Abstract
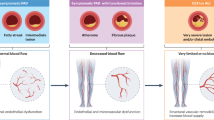
The most serious long-term effects of diabetes is peripheral artery disease (PAD) which increases the chance of developing diabetic foot ulcers, gangrene and even lower limb amputation. The clinical manifestations of PAD which are typically not revealed until symptoms like intermittent claudication, rest pain and ischemic gangrene develop, are not present in majority of diabetes mellitus patients with PAD due to diabetic peripheral neuropathy. Therefore, current study is aimed to evaluate the inflammatory and endothelial dysfunction markers with their correlation to biomarkers that can help for in-time diagnosis and efficient prognosis of developing diabetes-associated PAD. Enzyme-linked immunosorbent assay was used to evaluate the interlukin-6, interlukin-8, intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) in PAD with diabetes group, diabetic group and healthy individual group while biomarkers were measured by kit method. It was observed that serum IL-6, IL-8, ICAM and VCAM levels in type II diabetes mellitus (T2DM) with PAD patients were increased significantly (85.93, 597.08, 94.80 and 80.66) as compared to T2DM patients (59.52, 231.34, 56.88 and 50.19) and healthy individuals (4.81, 16.93, 5.55 and 5.16). The overall means for the parameters, IL-6, IL-8, ICAM, VCAM, urea, S/creatinine, CK-MB, AST, ALT, cholesterol, triglyceride, HDL, LDL, PT, aPTT, INR, HbA1C, and CRP within all groups were significantly (P < 0.05) different from each other. Therefore, it was concluded that the change in IL-6, IL-8, ICAM and VCAM can serve as an accurate diagnostic indicator and successful treatment.
Similar content being viewed by others
Introduction
Diabetes is a complicated chronic illness associated with persistent hyperglycemia due to impaired insulin production, impaired insulin sensitivity or both1,2. Increase in the incidence, disability and mortality rates of diabetes are being caused by the population’s changing dietary habits and lifestyle as well as the ongoing acceleration of the aging process, which has a significant impact on public health3. One of the most serious long-term effects of diabetes is peripheral artery disease (PAD) which increases the chance of developing diabetic foot ulcers, gangrene and even lower limb amputation4. Around 202 million individuals worldwide or 12–14% of the total population are reported to be affected with PAD5,6. It is more prevalent as people get older; affecting 10–25% of those over 55 and 40% of those over 80 and is linked to severe morbidity, mortality, and quality of life harm7,8. The clinical manifestations of PAD which are typically not revealed until symptoms like intermittent claudication, rest pain and ischemic gangrene appear, are not present in majority of diabetic patients with PAD because of diabetic peripheral neuropathy (DPN)4,9. The development of stenosis or aneurysms in non-coronary circulation can result from a number of pathophysiological events, although atherosclerosis is the most prevalent lesion that affects the aorta and its branches5,10.
The progressive buildup of lipids and inflammatory cells in the artery wall leads to the formation of atherosclerotic plaques11,12. The formation of atherosclerotic lesions is also greatly influenced by progressive endothelial dysfunction13. For the diagnosis of PAD, several plasma indicators of acute or chronic athero-inflammation, particularly inflammatory cytokines, acute indicators of endothelial dysfunction, subsets of mast cells, monocytes derived macrophages and all types of phase reactants play major role14,15. The classical inflammatory biomarkers have been found in numerous researches as vascular inflammation markers16. A non-specific marker of inflammation that rises with high concentrations of acute phase protein17,18, fibrinogen, a glycoprotein complex and a marker of thrombosis cascades, C-reactive protein (CRP) and inflammatory cytokines19. According to pathological stages and CV complications, it has been reported that circulating concentration of some cytokines such as CRP and interleukin (IL-6) are higher in PAD patients20,21,22,23. The levels of IL-6 and IL-8 are significantly higher in PAD patients13,24,25,26,27,28,29. IL-6 is linked to PAD severity25,27,30. In comparison to normal individual groups, PAD have been found to have higher levels of circulating soluble intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM)13,26,28,29,31,32. Particularly in the early phases of the disease or in diabetic patients, PAD frequently goes undiagnosed and untreated about 50% silent rates10. While some biomarkers have already found being used in clinical practice today, others need to be discovered and validated through research13,33,34,35.
The development and course of PAD are influenced by inflammation. Numerous potential inflammatory markers exists, such as established risk factors that promote atherogenicity via an inflammatory pathway. One of the biggest risk factors for PAD development is diabetes mellitus, which both directly and indirectly increases inflammatory pathways. Arterial hypertension caused by inflammatory markers, which affects around 80% of individuals with PAD may potentially be influenced by inflammation36,37,38,39. In fact vascular endothelial cells produce and express vascular cell adhesion molecule-1 in response to angiotensin II. Arterial smooth muscle cells (SMCs) release more pro-inflammatory cytokines, including interleukin (IL)-6, when exposed to angiotensin II Notably, every inflammatory molecule examined in these investigations contributes actively to peripheral pathogenesis rather than only acting as a signal of inflammation40,41. Nitric oxide (NO) released from endothelial cells is inhibited by CRP causing the severe atherosclerosis and loss of vascular flexibility. Increased levels of soluble intercellular adhesion molecule-1 (ICAM-1) have also been linked, independently, to the development of PAD, according to the Edinburgh Artery Study42.
This research is concentrated on chronic lower limb ischemia of atherosclerotic etiology known as lower extremity PAD. Our study is stressing the need for novel treatment targets, early diagnosis and prognostic markers and lower limb PAD due to the absence of accurate biomarkers for PAD assessment and crucial roles played by inflammation and vascular remodeling in the growth and rupture of atherosclerosis plaque.
Material and methods
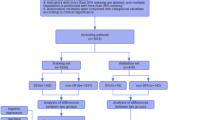
Sample collection and preparation
10 mL of venous blood samples from DM, DM plus PAD, and healthy individuals were collected who visited the Mayo Teaching Hospital of King Edward Medical University (KEMU), Lahore, according to WHO guidelines following the consent of the patients involved in the research43. Blood samples were divided into two experimental groups and one control group. Diabetic blood samples (D-S1 and D-S2) were included in group 1 while blood samples from diabetics with PAD (DP-S1 and DP-S2) were included in group 2, and blood samples from healthy individuals were labeled as a control. Subsequently, subsamples were prepared by drawing 3 mL of blood from each tube into a gel vial with yellow cap for biochemical profiling, 2 mL in a citrated vial with blue cap for coagulation profiling, and 5 mL in a sterile vial with red cap in ethylenediaminetetraacetic acid (EDTA) for HbA1c analysis44,45.
Interleukin-6 (IL-6)
Interleukin-6 is multifunctional cytokine that regulates immune responses, acute phase reactions and hematopoiesis and plays an important role in host defense mechanisms. Interleukin-6 was measured in serum samples via human IL-6 ELISA kit of Thermo Fischer Scientific. The serum was collected after centrifugation of blood at 4000 xg for 10 min. Lyophilized human IL-6 standard was reconstituted with sterile distilled water. Label seven sterile tubes, one for each standard marked as S1, S2, S3, S4, S5, S6 and S7. Prepared 1:2 serial dilutions for standard curve as follows; Take 225 μL of assay buffer (1x) into each tube. Pipette 225 μL of reconstituted standard (200 pg/ mL) into the first tube S1 and mix it gently. Take 225 μL of this dilution into 2nd tube S2 and mix thoroughly. Repeat serial dilutions 5 more times S33, S4, S5, S6 and S7. Lyophilized controls were reconstituted with distilled water. Pipette 100 μL of each standard dilution in the microwells.
Serum samples were diluted 1:2 (50 μL sample + 50 μL 1 × assay buffer) as add 50 μL assay buffer 1 × in samples well 50 μL serum, 50 μL high and low control and 100 μL in blank. An anti-human IL-6 antibody is adsorbed onto microwells of ELISA plate. Human IL-6 present in the serum or standard binds to antibodies adsorbed to the microwells. A 50 μL biotin-conjugated anti-human IL-6 antibody was added to all wells, incubated for 2 h at room temperature (18–25 °C) and bound to human IL-6 captured by the first antibody. Microwells were emptied and washed 4 times with wash buffer. A 100 μL of Streptavidin-HRP were added to all wells and incubated for 1 h at room temperature (18–25 °C). Microwells were emptied and washed 4 times with wash buffer. A 100 μL of TMB substrate solution was added to all wells and incubated at room temperature (18–25 °C) for 10 min. A 100 μL of stop solution was added to all wells and color density was measured at 450 nm in ELISA microplate reader46.
Interleukin-8 (IL-8)
Interleukin-8/Neutrophil-Actvating Peptide-1 selectively stimulates the ability of neutrophils and T-lymphocytes to invade injured or inflamed tissue. Interleukin-8 was measured in serum samples via human IL-8 ELISA kit of Thermo Fischer Scientific. The serum was collected after centrifugation of blood at 4000 xg for 10 min. Lyophilized human IL-8 standard was reconstituted with sterile distilled water. Label seven sterile tubes, one for each standard marked as S1, S2, S3, S4, S5, S6 and S7. Prepare 1:2 serial dilutions for standard curve as follows; Take 225 μL of assay buffer (1x) into each tube. Pipette out 225 μL of reconstituted standard (200 pg/ mL) into the first tube S1 and mix it gently. Take 225 μL of this dilution into 2nd tube S2 and mix thoroughly. Repeat serial dilutions 5 more times S3, S4, S5, S6 and S7. Lyophilized controls were reconstituted with distilled water. Pipette 100 μL of each standard dilution in the microwells.
Serum samples were diluted 1:2 (50 μL sample + 50 μL 1 × assay buffer) as add 50 μL assay buffer 1 × in samples well, 50 μL serum, 50 μL high and low control and 100 μL in blank. An anti-human IL-8 antibody is adsorbed onto microwells of ELISA plate. Human IL-8 present in the serum or standard binds to antibodies adsorbed to the microwells. A 50 μL biotin-conjugated anti-human IL-8 antibody was added to all wells, incubated for 2 h at room temperature (18–25 °C) and bound to human IL-8 captured by the first antibody. Microwells were emptied and washed 3 times with wash buffer. A 100 μL of Streptavidin-HRP were added to all wells and incubated for 1 h at room temperature (18–25 °C). Microwells were emptied and washed 3 times with wash buffer. A 100 μL of TMB substrate solution was added to all wells and incubated at room temperature (18–25 °C) for 10 min. A 100 μL of stop solution was added to all wells and color density was measured at 450 nm in ELISA microplate reader46.
Intercellular adhesion molecule-I (ICAM-I)
Intercellular Adhesion molecule-I (ICAM-I) is a member of the immunoglobulin supergene family and functions as a ligand for the lymphocyte function-associated antigen-1. ICAM-I was measured in serum samples via human ICAM-I ELISA kit of Thermo Fischer Scientific. The serum was collected after centrifugation of blood at 4000 xg for 10 min. Lyophilized human ICAM-I standard was reconstituted with sterile distilled water. Label four sterile tubes, one for each standard marked as S1, S2, S3 and S4. Prepare 1:2 serial dilutions for standard curve as follows; Take 225 μL of sample diluent into each tube. Pipette out 225 μL of reconstituted standard (1 = 100 ng/mL) into the first tube S1 and mix it gently. Take 225 μL of this dilution into 2nd tube S2 and mix thoroughly. Repeat serial dilutions 2 more times S3 and S4. Lyophilized controls were reconstituted with distilled water. Pipette 100 μL of each standard dilution in the microwells.
Serum samples were diluted 1:10 (10 μL sample + 90 μL sample diluent) as add 90 μL sample diluent in samples well with 10 μL serum, 10 μL high and low control and 100 μL in blank. An anti-human ICAM-I antibody is adsorbed onto microwells of ELISA plate. Human ICAM-I present in the serum or standard binds to antibodies adsorbed to the microwells. A 50 μL HRP-conjugated was added to all wells, incubated for 1 h at room temperature (18–25 °C) and bound to human ICAM-I captured by the first antibody. Microwells were emptied and washed 3 times with wash buffer. A 100 μL of Streptavidin-HRP were added to all wells and incubated for 1 h at room temperature (18–25 °C). Microwells were emptied and washed 3 times with wash buffer. A 100 μL of TMB substrate solution was added to all wells and incubated at room temperature (18–25 °C) for 10 min. A 100 μL of stop solution was added to all wells and color density was measured at 450 nm in ELISA microplate reader47.
Vascular cell adhesion molecule-I (VCAM-I)
Vascular cell adhesion molecule-I (VCAM-I) or CD106 is a member of the immunoglobulin supergene family. VCAM-I was measured in serum samples via human VCAM-I ELISA kit of Thermo Fischer Scientific. The serum was collected after centrifugation of blood at 4000 xg for 10 min. Lyophilized human VCAM-I standard was reconstituted with sterile distilled water. Label six sterile tubes, one for each standard marked as S1, S2, S3, S4, S5 and S6. Prepare 1:2 serial dilutions for standard curve as follows; Take 225 μL of sample diluent into each tube. Pipette out 225 μL of reconstituted standard (1 = 100 ng/ mL) into the first tube S1 and mix it gently. Take 225 μL of this dilution into 2nd tube S2 and mix thoroughly. Repeat serial dilutions 4 more times S3, S4, S5 and S6. Lyophilized controls were reconstituted with distilled water. Pipette 100 μL of each standard dilution in the microwells.
Serum samples were diluted 1:50 (10 μL sample + 490 μL assay buffer) as add 490 μL assay buffer in 10 μL serum, then take 100 μL prediluted sample in all sample wells, 10 μL high and low control and 100 μL in blank. An anti-human VCAM-I antibody is adsorbed onto microwells of ELISA plate. Human VCAM-I present in the serum or standard binds to antibodies adsorbed to the microwells. A 50 μL conjugate-mixture was added to all wells, incubated for 2 h at room temperature (18–25 °C) and bound to human VCAM-I captured by the first antibody. Microwells were emptied and washed 3 times with wash buffer. A 100 μL of TMB substrate solution was added to all wells and incubated at room temperature (18–25 °C) for 10 min. A 100 μL of stop solution was added to all wells and color density was measured at 450 nm in ELISA microplate reader47.
Estimation of glycan and glycosaminoglycan (HbA1c)
Measurement of hemoglobin-A1c was done on Beckman coulter system. Quantitative estimation of hemoglobin 1c concentration was performed in human whole blood. The HbA1c assay was carried out using the kit consisting of total hemoglobin reagent, HbA1c antibodies and HbA1c polyhepten and hemolyzing reagent48.
K2-EDTA whole blood was pre-treated with hemolyzing reagent in ratio of 1:100 and mixed well to obtain complete hemolysis of blood. Tetradecylammonium bromide (TTAB) in hemolyzing reagent eliminates the white blood cells. The concentration of both HbA1c and total hemoglobin were determined. HbA1c reagent was used to measure hemoglobin A1c concentration by turbidimetric immunoinhibition method. Hemoglobin A1c antibodies combine with HbA1c from sample to form soluble antigen–antibody complexes. Polyhepten then binds with excess antibodies and resulting agglutination complex was measured turbidimetrically at 450 nm49.
Coagulation profiling
To evaluate coagulopathy, prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured according to the previous protocol50. For every 100 µL of citrated plasma sample, 200 µL of PT reagents were added and the time of coagulation was measured. For aPTT estimation, 100 µL of aPTT reagent was added to 100 µL of citrated plasma sample followed by incubation at 37 °C for 3 min. Subsequently, 100 µL of warmed calcium chloride was added to initiate the clotting cascade and time of clot formation was measured50.
Measurement of C-reactive protein
The amperometric technique was used to measure the level of C-reactive protein in the 10 times diluted blood samples. After placing 30 µL of sample on immunosensor strips and 30 min of incubation, the electrodes were washed with PBS followed by dropping 50 µL of 0.5 mM ferrocyanide onto the electrodes. Ultimately, CRP concentration was determined using a standard calibration curve51.
Serum creatinine
Serum samples were collected and creatinine levels were measured using Beckman Coulter AU analyzer. Jaffe’s method was employed which depicts the end results by the formation of red or orange yellow chromophores. All the reagents were already prepared. The kit method containing reagent 1 (R1) and reagent 2 (R2) was used consisting of sodium hydroxide and picric acid, respectively. The total volume of 1000 µL of reagents was added to 50 µL of serum sample in a 4:1 (R1:R2) ratio followed by 3 min of incubation. After incubation, the absorbance was measured at 520/800 nm52,53.
Blood urea nitrogen
The measurement of blood urea nitrogen (BUN) was carried out in serum samples of patients by Beckman Coulter AU analyzer. At first urease enzyme was added to the serum sample in order to hydrolyze urea in to ammonia and carbon dioxide. Subsequently, l-glutamate dehydrogenase catalyzed the conversion of ammonia and α-oxoglutarate to glutamate. In the whole process, the hydrolysis of a urea molecule was associated with the oxidation of two molecules of NADH. At the end the absorbance was determined at 340 nm which increases with the falling levels of NADH in the reaction mixture54.
Creatine kinase MB
The plasma levels of creatine kinase-MB (CK-MB) were estimated by the kit method. For obtaining plasma, the blood samples were centrifuged for 15 min at 500 xg at room temperature. After obtaining plasma, both reagents were added according to the manufacturer’s instructions and the absorbance was recorded using a microplate reader54.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
The levels of AST are measured in serum samples that were stored at 15–20 ℃ and analyzed by using Beckman Coulter AU analyzer. 88 mmol/L of Tris buffer (pH 7.80), 900 U/L of lactate dehydrogenase (LDH), 260 nmol/L of l-aspartate, 600 U/L of malate dehydrogenase (MDH), 0.22 mmol/L of NADH and 12 mmol/L of α-oxoglutarate were used to measure AST. In this reaction, aspartate and α-oxoglutarate were catalyzed by AST to form oxaloacetate (OA) and l-glutamate. OA was reduced by MDH into l-malate in the presence of NADH which was converted in to NAD+. At the end of this reaction, the activity of AST was measured at 340 nm. The absorbance was found to decrease due to increase in the consumption of NADH indicating enhanced AST activity52.
ALT levels were also evaluated via Beckman Coulter AU analyzer with all ALT reagents prepared beforehand. At first amino group transfer reaction was catalyzed by ALT in which alanine and α-oxoglutarate were converted into glutamate and pyruvate. The reaction was further continued by the action of LDH on pyruvate in the presence of NADH to form lactate and NAD+. Afterwards, the absorbance was measured at 340 nm indicating the consumption of NADH and ALT activity52.
Lipid profile estimation
Total cholesterol
To evaluate the total cholesterol levels, isopropanol: NP40 v/v (9:1) was used to dissolve the sample. Subsequently, 10 µL/well of 100 U/mL of catalase solution and 40 µL/well of sample solution in a black 96-well microplate were added. The microplate was incubated for 15 min at 37 °C to remove any peroxidase in the reagent or sample. The second incubation for 15 min at 37 °C was carried out after the addition of 150 µL/well of 0.67 U/mL cholesterol esterase plus reagent A, containing 0.4 mM ADHP, 1.3 U/mL HRP, 0.1% Triton X-100, 5 mM cholic acid, 0.1 M potassium phosphate buffer (pH 7.4), into each well of the microplate. After the second incubation, the absorbance was measured at the excitation and emission wavelengths of 530 and 580 nm, respectively55.
Triglycerides
The GPO/POD method was used to determine triglyceride (TG) levels in the venous blood samples. Microbial lipases were added to the blood sample to initiate the hydrolysis of TGs yielding glycerol and free fatty acids (FFAs). Subsequently, glycerol was phosphorylated by glycerol kinase and glycerol-3-phosphate oxidase into glycerol-3-phosphate (G3P) and oxidizes G3P generating dihydroxyacetone phosphate (DAP) and hydrogen peroxide (H2O2), respectively. The end product will be red quinoneimine produced by the reaction of H2O2 with 4-aminoantipyrine and 4-chlorophenol. The absorbance measured will be proportional to the concentration of TGs in the sample evaluated56.
Low- and high-density lipoprotein
High-density lipoproteins (HDL) and low-density lipoproteins (LDL) were measured simultaneously by the homogeneous method29. For HDL, chylomicrons, LDL and very low-density lipoproteins (VLDL) were blocked by the formation of antigen–antibody complexes in the sample and HDL was measured by the enzymatic cholesterol.
In the case of LDL, two types of reagents were used to quantify LDL in the sample solution. At first, reagent 1, which contains cholesterol oxidase, cholesterol esterase, ascorbic acid, 4-amino-antipyrine, MES buffer (pH 6.3), peroxidase, and detergent 1, was added to each well with the sample solution. Two types of reactions occur which involve the solubilization of non-LDL proteins and degradation of released cholesterol by detergent 1 and enzymes, respectively. Secondly, reagent 2, containing detergent 2, MES buffer (pH 6.3), and N,N-bis-m-tolidine-disodium, was added to solubilize LDL which was later quantified57.
Statistical analysis
International Business Machine (IBM)—Statistical Package for Social Sciences (SPSS) version 23 was used for data analysis. Data was evaluated for overall means for various parameters using ANOVA. Significant difference of different parameters among various groups were evaluated using the repeated measures ANOVA (Dunnett’s T3 Post Hoc Test). The results of renal function tests, cardiac function test, ALT, lipid profile, coagulation profile, HbA1c and C-reactive proteins were correlated by healthy individuals, participants with diabetes mellitus (DM) and those with DM plus peripheral arterial disease (PAD) using the Pearson’s Correlation Coefficient (r value).
Ethical approval
The Research and Ethics Committee (Path/No.195/2021), Faculty of Pathology, King Edward Medical University, Lahore, Pakistan, approved the current study.
Results
Evaluation of inflammatory and endothelial dysfunction markers and metabolic characteristics in patients with type 2 diabetes mellitus (T2DM) plus peripheral arterial disease (PAD), T2DM and healthy individuals
The ANOVA revealed that the overall means for the parameters, IL-6, IL-8, ICAM, VCAM, urea, S/creatinine, CK-MB, AST, ALT, cholesterol, triglyceride, HDL, LDL, PT, aPTT, INR, HbA1C, and CRP within all groups were significantly (P < 0.05) different from each other.
However, repeated measures ANOVA (Dunnett’s T3 Post Hoc Test) between various groups revealed that the IL-6, IL-8, ICAM and VCAM in healthy persons were significantly lower than the all-other groups. The data presented in Table 1 shows the mean plus standard error mean of different groups. The results revealed that serum IL-6 level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (85.93) as compared to T2DM patients (59.52) and healthy individuals (4.81). Serum IL-8 level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (597.08) as compared to T2DM patients (231.34) and healthy individuals (16.93). Similarly, the results revealed that serum ICAM level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (94.80) as compared to T2DM patients (56.88) and healthy individuals (5.55). Serum VCAM level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (80.66) as compared to T2DM patients (50.19) and healthy individuals (5.16).
The results represented that serum urea level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (50.64) as compared to T2DM patients (25.67) and healthy individuals (20.21). Likewise serum creatinine level was elevated significantly in T2DM with PAD patients (1.52) as compared to T2DM patients (0.72) and healthy individuals (0.60). Cardiac enzymes (CK-MB and AST) shows significantly increased level in T2DM with peripheral arterial disease (PAD) patients (32.62 and 46.78) as compared to T2DM patients (15.83 and 25.35) and healthy individuals (12.93 and 19.92). The results in Table 1 demonstrated that serum liver enzymes (ALT and AST respectively) were also raised significantly in T2DM with peripheral arterial disease (PAD) patients (49.89 and 46.78) as compared to T2DM patients (20.02 and 25.35) and healthy individuals (18.85 and 19.92). Lipid profile (cholesterol, triglycerides, HDL, and LDL) showed significantly elevated level in T2DM with PAD patients (239.00, 417.43, 47.37 and 118.75) as compared to T2DM patients (201.75, 211.75, 42.89 and 115.43) and healthy individuals (144.78, 113.29, 53.42 and 106.00). The coagulation profile (PT, aPTT and INR) in the Table 1 represents the significantly elevated level in T2DM with PAD patients (15.10, 36.24 and 1.56) as compared to T2DM patients (13.45, 33.86 and 1.01) and healthy individuals (12.85, 32.92 and 0.98). The HbA1c level was significantly declined in T2DM patients (8.94) as compared to T2DM with PAD patients (9.05) and lower in healthy individuals (4.79). Serum CRP level was significantly inclined in T2DM with PAD (8.59) as compared to T2DM patients (1.43) and healthy individuals (0.50).
Pearson’s correlation of inflammatory and endothelial dysfunction markers and metabolic characteristics overall in those with type 2 diabetes mellitus (T2DM) plus peripheral arterial disease (PAD), T2DM and healthy individuals
The result of correlation coefficient of inflammatory and endothelial dysfunction markers of overall in (Table 2) IL-6 × IL-8 (r = 0.587; P < 0.01), IL-6 × ICAM (r = 0.819; P < 0.01), IL-6 × VCAM (r = 0.716; P < 0.01), IL-8 × ICAM (r = 0.750; P < 0.01), IL-8 × VCAM (r = 0.642; P < 0.01), and ICAM × VCAM (r = 0.799; P < 0.01) had a statistical significantly positive correlation.
The result of correlation coefficient of inflammatory marker (IL-6) with biochemical markers of overall in (Table 3) IL-6 × urea (r = 0.587; P < 0.01), IL-6 × S/creatinine (r = 0.474; P < 0.01), and IL-6 × CKMB (r = 0.445; P < 0.01), IL-6 × AST (r = 0.417; P < 0.01), IL-6 × ALT (r = 0.429; P < 0.01), IL-6 × cholesterol (r = 0.490; P < 0.01), IL-6 × triglyceride (r = 0.410; P < 0.01), IL-6 × LDL (r = 0.267; P < 0.05), IL-6 × PT (r = 0.303; P < 0.01), IL-6 × aPTT (r = 0.344; P < 0.01), IL-6 × HbA1C (r = 0.452; P < 0.01), IL-6 × CRP (r = 0.247; P < 0.05) had a statistical significantly positive correlation. While IL-6 × HDL and IL-6 × INR had no significant correlation.
The result of correlation coefficient of inflammatory marker (IL-6) with biochemical markers of overall in (Table 4) IL-8 × urea (r = 0.583; P < 0.01), IL-8 × S/creatinine (r = 0.521; P < 0.01), and IL-8 × CKMB (r = 0.458; P < 0.01), IL-8 × AST (r = 0.522; P < 0.01), IL-8 × ALT (r = 0.559; P < 0.01), IL-8 × cholesterol (r = 0.473; P < 0.01), IL-8 × triglyceride (r = 0.537; P < 0.01), IL-8 × PT (r = 0.425; P < 0.01), IL-8 × aPTT (r = 0.355; P < 0.01), IL-8 × HbA1C (r = 0.343; P < 0.01), IL-8 × CRP (r = 0.396; P < 0.05) had a statistical significantly positive correlation. While IL-8 × HDL, IL-8 × LDL and IL-8 × INR had no significant correlation.
The result of correlation coefficient of endothelial dysfunction marker (ICAM) with biochemical markers of overall in (Table 5) ICAM × urea (r = 0.632; P < 0.01), ICAM × S/creatinine (r = 0.552; P < 0.01), and ICAM × CKMB (r = 0.485; P < 0.01), ICAM × AST (r = 0.498; P < 0.01), ICAM × ALT (r = 0.470; P < 0.01), ICAM × cholesterol (r = 0.523; P < 0.01), ICAM × triglyceride (r = 0.503; P < 0.01), ICAM × LDL (r = 0.224; P < 0.05), ICAM × PT (r = 0.307; P < 0.01), ICAM × aPTT (r = 0.346; P < 0.01), ICAM × HbA1C (r = 0.441; P < 0.01), ICAM × CRP (r = 0.275; P < 0.05) had a statistical significantly positive correlation. While ICAM × HDL and ICAM × INR had no significant correlation.
The result of correlation coefficient of endothelial dysfunction marker (VCAM) with biochemical markers of overall in (Table 6) VCAM × urea (r = 0.499; P < 0.01), VCAM × S/creatinine (r = 0.464; P < 0.01), and VCAM × CKMB (r = 0.486; P < 0.01), VCAM × AST (r = 0.358; P < 0.01), VCAM × ALT (r = 0.394; P < 0.01), VCAM × cholesterol (r = 0.452; P < 0.01), VCAM × triglyceride (r = 0.474; P < 0.01), VCAM × LDL (r = 0.340; P < 0.05), VCAM × PT (r = 0.243; P < 0.01), VCAM × aPTT (r = 0.247; P < 0.01), VCAM × HbA1C (r = 0.489; P < 0.01), VCAM × CRP (r = 0.244; P < 0.05) had a statistical significantly positive correlation. While VCAM × HDL and VCAM × INR had no significant correlation.
Diagnostic performance of inflammatory and endothelial dysfunction markers in PAD + T2DM
The ROC curves represent a correct diagnostic performance for all four inflammatory and endothelial dysfunction markers. The area under curve (AUC, s) for IL-6, IL-8, ICAM and VCAM were higher than 0.8 (Fig. 1). AUCs for two inflammatory markers and two endothelial dysfunction markers were highly statistically significant (p < 0.005, 0.001) (Table 7).
Discussion
T2DM increases the susceptibility to complex pro-thrombotic state where endothelial dysfunction, platelet hyperactivity, changes to coagulation cascade and chronic low-grade inflammation play significant role58. Inflammatory biomarkers play important role in atherosclerosis59. IL-6 is released by fibroblasts, endothelial cells, macrophages, B-lymphocytes and Th2 lymphocytes and is pivotal in developing inflammatory response in PAD with diabetes60. IL-6 has played important role in the genesis of atherosclerosis and releasing of IL-8 by endothelial cells and macrophages61.
Numerous studies have examined the role of IL-6 in the pathophysiology of atherosclerosis particularly examining the relationship between the inflammatory process and peripheral artery disease (PAD). Numerous inflammatory molecules, sometimes referred to as acute-phase proteins, including CRP, fibrinogen synthesis, complement factor release, and serum amyloid A formation, are known to be stimulated by IL-6 in the liver61,62. IL-6 promotes the synthesis of MCP-1 and IL-8 by macrophages and endothelial cells, among other functions in the development and upkeep of atherosclerotic plaque. Additionally, IL-6 seems to be able to boost the synthesis of ICAM-1 by SMCs and increases the release of chemokines by the arterial wall's intimal cells. Furthermore, it facilitates leukocyte homing into atherosclerotic plaque. Lastly, IL-6 encourages SMCs to develop into foam cells. Research has demonstrated that IL-6 is a novel predictor of PAD and a valid marker for predicting the clinical course of the illness over a twelve-year span. Its function in PAD is actually well-defined, and evidence from community research indicates that it ought to be regarded as a predicting63.
Chronic consequences of diabetes mellitus (DM) include atherosclerosis in the arteries of the heart, brain and lower limbs. Leg ulcers and amputations can result from these issues that damage the lower limb arteries which is called PAD. Its burden is steadily increasing and contributes to diabetic’s mortality64. Peripheral artery disease (PAD) typically coexists with the conditions like diabetes, cardiac disease and respiratory illness. It is linked to the gradual loss of limb; claudication and gangrene witch ultimately lowers quality of life65. As a result, evaluation of a number of inflammatory biomarkers for the early diagnosis of PAD, in order to lower the risk of mortality and enhance their quality of life, is crucial.
Greater the exposure of the biomarkers IL-6 and IL-8 has resulted in a collection of research data on the role of interleukins (ILs) in PAD, specifically in relation to glycoproteins acting as proinflammatory substances that influence blood vessel walls when atherosclerotic plaque development is initiated and how it develops. The acute phase protein known as C-reactive protein (CRP) originates from liver cells. The secretion of interlukin-6 by macrophages and T-cells promotes its release60. The release of IL-8 from monocytes and macrophages depends critically on stimulated inflammatory circumstances. It is intriguing to observe that polymorph nuclear leukocytes significantly produce IL-866. Significant associations between IL-18, P-selectin, VCAM and other inflammatory markers including IL-1, IL-6 and CRP as well as adhesion molecules like ICAM67. In our study, the results revealed that serum IL-6 level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (85.93) as compared to T2DM patients (59.52) and healthy individuals (4.81). Serum IL-8 level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (597.08) as compared to T2DM patients (231.34) and healthy individuals (16.93).
The susceptibility to exhibit continuous atherosclerosis progression is one of the primary characteristics of peripheral artery disease. It is generally known that inflammation plays a crucial role in the emergence of atherosclerosis. However, there is no previously reported inflammatory biomarker for the identification of the risk of stratification or conclusive diagnosis of PAD68. T2DM has significant higher levels of ICAM and VCAM compared to controls, an adhesion molecule considered to play a role in atherosclerosis progression69. Our results agree with earlier study, ICAM and VCAM were all found to be positively linked with DM in a prior study by Bluher70 as compared to hyperglycemic and impaired glucose tolerant patients. A more recent study reported a rise in VCAM blood concentration of PAD patients71. According to another recent report, VCAM is a marker that is pathogenically related to diabetic nephropathy and is predictive of it68. The results of our study revealed that serum ICAM level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (94.80) as compared to T2DM patients (56.88) and healthy individuals (5.55). Serum VCAM level in type II diabetes mellitus (T2DM) with peripheral arterial disease (PAD) patients was increased significantly (80.66) as compared to T2DM patients (50.19) and healthy individuals (5.16).
To counteract the inflammation caused by other cytokines, IL-6 (as adipokine) levels gradually rise in DM, yet this can result in inflammation of vascular cells that leads to atherosclerosis72. Adipocytes in adipose tissue release IL-6 and IL-8 in obese individuals which induces insulin resistance particularly in hepatocytes and results in DM73. Due to the increased levels of cytokines and stress in DM, the levels of intercellular adhesion molecule (ICAM) and vascular cellular adhesion molecules (VCAM) are also increased. They are highly expressed in endothelial cells of arteries and trigger the release of new cytokines, which erodes the lining of the blood vessels74. IL-6, IL-8, ICAM and VCAM are rapidly rising in PAD causes the complications. So they induce endothelial abnormalities and harm the vascular system.
The levels of renal markers (BUN and serum creatinine) were also abnormally raised in DM plus PAD than in DM. BUN and creatinine are metabolic waste products that are normally excreted from kidneys, but under pathological conditions, they accumulate in the blood plasma74. In diabetics, the plasma level of creatinine drops due to decreased skeletal muscle volume; however, it rises in vascular disease indicating renal dysfunction and may increase or worsen atherosclerosis75,76. In critical limb ischemia, the level of BUN is also increased where it serves as a diabetic risk factor, a biomarker for hemodynamic changes, and an indicator of survival chances in patients77. In our study, the result of correlation coefficient of inflammatory markers (IL-6, IL-8) and endothelial dysfunction marker (ICAM, VCAM) with biochemical marker urea and creatinine of overall (IL-6, IL-8, ICAM, VCAM × urea, creatinine) had a statistical significantly positive correlation.
CRP is an indicator of cardiac dysfunction and functions as an acute phase reactant in blood plasma78. Its production is stimulated by an IL-6 mediated inflammatory signal in hepatocytes and adipose tissues; therefore, enhanced levels represent an inflammation or tissue injury79,80. As the level of CRP rises in blood so does the progression of atherosclerosis. It is because being acute phase reactant it activates platelets, complements system, initiates thrombus formation, and modifies innate immunity81. In our study, the result of correlation coefficient of inflammatory markers (IL-6, IL-8) and endothelial dysfunction marker (ICAM, VCAM) with biochemical marker AST, CKMB and CRP of overall (IL-6, IL-8, ICAM, VCAM × AST, CKMB and CRP) had a statistical significantly positive correlation.
Persistent glycemic control and prognosis of the T2DM therapy are critically associated with the levels of HbA1c, where, they represent the mean blood levels of glucose over a period of 3–6 months82,83. HbA1c levels are essentially elevated in T2DM as demonstrated by our results and agree with previous research82. Therefore, HbA1c can serve as an independent determinant for investigating diabetics that have the chance to develop PAD84. The association of HbA1c with PAD is due to increased glucose concentration over a long period of time thus activating platelets and protein kinase C pathway that induces pro-inflammatory condition leading to enhanced oxidation stress and endothelial dysfunction. Moreover, this consistent hyperglycemic condition also causes stiffness in the arteries83. In our study, the result of correlation coefficient of inflammatory markers (IL-6, IL-8) and endothelial dysfunction marker (ICAM, VCAM) with biochemical marker glycolytic hemoglobin (HbA1c) of overall (IL-6, IL-8, ICAM, VCAM × HbA1c) had a statistical significantly positive correlation.
Furthermore, the PT and aPTT (coagulation profile) level doesn’t increase preferably in diabetics plus PAD as compared to the control group and diabetics group. Unlike, enhanced fibrinogen levels are reported in diabetic patients as compared to non-diabetics85. Furthermore, coagulation profile indicates the risk of atherothrombotic events, the main cause of the development of cardiovascular complications86. In our study, the result of correlation coefficient of inflammatory markers (IL-6, IL-8) and endothelial dysfunction marker (ICAM, VCAM) with coagulation markers (PT, aPTT) of overall (IL-6, IL-8, ICAM, VCAM × PT, APTT) had a statistical significantly positive correlation.
Liver function tests were performed to estimate the levels of AST and ALT. Both the enzyme levels were elevated in diabetics and diabetics plus PAD. The level of AST and ALT significantly increases among diabetics and non-diabetics (control). These results agree to those reported previously87. Contrarily, when diabetics develop PAD symptoms, marked elevation in the levels of AST and ALT occurs. Diabetes with PAD can also be linked with abnormalities in the lipid profile of patients. The levels of triglycerides are elevated while low levels of HDL were observed in our investigations which are in accordance with the literature reports88. In our study, the result of the correlation coefficient of inflammatory markers (IL-6, IL-8) and endothelial dysfunction marker (ICAM, VCAM) with biochemical markers (AST, ALT, cholesterol, triglycerides, LDL) of overall (IL-6, IL-8, ICAM, VCAM × AST, ALT, cholesterol, triglycerides, LDL) had a statistical significantly positive correlation.
Although the level of all the tested parameters IL-6, IL-8, ICAM, and VCAM increased in DM plus PAD except and HDL levels, which are somehow, decreased from the control values. Any variation in markers of interest in healthy individuals, diabetics and diabetics plus PAD can be easily observed from Tables 1, 2, 3, 4, 5 and 6. A remarkable increase in IL-6, IL-8, ICAM and VCAM depicts that these analytes can be utilized as biomarkers in the diagnosis and prognosis of PAD associated with DM.
The clinical manifestations of PAD include claudication and pain at rest which progresses to ulceration and gangrene in later stages. Nonetheless, diabetic neuropathy may also be a secondary cause of leg pain and functional impairment89. One tool for categorizing the clinical phases of symptomatic PAD is the Fontaine scale. Fontaine stages IIa and IIb have mild and moderate to severe impairment claudication, respectively; Fontaine stage-III patients have symptoms at rest; and Fontaine stage-IV patients have considerable tissue loss (ulcers or gangrene). Patients at Fontaine stage-I have PAD but are asymptomatic90. Significant elevation of inflammatory and endothelial dysfunction markers include IL-6, IL-8, ICAM and VCAM can diagnose PAD patients even at Fontaine stage-I. Along these lines, machine learning techniques could be helpful in combing many biomarkers with clinical and functional factors to provide prediction algorithms that are more accurate. This will result in more precise PAD risk assessment, earlier PAD diagnosis and more individualized medication or surgery regimens91.
Conclusion
In summary, diabetics can progressively develop PAD that damages their vascular system and increases the risk of cardiovascular disorders and lower limb amputations. Despite the incidence of PAD, poor therapeutic strategies have been used due to the lack of knowledge about the PAD clinical manifestations and limited markers for its early diagnosis, progression and prognostic evaluation in targeted manner. Keeping these problems in mind, we investigated a number of inflammatory and endothelial dysfunction markers along with biomarkers and found drastic alterations in the levels of inflammatory and endothelial dysfunction markers and some biomarkers in diabetics plus PAD as compared to diabetics only. These inflammatory and endothelial dysfunction markers include IL-6, IL-8, ICAM and VCAM. Among these biomarkers, marked increase in IL-6, IL-8, ICAM and VCAM were significantly higher in diabetics plus PAD as compared to DM and healthy individuals. In order to prevent the PAD in patients with DM, doctors may view these characteristics as useful diagnostic and prognostic criteria. The patients can be advised by clinicians to check their IL-6, IL-8, ICAM and VCAM from clinical pathology laboratory.
The current research also pointed out the interesting objectives particularly considering that vasoactive drugs commonly used in PAD treatment proved not to fulfill all the therapeutic targets. Further research is needed on additional inflammatory biomarkers in PAD to improve the database for their mechanisms and on novel effective therapies.
Data availability
All data generated or analyzed during this study are included in the published article.
References
Diabetes, A. A. Updates to the standards of medical care in diabetes-2018. Diabetes Care 41(9), 2045–2047. https://doi.org/10.2337/dc18-su09 (2018).
World Health Organization. (2018). Global report on diabetes. 2016.
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14(2), 88–98. https://doi.org/10.1038/nrendo.2017.151 (2018).
Yang, S. L. et al. Pathophysiology of peripheral arterial disease in diabetes mellitus. J. Diabetes 9(2), 133–140. https://doi.org/10.1111/1753-0407.12474 (2017).
Frank, U. et al. ESVM guideline on peripheral arterial disease. Vasa https://doi.org/10.1024/0301-1526/a000834 (2019).
Fowkes, F. G. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 382(9901), 1329–1340. https://doi.org/10.1016/S0140-6736(13)61249-0 (2013).
Hajibandeh, S. et al. Prognostic significance of ankle brachial pressure index: A systematic review and meta-analysis. Vascular 25(2), 208–224. https://doi.org/10.1177/1708538116658392 (2017).
Jirak, P. et al. Analysis of novel cardiovascular biomarkers in patients with peripheral artery disease. Minerva Med. 109(6), 443–450. https://doi.org/10.23736/S0026-4806.18.05628-8 (2018).
Firnhaber, J. M. & Powell, C. S. Lower extremity peripheral artery disease: Diagnosis and treatment. Am. Fam. Phys. 99(6), 362–369 (2019).
Gerhard-Herman, M. D. et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 135(12), e686-725. https://doi.org/10.1161/CIR.0000000000000470 (2017).
Marchio, P. et al. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell Longev. 2019, 8563845. https://doi.org/10.1155/2019/8563845 (2019).
Ruparelia, N. & Choudhury, R. Inflammation and atherosclerosis: What is on the horizon?. Heart 106(1), 80–85. https://doi.org/10.1136/heartjnl-2018-314230 (2020).
Signorelli, S. S. et al. Plasma levels of inflammatory biomarkers in peripheral arterial disease: Results of a cohort study. Angiology 67(9), 870–874. https://doi.org/10.1177/0003319716633339 (2016).
Raggi, P. et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 276, 98–108. https://doi.org/10.1016/j.atherosclerosis.2018.07.014 (2018).
Bäck, M., Yurdagul, A. Jr., Tabas, I., Öörni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16(7), 389–406. https://doi.org/10.1038/s41569-019-0169-2 (2019).
Erren, M. et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler. Thromb. Vasc. Biol. 19(10), 2355–2363. https://doi.org/10.1161/01.atv.19.10.2355 (1999).
Kiyani, M. M. et al. Erythrocyte sedimentation rate in diabetic and non-diabetic patients of cardiovascular disease. J. Teknol Laboratorium 8(1), 18–22. https://doi.org/10.29238/teknolabjournal.v8i1.159 (2019).
Erikssen, G. et al. Erythrocyte sedimentation rate: A possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. Eur. Heart J. 21(19), 1614–1620. https://doi.org/10.1053/euhj.2000.2148 (2000).
Ibaram, D. R. S. Role of C-Reactive protein in peripheral arterial disease. Int. J. Inn. Res. 3(12), 2428–2432. https://doi.org/10.23958/ijirms/vol03-i12/513 (2018).
Kremers, B. et al. Plasma biomarkers to predict cardiovascular outcome in patients with peripheral artery disease: A systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 40(9), 2018–2032. https://doi.org/10.1161/ATVBAHA.120.314774 (2020).
Singh, T. P., Morris, D. R., Smith, S., Moxon, J. V. & Golledge, J. Systematic review and meta-analysis of the association between C-reactive protein and major cardiovascular events in patients with peripheral artery disease. Eur. J. Vasc. Endovasc. Surg. 54(2), 220–233. https://doi.org/10.1016/j.ejvs.2017.05.009 (2017).
Hazarika, S. & Annex, B. H. Biomarkers and genetics in peripheral artery disease. Clin. Chem. 63(1), 236–244. https://doi.org/10.1373/clinchem.2016.263798 (2017).
Busti, C., Falcinelli, E., Momi, S. & Gresele, P. Matrix metalloproteinases and peripheral arterial disease. Intern. Emerg. Med. 5, 13–25. https://doi.org/10.1007/s11739-009-0283-y (2010).
Saenz-Pipaon, G. et al. Functional and transcriptomic analysis of extracellular vesicles identifies calprotectin as a new prognostic marker in peripheral arterial disease (PAD). J. Extracell. Vesicles 9(1), 1729646. https://doi.org/10.1080/20013078.2020 (2020).
Valkova, M., Lazurova, I., Petrasova, D., Frankovicova, M. & Dravecka, I. Humoral predictors of ankle-brachial index in patients with peripheral arterial disease and controls. Bratisl Lek Listy 119(10), 646–650. https://doi.org/10.4149/BLL_2018_114 (2018).
Pande, R. L. et al. Association of monocyte tumor necrosis factor α expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J. Vasc. Surg. 61(1), 155–161. https://doi.org/10.1016/j.jvs.2014.06.116 (2015).
Beckman, J. A., Preis, O., Ridker, P. M. & Gerhard-Herman, M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death). Am. J. Cardiol. 96(10), 1374–1378. https://doi.org/10.1016/j.amjcard.2005.07.041 (2005).
Engelberger, R. P. et al. Biological variation of established and novel biomarkers for atherosclerosis: Results from a prospective, parallel-group cohort study. Clin. Chim. Acta. 447, 16–22. https://doi.org/10.1016/j.cca.2015.05.003 (2015).
Signorelli, S. S. et al. High circulating levels of cytokines (IL-6 and TNFalpha), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc. Med. 8(1), 15–19. https://doi.org/10.1191/1358863x03vm466oa (2003).
Igari, K., Kudo, T., Toyofuku, T. & Inoue, Y. Relationship of inflammatory biomarkers with severity of peripheral arterial disease. Int. J. Vasc. Med. 2016, 6015701. https://doi.org/10.1155/2016/6015701 (2016).
Unlü, Y., Karapolat, S., Karaca, Y. & Kiziltunç, A. Comparison of levels of inflammatory markers and hemostatic factors in the patients with and without peripheral arterial disease. Thromb. Res. 117(4), 357–364. https://doi.org/10.1016/j.thromres.2005.03.019 (2006).
Edlinger, C. et al. Disease-specific characteristics of vascular cell adhesion molecule-1 levels in patients with peripheral artery disease. Heart Vessels 34(6), 976–983. https://doi.org/10.1007/s00380-018-1315-1 (2019).
Khawaja, F. J. & Kullo, I. J. Novel markers of peripheral arterial disease. Vasc. Med. 14(4), 381–392. https://doi.org/10.1177/1358863X09106869 (2009).
Shirai, R. et al. Neopterin counters vascular inflammation and atherosclerosis. J. Am. Heart Assoc. 7(3), e007359. https://doi.org/10.1161/JAHA.117.007359 (2018).
Manfredini, F. et al. Biomarkers of muscle metabolism in peripheral artery disease: A dynamic NIRS-assisted study to detect adaptations following revascularization and exercise training. Diagnostics 10(5), 312. https://doi.org/10.3390/diagnostics10050312 (2020).
Tzoulaki, I. et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh artery study. Circulation 112, 976–983. https://doi.org/10.1161/CIRCULATIONAHA.104.513085 (2005).
Hoogeveen, R. C. et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis risk in communities study. Atherosclerosis 183, 301–307. https://doi.org/10.1016/j.atherosclerosis.2005.03.007 (2005).
Cassar, K., Bachoo, P., Ford, I., Greaves, M. & Brittenden, J. Markers of coagulation activation, endothelial stimulation and inflammation in patients with peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 29, 171–176. https://doi.org/10.1016/j.ejvs.2004.11.001 (2005).
Tzoulaki, I. et al. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur. Heart J. 28, 354–362. https://doi.org/10.1093/eurheartj/ehl441 (2007).
Rahman, I. & Adcock, I. M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 28, 219–242. https://doi.org/10.1183/09031936.06.00053805 (2006).
Griendling, K. K., Ushio-Fukai, M., Lassegue, B. & Alexander, R. W. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 29, 366–373. https://doi.org/10.1161/01.HYP.29.1.366 (1997).
Kranzhofer, R. et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1623–1629. https://doi.org/10.1161/01.ATV.19.7.1623 (1999).
Signorelli, S. S., Fiore, V. & Malaponte, G. Inflammation and peripheral arterial disease: The value of circulating biomarkers. Int. J. Mol. Med. 33(4), 777–783. https://doi.org/10.3892/ijmm.2014.1657 (2014).
Fu, Z., Gilbert, E. R. & Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 9(1), 25–53. https://doi.org/10.2174/1573399811309010025 (2013).
World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy (World Health Organization, 2010).
Zhang, H., Wang, Z., Wang, F., Wang, C. & Zhang, H. IL-6 and IL-8 are involved in JMJD2A-regulated malignancy of ovarian cancer cells. Arch. Biochem. Biophys. 684, 108334. https://doi.org/10.1016/j.abb.2020.108334 (2020).
Jing, S. H., Gao, X., Yu, B. & Qiao, H. Raf kinase inhibitor protein (RKIP) inhibits tumor necrosis factor-α (TNF-α) induced adhesion molecules expression in vascular smooth muscle bells by suppressing (nuclear transcription factor-κB (NF-kappaB) pathway. Med. Sci. Monit. 23, 4789–4797. https://doi.org/10.12659/msm.903661 (2017).
Burtis, C. A., Ashwood, E. R. & Tietz, N. W. Tietz Textbook of Clinical Chemistry (Saunders, 1999).
Zhang, L. Progress in Molecular Biology and Translational Science: Glycans and Glycosaminoglycans as Clinical Biomarkers and Therapeutics-Part B (Academic Press, 2019).
Rizzo, F., Papasouliotis, K., Crawford, E., Dodkin, S. & Cue, S. Measurement of prothrombin time (PT) and activated partial thromboplastin time (APTT) on canine citrated plasma samples following different storage conditions. Res. Vet. Sci. 85(1), 166–170. https://doi.org/10.1016/j.rvsc.2007.10.001 (2008).
Thangamuthu, M., Santschi, C. & Martin, O. J. F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors 8(2), 34. https://doi.org/10.3390/bios8020034 (2018).
Abdulazeez, N. et al. Effects of Moringa oleifera aqueous leaf extract on some serum-biochemical constituents on Wister rats. Am. J. Life Sci. 8(5), 96–101. https://doi.org/10.11648/j.ajls.20200805.12 (2020).
Liu, W. S. et al. Serum creatinine determined by Jaffe, enzymatic method, and isotope dilution-liquid chromatography-mass spectrometry in patients under hemodialysis. J. Clin. Lab. Anal. 26(3), 206–214. https://doi.org/10.1002/jcla.21495 (2012).
Chamera, T. et al. Post-effort changes in activity of traditional diagnostic enzymatic markers in football players’ blood. J. Med. Biochem. 34(2), 179–190. https://doi.org/10.2478/jomb-2014-0035 (2015).
Robinet, P., Wang, Z., Hazen, S. L. & Smith, J. D. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J. Lipid. Res. 51(11), 3364–3369. https://doi.org/10.1194/jlr.D007336 (2010).
Penumarthy, S., Penmetsa, G. S. & Mannem, S. Assessment of serum levels of triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol in periodontitis patients. J. Indian Soc. Periodontol. 17(1), 30–35. https://doi.org/10.4103/0972-124X.107471 (2013).
Fawwad, A., Sabir, R., Riaz, M., Moin, H. & Basit, A. Measured versus calculated LDL-cholesterol in subjects with type 2 diabetes. Pak. J. Med. Sci. 32(4), 955–960. https://doi.org/10.12669/pjms.324.9896 (2016).
Paneni, F., Costantino, S. & Cosentino, F. Insulin resistance, diabetes, and cardiovascular risk. Curr. Atheroscler. Rep. 16(7), 419. https://doi.org/10.1007/s11883-014-0419-z (2014).
Wolf, J., Rose-John, S. & Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 70(1), 11–20. https://doi.org/10.1016/j.cyto.2014.05.024 (2014).
Signorelli, S., Marino, E. & Scuto, S. Inflammation and peripheral arterial disease. J 2(2), 142–151. https://doi.org/10.3390/j2020012 (2019).
McDermott, M. M. et al. Relation of interleukin-6 and vascular cellular adhesion molecule-1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am. J. Cardiol. 107(9), 1392–1398. https://doi.org/10.1016/j.amjcard.2011.01.007 (2011).
Akira, S., Taga, T. & Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 54, 1–78. https://doi.org/10.1016/S0065-2776(08)60532-5 (1993).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 21, 331–344. https://doi.org/10.1016/j.cytogfr.2010.09.002 (2010).
Wondmkun, Y. T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 13, 3611–3616. https://doi.org/10.2147/DMSO.S275898 (2020).
Williams, N., Cundy, P. J. & Eastwod, D. M. Surgical management of thoracolumbar kyphosis in patients with mucopolysaccharidosis: A systematic review. Spine 42(23), 1817–1825. https://doi.org/10.1097/BRS.0000000000002242 (2017).
Marino, F. et al. Angiotensin type 1 receptor expression and interleukin-8 production in polymorphonuclear leukocytes of patients with peripheral arterial disease. J. Cardiovasc. Pharmacol. 54(6), 520–525. https://doi.org/10.1097/FJC.0b013e3181bfadfd (2009).
Al-Rubeaan, K., Nawaz, S. S., Youssef, A. M., Al Ghonaim, M. & Siddiqui, K. IL-18, VCAM-1 and P-selectin as early biomarkers in normoalbuminuric Type 2 diabetes patients. Biomark. Med. 13(6), 467–478. https://doi.org/10.2217/bmm-2018-0359 (2019).
Ceasovschih, A. et al. Biomarker utility for peripheral artery disease diagnosis in real clinical practice: A prospective study. Diagnostics 10(9), 723. https://doi.org/10.3390/diagnostics10090723 (2020).
Wautier, J. L. & Wautier, M. P. Blood cells and vascular cell interactions in diabetes. Clin. Hemorheol. Microcirc. 25(2), 49–53 (2001).
Blüher, M., Unger, R., Rassoul, F., Richter, V. & Paschke, R. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 45(2), 210–216. https://doi.org/10.1007/s00125-001-0723-3 (2002).
Ruszkowska-Ciastek, B. et al. Effect of uncontrolled hyperglycemia on levels of adhesion molecules in patients with diabetes mellitus type 2. J. Zhejiang Univ. Sci. B 16(5), 355–361. https://doi.org/10.1631/jzus.B1400218 (2015).
Karimi, Z. et al. Intercellular adhesion molecule-1 in diabetic patients with and without microalbuminuria. Diabetes Metab. Syndr. 12(3), 365–368. https://doi.org/10.1016/j.dsx.2017.12.028 (2018).
Hou, T. et al. Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev. 4(3), 179–192. https://doi.org/10.2174/157340308785160570 (2008).
Dabla, P. K. Renal function in diabetic nephropathy. World J. Diabetes 1(2), 48–56. https://doi.org/10.4239/wjd.v1.i2.48 (2010).
Mlekusch, W. et al. Serum creatinine predicts mortality in patients with peripheral artery disease: Influence of diabetes and hypertension. Atherosclerosis 175(2), 361–367. https://doi.org/10.1016/j.atherosclerosis.2004.04.008 (2004).
Gary, T. et al. Elevated blood urea nitrogen is associated with critical limb ischemia in peripheral arterial disease patients. Medicine 94(24), e948. https://doi.org/10.1097/MD.0000000000000948 (2015).
Saenz-Pipaon, G. et al. The role of circulating biomarkers in peripheral arterial disease. Int. J. Mol. Sci. 22(7), 3601. https://doi.org/10.3390/ijms22073601 (2021).
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: A critical update. J. Clin. Invest. 111(12), 1805–1812. https://doi.org/10.1172/JCI18921 (2003).
Calabro, P., Chang, D. W., Willerson, J. T. & Yeh, E. T. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: Linking obesity to vascular inflammation. J. Am. Coll. Cardiol. 46(6), 1112–1113. https://doi.org/10.1016/j.jacc.2005.06.017 (2005).
Koenig, W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int. J. Cardiol. 168(6), 5126–5134. https://doi.org/10.1016/j.ijcard.2013.07.113 (2013).
Badimon, L. et al. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 9, 430. https://doi.org/10.3389/fimmu.2018.00430 (2018).
Shatnawi, N. J. et al. The relation of anatomical distribution of symptomatic peripheral arterial disease (PAD) with HbA1c level in patients with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 12, 20420188211000504. https://doi.org/10.1177/20420188211000504 (2021).
Arya, S. et al. High hemoglobin A1c associated with increased adverse limb events in peripheral arterial disease patients undergoing revascularization. J. Vasc. Surg. 67(1), 217-228.e1. https://doi.org/10.1016/j.jvs.2017.06.101 (2018).
Yang, C. P. et al. Fasting plasma glucose variability and HbA1c are associated with peripheral artery disease risk in type 2 diabetes. Cardiovasc. Diabetol. 19(1), 4. https://doi.org/10.1186/s12933-019-0978-y (2020).
Bembde, A. S. A study of plasma fibrinogen level in type-2 diabetes mellitus and its relation to glycemic control. Indian J. Hematol. Blood Transfus. 28(2), 105–108. https://doi.org/10.1007/s12288-011-0116-9 (2012).
Agarwal, C. et al. Association of coagulation profile with microvascular complications and glycemic control in type 2 diabetes mellitus-a study at a tertiary care center in Delhi. Hematol. Transfus. Cell Ther. 41(1), 31–36. https://doi.org/10.1016/j.htct.2018.05.002 (2019).
Mandal, A. et al. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus 10(11), e3626. https://doi.org/10.7759/cureus.3626 (2018).
Bhowmik, B. et al. Serum lipid profile and its association with diabetes and prediabetes in a rural Bangladeshi population. Int. J. Environ. Res. Public Health 15(9), 1944. https://doi.org/10.3390/ijerph15091944 (2018).
Frank, U. et al. ESVM guideline on peripheral arterial disease. Vasa 48(Suppl. 102), 1–79. https://doi.org/10.1024/0301-1526/a000834 (2019).
Olinic, D. M. et al. Epidemiology of peripheral artery disease in Europe: VAS educational paper. Int. Angiol. 37, 327–334. https://doi.org/10.23736/s0392-9590.18.03996-2 (2018).
Achim, A. et al. Approaches to peripheral artery disease in diabetes: Are there any differences?. Int. J. Environ. Res. Public Health 19(16), 9801. https://doi.org/10.3390/ijerph19169801 (2022).
Acknowledgements
The authors would like to extend their sincere appreciation to the researchers supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the Project Number (RSP2024R457).
Funding
The authors would like to extend their sincere appreciation to the researchers supporting the Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the Project Number (RSP2024R457).
Author information
Authors and Affiliations
Contributions
S.Z.: conceptualization: formal analysis: investigation: resources: data curation: writing—review and editing: visualization: supervision; S.A.: methodology: formal analysis: investigation: writing—original draft; I.K.: formal analysis: investigation: resources: writing—review and editing: visualization; Y.A.B.J.: resources: data curation: writing—review and editing: visualization: funding acquisition; G.F.W.: resources: writing—review and editing: visualization: All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaib, S., Ahmad, S., Khan, I. et al. An evaluation of inflammatory and endothelial dysfunction markers as determinants of peripheral arterial disease in those with diabetes mellitus. Sci Rep 14, 15348 (2024). https://doi.org/10.1038/s41598-024-65188-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-65188-w
Keywords
This article is cited by
-
Mechanisms and clinical translation of ICOS/ICOSL signaling pathway blockade in delaying vascular complications of type 2 diabetes
Diabetology & Metabolic Syndrome (2025)