirmed based on skewness and kurtosis values. The homogeneity of variances was verified using Levene’s test. Between-group comparisons were based on Pearson’s chi-square test, Student’s t-test for independent groups, Welch’s t-test for independent groups, and the Mann–Whitney U-test. The significance of within-group changes over time was verified using t-tests for dependent groups and Wilcoxon’s test. Statistical significance for all analyses was set at α = 0.05.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Investigators, A. G. I. S. The advanced glaucoma intervention study, 6: Effect of cataract on visual field and visual acuity. The AGIS investigators. Arch. Ophthalmol. 118, 1639–1652 (2000).
Gedde, S. J. et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology 125, 650–663 (2018).
Simsek, T. & Bilgeç, M. D. Ahmed glaucoma valve implantation versus suprachoroidal silicone tube implantation following the injection of bevacizumab into the anterior chamber in patients with neovascular glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 257, 799–804 (2019).
Musch, D. C., Gillespie, B. W., Lichter, P. R., Niziol, L. M. & Janz, N. K. Visual field progression in the collaborative initial glaucoma treatment study the impact of treatment and other baseline factors. Ophthalmology 116, 200–207 (2009).
Saheb, H. & Ahmed, I. I. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 23, 96–104 (2012).
Caprioli, J. et al. Special commentary: Supporting innovation for safe and effective minimally invasive glaucoma surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 122, 1795–1801 (2014).
Fellman, R. L. et al. American glaucoma society position paper: Microinvasive glaucoma surgery. Ophthalmol. Glaucoma 3, 1–6 (2020).
Jabłońska, J., Lewczuk, K., Konopińska, J., Mariak, Z. & Rękas, M. Microinvasive glaucoma surgery: A review and classification of implant-dependent procedures and techniques. Acta Ophthalmol. 100, e327–e338 (2022).
Gonnermann, J. et al. Contralateral eye comparison study in MICS & MIGS: Trabectome vs. iStent inject. Graefes Arch. Clin. Exp. Ophthalmol. 255, 359–365 (2017).
Lewczuk, K., Jabłońska, J., Konopińska, J., Mariak, Z. & Rękas, M. Schlemm’s canal: The outflow ‘vessel’. Acta Ophthalmol. 100, e881–e890 (2022).
Yuan, F. et al. Mathematical modeling of outflow facility increase with trabecular meshwork bypass and schlemm canal dilation. J. Glaucoma 25, 355–364 (2016).
Aghayeva, F. A., Chronopoulos, P., Schuster, A. K., Pfeiffer, N. & Hoffmann, E. M. Inter-eye relationship of intraocular pressure change after unilateral trabeculectomy, filtering canaloplasty, or PreserFlo™ microshunt implantation. Graefes Arch. Clin. Exp. Ophthalmol. 259(10), 3045–3053. https://doi.org/10.1007/s00417-021-05188-y (2021).
Arrieta, E. A. et al. Clinicopathologic correlations of poly-(styrene-b-isobutylene-b-styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalm. Surg. Lasers Imaging 42(4), 338–345 (2011).
Saeed, E., Gołaszewska, K., Dmuchowska, D. A., Zalewska, R. & Konopińska, J. The PreserFlo microshunt in the context of minimally invasive glaucoma surgery: A narrative review. Int. J. Environ. Res. Public Health 20(4), 2904 (2023).
Acosta, A. C. et al. A newly designed glaucoma drainage implant made of poly(styrene-b-isobutylene-b-styrene): Biocompatibility and function in normal rabbit eyes. Arch. Ophthalmol. 124(12), 1742–1749 (2006).
Qidwai, U., Jones, L. & Ratnarajan, G. A comparison of iStent combined with phacoemulsification and endocyclophotocoagulation (ICE2) with the PreserFlo MicroShunt and XEN-45 implants. Ther. Adv. Ophthalmol. 14, 25158414221125696 (2022).
Paletta Guedes, R. A., Gravina, D. M., Paletta Guedes, V. M. & Chaoubah, A. iStent inject (second-generation trabecular microbypass) versus nonpenetrating deep sclerectomy in association with phacoemulsification for the surgical treatment of open-angle glaucoma and cataracts: 1-year results. J. Glaucoma 29(10), 905–911. https://doi.org/10.1097/IJG.0000000000001576 (2020).
Mathew, D. J. et al. Adherence to world glaucoma association guidelines for surgical trials in the era of microinvasive glaucoma surgeries. Ophthalmol. Glaucoma 2(2), 78–85 (2019).
Kozera, M., Konopinska, J., Mariak, Z. & Rekas, M. Effectiveness of iStent trabecular micro-bypass system combined with phacoemulsification vs. phacoemulsification alone in patients with glaucoma and cataract depending on the initial intraocular pressure. Ophthalm. Res. 64, 327 (2020).
Konopinska, J., Kozera, M., Krasnicki, P., Mariak, Z. & Rekas, M. The effectiveness of first-generation iStent microbypass implantation depends on initial intraocular pressure: 24-month follow-up-prospective clinical trial. J. Ophthalmol. 2020, 8164703 (2020).
Spiegel, D. et al. Coexistent primary open-angle glaucoma and cataract: Interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur. J. Ophthalmol. 19(3), 393–399 (2009).
Belovay, G. W., Naqi, A., Chan, B. J., Rateb, M. & Ahmed, I. I. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J. Cataract Refract. Surg. 38(11), 1911–1917 (2012).
Fea, A. M. et al. Effectiveness of microshunt in patients with primary open-angle and pseudoexfoliative glaucoma: A retrospective european multicenter study. Ophthalmol. Glaucoma 5(2), 210–218. https://doi.org/10.1016/j.ogla.2021.08.005 (2022) (Epub 2021 Aug 31).
Beckers, H. J. M. et al. Safety and effectiveness of the PRESERFLO microshunt in primary open-angle glaucoma: Results from a 2-year multicenter study. Ophthalmol. Glaucoma 5(2), 195–209. https://doi.org/10.1016/j.ogla.2021.07.008 (2022).
Pillunat, K. R. et al. PRESERFLO™ MicroShunt versus trabeculectomy: First results on efficacy and safety. Acta Ophthalmol. 100(3), e779–e790. https://doi.org/10.1111/aos.14968 (2022) (Epub 2021 Jul 31).
Hayashi, K., Hayashi, H., Nakao, F. & Hayashi, F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology 107, 698–703 (2000).
Damji, K. F. et al. Intraocular pressure following phacoemulsification in patients with and without exfoliation syndrome: A 2 year prospective study. Br. J. Ophthalmol. 90, 1014–1018 (2006).
Suzuki, R., Tanaka, K., Sagara, T. & Fujiwara, N. Reduction of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation. Ophthalmologica 208, 254–258 (1994).
Suzuki, R., Kuroki, S. & Fujiwara, N. Ten-year follow-up of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation performed by the same surgeon. Ophthalmologica 211, 79–83 (1997).
Cekiç, O. & Batman, C. The relationship between capsulorhexis size and anterior chamber depth relation. Ophthalm. Surg. Lasers 30, 185–190 (1999).
Hayashi, K., Hayashi, H., Nakao, F. & Hayashi, F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J. Cataract Refract. Surg. 27, 1779–1786 (2001).
Stawowski, Ł et al. Comparison of ExPress mini-device implantation alone or combined with phacoemulsification for the treatment of open-angle glaucoma. J. Ophthalmol. 2015, 613280 (2015).
Link, S., Häring, G. & Hedderich, J. Effect of phacoemulsification and posterior chamber lens implantation on intraocular pressure in patients with and without open-angle glaucoma. Ophthalmologe 97, 402–406 (2000).
Tarongoy, P., Ho, C. L. & Walton, D. S. Angle-closure glaucoma: The role of the lens in the pathogenesis, prevention, and treatment. Surv. Ophthalmol. 54, 211–225 (2009).
Spaeth, G. L. European glaucoma society terminology and guidelines for glaucoma. Br. J. Ophthalmol. 105(1), 1–169 (2021).
Arriola-Villalobos, P. et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br. J. Ophthalmol. 96, 645–649 (2012).
Pinchuk, L. et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B Appl. Biomater. 105, 211–221 (2017).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.K. methodology, J.K.; software, E.S.; validation, K.G.; formal analysis, E.S. and J.K.; investigation, E.S.; data curation, K.G.; writing—original draft preparation, J.K.; writing—review and editing, E.S; visualization, K.G.; supervision, J.K.; project administration, J.K.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Konopińska, J., Gołaszewska, K. & Saeed, E. Minimally invasive bleb surgery versus minimally invasive glaucoma surgery: a 12-month retrospective study. Sci Rep 14, 12850 (2024). https://doi.org/10.1038/s41598-024-61811-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-61811-y