Abstract
A novel palladium-loaded yolk-shell structured nanomaterial with magnetite core and phenylene-based periodic mesoporous organosilica (PMO) shell (Fe3O4@YS-Ph-PMO/Pd) nanocatalyst was synthesized for the reduction of nitrobenzenes. The Fe3O4@YS-Ph-PMO/Pd was prepared through cetyltrimethylammonium bromide (CTAB) directed condensation of 1,4-bis(triethoxysilyl)benzene (BTEB) around Fe3O4@silica nanoparticles followed by treatment with palladium acetate. This nanocatalyst was characterized by using Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA), low-angle and wide-angle powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and vibrating sample magnetometer (VSM) analyses. These analyses showed a magnetic nanomaterial with high chemical and thermal stability for the designed composite. The Fe3O4@YS-Ph-PMO/Pd nanocomposite was employed as a powerful and highly recoverable catalyst in the green reduction of nitroarenes in H2O at room temperature. A variety of nitroarene derivatives were applied as substrate in the presence of 0.9 mol% of Fe3O4@YS-Ph-PMO/Pd catalyst. All nitroarenes were selectively converted to their corresponding amines with high to excellent yields (92–96%) within short reaction times (10–18 min). This catalyst was recovered and reused at least 11 times without significant decrease in efficiency and stability.
Similar content being viewed by others
Introduction
In the recent years, the yolk-shell (YS) structured nanomaterials have demonstrated a new type of structures containing a void between core and shell. These materials have received increasing attention owing to tunable their physiochemical properties and also high capability in the adsorption and catalytic processes1,2,3,4,5,6,7,8,9. These nanomaterials have a lot of application in areas of data storage, catalysis and environmental remediation5,6,10,11,12. Due to the aforementioned notes and also widespread applications of YSs in green chemistry, various methods such as Kirkendall, etching, ship in bottle and Ostwald have been employed for the synthesis of these materials13,14,15,16. Among these, selective etching is very interested in between researchers17,18. YSs with magnetite core and PMO shell (Mag@YS-PMOs) have attracted much research attention because of their combined properties of YSs, magnetic materials and ordered mesoporous structures12,19,20,21,22. The Mag@YS-PMOs have the advantages of magnetic nanoparticles such as easy magnetic separation, high dispersion ability in aqueous media as well as high chemical reactivity and stability. In addition, these have also the advantages of PMOs such as highly-ordered mesostructure, excellent loading of uniformly distributed organic functions in their framework and high moisture stability12,19,23. These characteristics make Mag@YS-PMOs as promising applicant for supporting metals in different chemical processes5,6,24,25.
On the other hand, aromatic amines are very important in herbicide, dye, agrochemical and pesticide industries26,27,28. In addition, they are substrates for different intermediates such as diazonium salts, isocyanate, azo and amide molecules29,30. Recently, sodium borohydride, NaBH4, has been suggested as a new fuel source for supplying hydride ions to reduce nitroarene compounds to the corresponding amines under mild conditions. However, NaBH4 exhibits limited capability in the absence of additives31,32,33,34,35. Therefore, this reagent is usually used in the presence of metallic catalysts. Another important and conventional method for preparing the aromatic amines is the reduction of corresponding nitroarenes via catalytic hydrogenation36,37. The most metallic complexes and metallic nanoparticles applied for catalytic reduction of nitroarenes are based on Rh, Ru, Pt, Pd, Au, Cu, Ir and Ni38,39,40. Some of recently developed catalytic systems in this matter are Fe3O4/Ni MNPs41, Rh–Fe3O4 heterodimer42, Cu/Fe2O4–G43, graphene-Fe3O444, Au–GO45, Fe3O4@GO46, Ag@Ni47 and NiFe2O4@Cu48. However, the homogeneous protocols for reduction of nitroarenes have different disadvantages such as separation of catalyst and product. Therefore, it is still necessary to design an effective catalytic system resolving these issues.
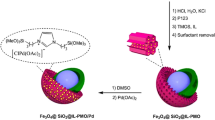
According to the advantages of Mag@YS-PMO-based nanocatalysts as well as the importance of reduction of nitroarenes in green chemistry, in continuation of our previous works49,50,51, herein, a YS nanomaterial with magnetite core and PMO shell-supported palladium species (Fe3O4@YS-Ph-PMO/Pd) is synthesized through sol–gel mediated hydrolysis and co-condensation of 1,4-bis(triethoxysilyl)benzene (BTEB) around Fe3O4@SiO2 cores using CTAB surfactant followed by treatment with palladium acetate (Fig. 1). Furthermore, the physiochemical properties of the synthesized Fe3O4@YS-Ph-PMO/Pd nanocatalyst were studied by using FT-IR, TGA, PXRD, SEM, TEM and VSM techniques. After characterization, the catalytic efficiency of this magnetic nanomaterial was investigated in the green reduction of nitroarenes under moderate conditions.
Experimental
General
All chemicals and reagents such as iron (II) chloride tetrahydrate, iron (III) chloride hexahydrate, ammonia, 1,4-bis(triethoxysilyl)benzene, NaBH4, cetyltrimethylammonium bromide (CTAB) and all applied nitroarenes and alcohols were purchased from Fluka, Merck and Aldrich companies. Solvents were dried and purified following standard procedures. The characterization of the materials was conducted using instruments previously reported52,53. The purity determination of the products and reaction monitoring were carried out by using TLC on silica gel polygram SILG/UV 254 plates.
Preparation of Fe3O4@YS-Ph-PMO
For the preparation of Fe3O4@YS-Ph-PMO nanomaterial, firstly, Fe3O4 and silica coated magnetite nanoparticles (Fe3O4@SiO2) were synthesized according to known procedures23. Then, 100 mg of Fe3O4@SiO2 microspheres were added into a flask containing EtOH (60 mL) and H2O (80 mL). This mixture was homogenized for 25 min under ultrasound irradiations. Next, CTAB (140 mg) and NH3 (25%, 2 mL) were added while stirring at RT for 30 min. After that, BTEB (0.3 mL) was added and it was further stirred at RT for 2 h. This mixture was heated statically at 100 °C for 24 h. The obtained Fe3O4@SiO2@CTAB@Ph-PMO product was dispersed in a basic solution (H2O:Na2CO3, 80:4). The resulting mixture was heated to 50 °C for 4 h to eliminate the SiO2 shell. The CTAB surfactant was successfully eliminated by refluxing of as-made material in an acidic EtOH solution (EtOH:HCl 100:2). The final product was dried at 70 °C for 12 h and denoted as Fe3O4@YS-Ph-PMO.
Preparation of Fe3O4@YS-Ph-PMO/Pd
For this, Fe3O4@YS-Ph-PMO (0.4 g) was added to an aqueous solution of palladium acetate (0.05 M, 14 mL). Then, this mixture was stirred at RT for 24 h. The product was magnetically collected, washed completely with H2O, dried at 70 °C for 7 h and denoted as Fe3O4@YS-Ph-PMO/Pd.
Procedure for the reduction of nitrobenzenes
For this, nitrobenzene (1 mmol), Fe3O4@YS-Ph-PMO/Pd (0.9 mol %) and H2O (10 mL) were added into a reaction vessel. Then, an aqueous solution of NaBH4 (3 mmol) was added while stirring at RT. After completion of reaction, the catalyst was magnetically separated and the amine product was obtained after extraction with EtOAc and evaporation of solvent. The IR and NMR spectra of prepared aminobenzenes are available in the supporting information.
Results and discussion
The preparation of Fe3O4@YS-Ph-PMO/Pd nanocatayst with yolk-shell structure is shown in Fig. 1. At first, the magnetic silica (Fe3O4@SiO2) was prepared by chemical modification of Fe3O4 nanoparticles with tetramethoxysilane (TMOS). Then, 1,4-bis(triethoxysilyl)benzene (BTEB) was hydrolyzed and co-condensed on the Fe3O4@SiO2 spheres in the presence of CTAB surfactant in a basic ammonia–water–ethanol solution through sol–gel process. Then, the silica layer was removed in an aqueous solution of Na2CO3 through an etching process. After that, the CTAB surfactant was removed by a Soxhlet apparatus to give a yolk-shell structured material called Fe3O4@YS-Ph-PMO. The resulting material was then treated with a sub-stoichiometric amount of Pd(OAc)2 in water to produce the Fe3O4@YS-Ph-PMO/Pd nanocatalyst.
The Fourier transform infrared (FT-IR) spectra of prepared materials are shown in Fig. 2. For all samples, the Fe–O bond is cleared at 582 cm−1. The band about 3552 cm−1 is due to the O–H bonds of material surface. For Fe3O4@SiO2, Fe3O4@SiO2@CTAB@YS-Ph-PMO and Fe3O4@YS-Ph-PMO/Pd, the peaks at 1122 and 925 cm−1 are attributed to Si–O-Si bonds. The bands at 2924 and 2844 cm−1 are related to the C–H vibrations of CTAB surfactant (Fig. 2C). Interestingly, for Fe3O4@YS-Ph-PMO/Pd, the latter peaks are eliminated (Fig. 2D), indicating the successful removal of CTAB surfactant during extraction process. For Fe3O4@SiO2@CTAB@YS-Ph-PMO and Fe3O4@YS-Ph-PMO/Pd, the peaks observed at 3100 and 1620 cm−1 are, respectively, correspond to C–H and C=C vibrations of phenyl rings. These confirm the successful formation of Ph-PMO shell on magnetite NPs. Notably, the Fe–O absorption peaks of Fe3O4 and Fe3O4@SiO2 exhibited a slight red shift compared to Fe3O4@SiO2@CTAB@Ph-PMO and Fe3O4@YS-Ph-PMO/Pd nanomaterials. This shift is in line with the Bouguer-Beer-Lambert (BBL) law, where spectral positions correspond to sample thickness and absorbing entity concentration. The presence of silica, CTAB, and PMO layers on Fe3O4 nanoparticles contributes to this red shift, indicating a modified chemical environment surrounding the nanoparticles54,55,56. Moreover, an increase in layer thickness enhances this effect57,58,59.
Figure 3 shows the wide-angle powder X-ray diffraction (PXRD) patterns of Fe3O4, Fe3O4@SiO2, Fe3O4@YS-Ph-PMO and Fe3O4@YS-Ph-PMO/Pd nanomaterials. The PXRD patterns of all the materials showed six sharp peaks at 2θ of 30.38, 35.65, 43.34, 53.9, 57.40 and 62.98°, corresponding to the Miller indices of 220, 311, 400, 422, 511, and 440, respectively, which is completely matched with that of the Fe3O4 standard sample (JCPDS file No. 19-0629)60. This finding proves that the crystalline structure of the magnetic iron oxide NPs is preserved during the modification processes. Additionally, a continuous decrease in the intensity of Fe3O4 peaks was observed, indicating the successful modification of magnetic iron oxide with the organic and inorganic species. After coating with silica, a new and broad peak at 2θ = 20–25 appeared, which is attributed to the presence of amorphous silica (Fig. 3B). This peak disappeared in both Fe3O4@YS-Ph-PMO and Fe3O4@YS-Ph-PMO/Pd (Fig. 3C and D), confirming the successful removal of the silica layer through the etching process.
The low-angle powder X-ray diffraction (PXRD) pattern of the Fe3O4@YS-Ph-PMO/Pd is shown in Fig. 4. This illustrates a broad peak at 2θ = 2.2°, which is characteristic of ordered mesoporous structures. This pattern confirm well formation of Ph-PMO shell on magnetite NPs.
Thermal stability of Fe3O4@YS-Ph-PMO/Pd was studied by using thermal gravimetric analysis (TGA) (Fig. 5). This showed a low weight loss at temperature below 100 °C, corresponding to removal of adsorbed solvents. The second weight loss observed between 100 to 450 °C is related to the elimination of the remaining CTAB surfactant. The main weight loss, cleared at 451–620 °C, is corresponded to the removal of organic (phenylene) moieties incorporated in the mesoporous shell. These data successfully prove the high thermal stability of the designed nanocatalyst.
The magnetic properties of materials were investigated by vibrating sample magnetometer (VSM) (Fig. 6). The saturated magnetization values of the Fe3O4 (Fig. 6A), Fe3O4@SiO2 (Fig. 6B) and Fe3O4@YS-Ph-PMO/Pd (Fig. 6C) were found to be 65, 30 and 14 emu g−1, respectively. The decrease in magnetic properties following the modification processes is attributed to the well coating of SiO2 and PMO shells around Fe3O4 NPs. The magnetic separation capability of the Fe3O4@YS-Ph-PMO/Pd nanocomposite was also evaluated by introducing an external magnet near the reaction vessel, as depicted in Fig. 6b. As demonstrated, these nanomaterials can be efficiently collected by using an external magnet within seconds, confirming the high magnetic properties of the designed catalyst, rendering it readily recoverable.
The scanning electron microscopy (SEM) was performed to study the morphology of the particles at different steps of nanocatalyst preparation. According to this analysis, a uniform spherical morphology was observed for all prepared nanomaterials with an increase in size observed at each step (Fig. 7). The progressive increase in particle size after each step confirms the successful formation of the shell and modification of magnetite nanoparticles, as outlined in Fig. 1.
The TEM image of the Fe3O4@YS-Ph-PMO/Pd nanocatalyst showed spherical particles with a black core (magnetite NPs) and a grey shell (PMO layer) for the designed nanocomposite (Fig. 8). Notably, recently, the similar TEM images have been reported for a number of yolk-shell structured magnetic nanocomposites61,62.
The Fe3O4@YS-Ph-PMO/Pd nanocatalyst was then employed in the reduction of nitroarenes in the presence of NaBH4 (Table 1). The effect of different parameters was studied in the reduction of nitrobenzene as a reaction model. As shown, in the absence of catalyst, along with 3 mmol of NaBH4, no reduction was observed (Table 1, entry 1). However, after addition of the catalyst, the reaction proceeded successfully, and the highest yield achieved using 0.9 mol% of the Fe3O4@YS-Ph-PMO/Pd (Table 1, entry 3). Next, the effect of different solvents including methanol, ethanol and water was tested. As depicted in Table 1, the use of MeOH, EtOH, aqueous methanol or ethanol, as well as solvent-free media, resulted in low to moderate yields (Table 1, entries 5–9). Pleasantly, in aqueous media at RT, the reaction was completed and excellent yield of aniline was obtained. To investigate the neat effect of Pd, the efficiency of Fe3O4@YS-Ph-PMO/Pd was compared with Fe3O4 and Fe3O4@YS-Ph-PMO nanomaterials. This demonstrated that when using Pd-free Fe3O4 and Fe3O4@Ph-PMO materials, the reaction did not progress, and only a trace conversion (< 4%) was observed (Table 1, entries 10 and 11). This confirms that the reduction process is catalyzed by supported palladium species. Based on the aforementioned results, NaBH4 (3 mmol), H2O solvent (3 mL), Fe3O4@YS-Ph-PMO/Pd catalyst (0.9 mol %) and RT were chosen as the optimum conditions (Table 1, entry 3).
The efficiency and the scope of the Fe3O4@YS-Ph-PMO/Pd nanocatalyst were next studied using a variety of nitrobenzenes (Table 2). As shown, all nitroarenes deliver corresponding anilines in high yields. Importantly, in all cases, it was found that the aniline derivatives were the only products of the reactions. These results demonstrate high selectivity of the designed catalytic system.
Recoverability and reusability of the Fe3O4@YS-Ph-PMO/Pd nanocatalyst in the reduction of nitrobenzene were also examined. For this, after completion of the reaction, the Fe3O4@YS-Ph-PMO/Pd catalyst was magnetically separated, washed and reused in the next run. It was found that the Fe3O4@YS-Ph-PMO/Pd catalyst can be recovered and reused for at least 11 times with no important decrease in efficiency (Fig. 9).
A leaching experiment was then accomplished on the model reaction to investigate the leaching behavior of the palladium species under applied conditions. For this, after a conversion of about 40%, the Fe3O4@YS-Ph-PMO/Pd catalyst was magnetically separated and the residue mixture was allowed to progress under the optimized conditions. Notably, after 140 min, no further product was observed indicating that the removal of the catalyst resulted in a complete stop of the conversion of nitrobenzene to aniline (Fig. 10). These results confirm no leaching of palladium species and also demonstrate the high stability of the designed catalyst under applied conditions.
The catalytic performance of Fe3O4@YS-Ph-PMO/Pd was also compared with former heterogeneous catalysts in the reduction of nitrobenzene (Table 3). The result showed that the efficiency of Fe3O4@YS-Ph-PMO/Pd is much better than previous catalytic systems, particularly in terms of reaction temperature and recovery times.
Conclusion
In this work, the preparation, characterization and application of a new nanocatalyst named Fe3O4@YS-Ph-PMO/Pd were developed. The FT-IR and TG analyses clearly demonstrated high stability and well incorporation/immobilization of expected organic and inorganic moieties onto/into prepared nanomaterial. The SEM and TEM images showed a spherical morphology for the designed catalyst. The VSM analysis confirmed well-magnetic properties of the catalyst. The wide-angle PXRD analysis confirmed high stability of crystalline structure of magnetite NPs during catalyst preparation steps. The Fe3O4@YS-Ph-PMO/Pd was powerfully used in the reduction of nitrobenzenes giving corresponding anilines in high yield and selectivity. The leaching and recovery experiments confirmed that the designed catalyst operate in a heterogeneous manner. Other advantages of this methodology were the use of water as green solvent, performing reactions at RT, short reaction times, clean conditions, as well as high recoverability, durability and stability of the designed catalyst. Some applications of this catalytic system in other organic processes are underway in our laboratory.
Data availability
All data and materials are included in the manuscript.
References
Zhao, Y. & Jiang, L. Hollow micro/nanomaterials with multilevel interior structures. Adv. Mater. 21, 3621–3638 (2009).
Lou, X. W. D., Archer, L. A. & Yang, Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater. 20, 3987–4019 (2008).
Liu, J., Qiao, S. Z., Hu, Q. H. & Lu, G. Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 7, 425–443 (2011).
An, K. & Hyeon, T. Synthesis and biomedical applications of hollow nanostructures. Nano Today 4, 359–373 (2009).
Liu, J. et al. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 47, 12578–12591 (2011).
Zou, H. et al. An organosilane-directed growth-induced etching strategy for preparing hollow/yolk–shell mesoporous organosilica nanospheres with perpendicular mesochannels and amphiphilic frameworks. J. Mater. Chem. A 2, 12403–12412 (2014).
Mirbagheri, R., Elhamifar, D. & Shaker, M. Yolk–shell structured magnetic mesoporous silica: A novel and highly efficient adsorbent for removal of methylene blue. Sci. Rep. 11, 23259. https://doi.org/10.1038/s41598-021-02699-w (2021).
Neysi, M. & Elhamifar, D. Yolk-shell structured magnetic mesoporous organosilica supported ionic liquid/Cu complex: An efficient nanocatalyst for the green synthesis of pyranopyrazoles. Front. Chem. 11, 125 (2023).
Peng, H. et al. Multifunctional yolk-shell structured magnetic mesoporous polydopamine/carbon microspheres for photothermal therapy and heterogenous catalysis. ACS Appl. Mater. Interfaces 14, 23888–23895 (2022).
Liu, J., Bai, S., Zhong, H., Li, C. & Yang, Q. Tunable assembly of organosilica hollow nanospheres. J. Phys. Chem. C 114, 953–961 (2009).
Liu, J. et al. Organic−inorganic hybrid hollow nanospheres with microwindows on the shell. Chem. Mater. 20, 4268–4275 (2008).
Wei, Y. et al. A versatile in situ etching-growth strategy for synthesis of yolk–shell structured periodic mesoporous organosilica nanocomposites. RSC Adv. 6, 51470–51479 (2016).
Song, J.-C., Xue, F.-F., Zhang, X.-X., Lu, Z.-Y. & Sun, Z.-Y. Synthesis of yolk–shell mesoporous silica nanoparticles via a facile one-pot approach. Chem. Commun. 53, 3761–3764 (2017).
Liu, B. & Zeng, H. C. Symmetric and asymmetric Ostwald ripening in the fabrication of homogeneous core–shell semiconductors. Small 1, 566–571 (2005).
Ding, S. et al. Formation of SnO2 hollow nanospheres inside mesoporous silica nanoreactors. J. Am. Chem. Soc. 133, 21–23 (2010).
Yin, Y. et al. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004).
Chen, D., Li, L., Tang, F. & Qi, S. Facile and scalable synthesis of tailored silica “nanorattle” structures. Adv. Mater. 21, 3804–3807 (2009).
Zhu, Y., Kockrick, E., Ikoma, T., Hanagata, N. & Kaskel, S. An efficient route to rattle-type Fe3O4@ SiO2 hollow mesoporous spheres using colloidal carbon spheres templates. Chem. Mater. 21, 2547–2553 (2009).
Yang, Y., Liu, J., Li, X., Liu, X. & Yang, Q. Organosilane-assisted transformation from core–shell to yolk–shell nanocomposites. Chem. Mater. 23, 3676–3684 (2011).
Wang, X., Feng, J., Bai, Y., Zhang, Q. & Yin, Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 116, 10983–11060 (2016).
Purbia, R. & Paria, S. Yolk/shell nanoparticles: Classifications, synthesis, properties, and applications. Nanoscale 7, 19789–19873 (2015).
Yang, L., Guo, H., Wang, L. & Zhang, J. A facile “polystyrene-dissolving” strategy to hollow periodic mesoporous organosilica with flexible structure-tailorability. Micropor. Mesopor. Mat 239, 173–179 (2017).
Dai, J. et al. Yolk–shell Fe 3 O 4@ SiO 2@ PMO: Amphiphilic magnetic nanocomposites as an adsorbent and a catalyst with high efficiency and recyclability. Green Chem. 19, 1336–1344 (2017).
Wang, H. et al. Preparation of highly dispersed W/Al2O3 hydrodesulfurization catalysts via a microwave hydrothermal method: Effect of oxalic acid. Arab. J. Chem. 9, 18–24. https://doi.org/10.1016/j.arabjc.2014.11.023 (2016).
Liu, J., Qiao, S. Z., Budi-Hartono, S. & Lu, G. Q. M. Monodisperse yolk–shell nanoparticles with a hierarchical porous structure for delivery vehicles and nanoreactors. Angew. Chem. 122, 5101–5105 (2010).
Pandarus, V., Ciriminna, R., Beland, F. & Pagliaro, M. A new class of heterogeneous platinum catalysts for the chemoselective hydrogenation of nitroarenes. Adv. Synth. Catal. 353, 1306–1316 (2011).
Mikami, Y. et al. Highly chemoselective reduction of nitroaromatic compounds using a hydrotalcite-supported silver-nanoparticle catalyst under a CO atmosphere. Chem. Lett. 39, 223–225 (2010).
Ono, N. The Nitro-Aldol (Henry) Reaction. The Nitro Group in Organic Synthesis 30 (Wiley-VCH, 2001).
Lawrence, S. A. Amines: Synthesis, Properties and Applications (Cambridge University Press, 2004).
Mohan, V. et al. Advantage of Ni/SBA-15 catalyst over Ni/MgO catalyst in terms of catalyst stability due to release of water during nitrobenzene hydrogenation to aniline. Catal. Commun. 18, 89–92 (2012).
Zengin, N., Göksu, H. & Şen, F. Chemoselective hydrogenation of aromatic nitro compounds in the presence of homogeneous Pd based catalysts. Chemosphere 282, 130887 (2021).
Loghmani, M. H. & Shojaei, A. F. Hydrogen production through hydrolysis of sodium borohydride: Oleic acid stabilized Co–La–Zr–B nanoparticle as a novel catalyst. Energy 68, 152–159 (2014).
Shil, A. K., Sharma, D., Guha, N. R. & Das, P. Solid supported Pd (0): An efficient recyclable heterogeneous catalyst for chemoselective reduction of nitroarenes. Etrahedron. Lett. 53, 4858–4861 (2012).
Fountoulaki, S. et al. Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal. 4, 3504–3511 (2014).
Schlesinger, H. et al. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1. J. Am. Chem. Soc. 75, 215–219 (1953).
Saavedra, J. Z. et al. Reaction of InCl3 with various reducing agents: InCl3–NaBH4-mediated reduction of aromatic and aliphatic nitriles to primary amines. J. Org. Chem. 77, 221–228 (2011).
Wang, P., Liu, H., Niu, J., Li, R. & Ma, J. Entangled Pd complexes over Fe 3 O 4@ SiO 2 as supported catalysts for hydrogenation and Suzuki reactions. Catal. Sci. Tech. 4, 1333–1339 (2014).
Lin, F.-H. & Doong, R.-A. Catalytic nanoreactors of Au@ Fe3O4 yolk-shell nanostructures with various au sizes for efficient nitroarene reduction. J. Phys. Chem. C 121, 7844–7853 (2017).
Lopes, R., Pereira, M. M. & Royo, B. Selective reduction of nitroarenes with silanes catalyzed by nickel N-heterocyclic carbene complexes. ChemCatChem 9, 3073–3077 (2017).
Kadam, H. K. & Tilve, S. G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 5, 83391–83407 (2015).
Gawande, M. B. et al. Regio-and chemoselective reduction of nitroarenes and carbonyl compounds over recyclable magnetic ferrite nickel nanoparticles (Fe3O4 Ni) by using glycerol as a hydrogen source. Chem.-A Eur. J. 18, 12628–12632 (2012).
Jang, Y. et al. Simple one-pot synthesis of Rh–Fe 3 O 4 heterodimer nanocrystals and their applications to a magnetically recyclable catalyst for efficient and selective reduction of nitroarenes and alkenes. Chem. Commun. 47, 3601–3603 (2011).
Zhang, H., Gao, S., Shang, N., Wang, C. & Wang, Z. Copper ferrite–graphene hybrid: A highly efficient magnetic catalyst for chemoselective reduction of nitroarenes. RSC Adv. 4, 31328–31332 (2014).
Feng, C., Zhang, H.-Y., Shang, N.-Z., Gao, S.-T. & Wang, C. Magnetic graphene nanocomposite as an efficient catalyst for hydrogenation of nitroarenes. Chin. Chem. Lett. 24, 539–541 (2013).
Choi, Y. et al. Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J. Mater. Chem. 21, 15431–15436 (2011).
He, G. et al. Fe 3 O 4@ graphene oxide composite: A magnetically separable and efficient catalyst for the reduction of nitroarenes. Mater. Res. Bull. 48, 1885–1890 (2013).
Gawande, M. B. et al. First application of core-shell Ag@ Ni magnetic nanocatalyst for transfer hydrogenation reactions of aromatic nitro and carbonyl compounds. RSC Adv. 3, 1050–1054 (2013).
Zeynizadeh, B., Mohammadzadeh, I., Shokri, Z. & Hosseini, S. A. Synthesis and characterization of NiFe 2 O 4@ Cu nanoparticles as a magnetically recoverable catalyst for reduction of nitroarenes to arylamines with NaBH 4. J. Colloid Interface Sci. 500, 285–293 (2017).
Mirbagheri, R., Elhamifar, D. & Hajati, S. Ru-containing magnetic yolk–shell structured nanocomposite: A powerful, recoverable and highly durable nanocatalyst. RSC Adv. 11, 10243–10252 (2021).
Norouzi, M. & Elhamifar, D. Magnetic yolk-shell structured methylene and propylamine based mesoporous organosilica nanocomposite: A highly recoverable and durable nanocatalyst with improved efficiency. Colloids Surfaces A: Physicochem. Eng. Aspects 615, 126226 (2021).
Haydari, Z., Elhamifar, D., Shaker, M. & Norouzi, M. Magnetic nanoporous MCM-41 supported melamine: A powerful nanocatalyst for synthesis of biologically active 2-amino-3-cyanopyridines. Appl. Surface Sci. Adv. 5, 100096 (2021).
Elhamifar, D., Ramazani, Z., Norouzi, M. & Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: A novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo[b]pyrans under ultrasonic conditions. J. Colloid Interface Sci. 511, 392–401. https://doi.org/10.1016/j.jcis.2017.10.013 (2018).
Norouzi, M., Elhamifar, D., Mirbagheri, R. & Ramazani, Z. Synthesis, characterization and catalytic application of a novel ethyl and boron sulfonic acid based bifunctional periodic mesoporous organosilica. J. Taiwan Inst. Chem. Eng. 89, 234–244. https://doi.org/10.1016/j.jtice.2018.05.011 (2018).
Asadbegi, S., Bodaghifard, M. A. & Mobinikhaledi, A. Poly N, N-dimethylaniline-formaldehyde supported on silica-coated magnetic nanoparticles: A novel and retrievable catalyst for green synthesis of 2-amino-3-cyanopyridines. Res. Chem. Intermed. 46, 1629–1643. https://doi.org/10.1007/s11164-017-3200-4 (2020).
Akhavan, M., Foroughifar, N., Pasdar, H., Khajeh-Amiri, A. & Bekhradnia, A. Copper(II)-complex functionalized magnetite nanoparticles: A highly efficient heterogeneous nanocatalyst for the synthesis of 5-arylidenthiazolidine-2,4-diones and 5-arylidene-2-thioxothiazolidin-4-one. Trans. Metal Chem. 42, 543–552. https://doi.org/10.1007/s11243-017-0159-3 (2017).
Neysi, M., Zarnegaryan, A. & Elhamifar, D. Core–shell structured magnetic silica supported propylamine/molybdate complexes: An efficient and magnetically recoverable nanocatalyst. New J. Chem. 43, 12283–12291. https://doi.org/10.1039/C9NJ01160A (2019).
Mayerhöfer, T. G., Pahlow, S., Hübner, U. & Popp, J. R. CaF2: An ideal substrate material for infrared spectroscopy?. Anal. Chem. 92, 9024–9031 (2020).
Mayerhöfer, T. G. & Popp, J. The electric field standing wave effect in infrared transflection spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 191, 283–289 (2018).
Mayerhöfer, T. G., Pahlow, S., Hübner, U. & Popp, J. Removing interference-based effects from the infrared transflectance spectra of thin films on metallic substrates: A fast and wave optics conform solution. Analyst 143, 3164–3175 (2018).
Kassaee, M., Masrouri, H. & Movahedi, F. Sulfamic acid-functionalized magnetic Fe3O4 nanoparticles as an efficient and reusable catalyst for one-pot synthesis of α-amino nitriles in water. Appl. Catal. A 395, 28–33 (2011).
Yue, Q. et al. Plasmolysis-inspired nanoengineering of functional yolk–shell microspheres with magnetic core and mesoporous silica shell. J. Am. Chem. Soc. 139, 15486–15493 (2017).
Yu, L. et al. Nonsacrificial self-template synthesis of colloidal magnetic yolk–shell mesoporous organosilicas for efficient oil/water interface catalysis. Small 15, 1805465 (2019).
Jin, C. et al. Versatile rattle-type magnetic mesoporous silica spheres, working as adsorbents and nanocatalyst containers. J. Sol-Gel Sci. Technol. 77, 279–287 (2016).
Papadas, I. T., Fountoulaki, S., Lykakis, I. N. & Armatas, G. S. Controllable synthesis of mesoporous iron oxide nanoparticle assemblies for chemoselective catalytic reduction of nitroarenes. Chem. Eur. J. 22, 4600–4607 (2016).
Davarpanah, J. & Kiasat, A. R. Catalytic application of silver nanoparticles immobilized to rice husk-SiO 2-aminopropylsilane composite as recyclable catalyst in the aqueous reduction of nitroarenes. Catal. Commun. 41, 6–11 (2013).
Nasab, M. J. & Kiasat, A. R. Multifunctional Fe 3 O 4@ n SiO 2@ m SiO 2/Pr-Imi-NH 2· Ag core–shell microspheres as highly efficient catalysts in the aqueous reduction of nitroarenes: Improved catalytic activity and facile catalyst recovery. RSC Adv. 6, 41871–41877 (2016).
Duan, Y. et al. Synthesis of Pd/SBA-15 catalyst employing surface-bonded vinyl as a reductant and its application in the hydrogenation of nitroarenes. RSC Adv. 7, 3443–3449 (2017).
Liu, C., Tan, R., Yu, N. & Yin, D. Pt–Pd bi-metal nanoparticles captured and stabilized by imine groups in a periodic mesoporous organosilica of SBA-15 for hydrogenation of nitrobenzene. Micropor. Mesopor. Mater. 131, 162–169 (2010).
Acknowledgements
The authors thank Yasouj University and Iran National Science Foundation (INSF) for supporting this work.
Author information
Authors and Affiliations
Contributions
M. N.: Writing Original Draft, Investigation, Resources, Formal analysis. D. E.: Conceptualization, Writing, Review & Editing, Supervision, Visualization. S. K.: Formal Analysis, Revising Original Draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Norouzi, M., Elhamifar, D. & Kargar, S. Magnetic yolk-shell structured periodic mesoporous organosilica supported palladium as a powerful and highly recoverable nanocatalyst for the reduction of nitrobenzenes. Sci Rep 14, 16262 (2024). https://doi.org/10.1038/s41598-024-66883-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-66883-4
Keywords
This article is cited by
-
Doughnut-like wrinkled fibrous nano silica architecture as a novel and effective reusable green heterogeneous nanocatalyst for the Hantzsch reaction
Scientific Reports (2025)
-
Cu Nanoparticles Decorated on Magnetic NH2-MIL-101(Fe): A Highly Active Catalyst for C–N Coupling and Reductive Degradation of Dyes
Catalysis Letters (2025)
-
SiO₂@Fe3O4@Purine-SO3H: a robust solid acid nanocatalyst for accelerated Biginelli condensation
Research on Chemical Intermediates (2025)