Abstract
The objective of the present study was to evaluate the risk factors and outcomes associated with hyponatremia in patients with Guillain-Barré syndrome (GBS). We retrospectively studied 80 consecutive patients with GBS who visited our hospital and compared clinical, laboratory, and electrophysiological findings of patients with and without hyponatremia. Disability was evaluated using the Hughes grading system. Of the 80 patients, 18 (23%) had hyponatremia. Hyponatremia was significantly associated with older age (P = 0.003), urinary retention (P < 0.0001), Hughes grade ≥ 4 at admission and nadir (P = 0.003 and P < 0.001, respectively), acute inflammatory demyelinating polyneuropathy subtype (P = 0.017), sepsis (P = 0.001), mechanical ventilator support (P = 0.013), longer hospitalization length of stay (P < 0.0001), and inability to walk independently at 6 months (P < 0.001). Multivariate analysis performed to assess the risk factors of hyponatremia revealed that urinary retention (odds ratio [OR] 30.7, 95% confidence interval [CI] 3.6–264.4; P = 0.002) and mechanical ventilator support (OR 13.8, 95% CI 1.6–118.0; P = 0.017) were significant independent risk factors of hyponatremia. In assessing the outcomes of patients with hyponatremia, multivariate analysis showed that hyponatremia was independently associated with hospitalization length of stay ≥ 60 days and inability to walk independently at 6 month, with the former showing statistical significance but the latter not (OR 9.3, 95% CI 1.8–47.7; P = 0.007 and OR 4.9, 95% CI 0.9–26.3; P = 0.066, respectively). Therefore, we demonstrate that, along with mechanical ventilator support, urinary retention—possibly indicating autonomic dysfunction—is a risk factor of hyponatremia in GBS. Moreover, we confirm that hyponatremia is associated with poor outcome in GBS.
Similar content being viewed by others
Introduction
Guillain–Barré syndrome (GBS) is an immune-mediated peripheral neuropathy that features a broad spectrum of clinical variants1,2,3,4,5. Although abnormal results for cerebrospinal fluid test in GBS are well known, serum abnormalities have also been reported. One such serum abnormality in GBS is hyponatremia6,7,8,9,10,11,12. However, the clinical features of GBS with hyponatremia have not been clarified. Regarding the risk factors, previous studies have reported that disease severity and older age were associated with hyponatremia in GBS6,8,9. Autonomic dysfunction was also associated with hyponatremia in GBS and was especially examined because of its potentially important role in the pathogenesis10. However, interrelationships were observed between these possible risk factors, and the independence of these risk factors was not established. Some studies have also reported that hyponatremia is associated with poor outcome in GBS6,8,9,11,12. Nevertheless, many factors are associated with poor outcomes; therefore, the independent association of hyponatremia with poor outcomes needs to be verified.
In this study, we examined the clinical features of GBS with hyponatremia. First, we investigated whether previously identified risk factors, especially autonomic dysfunction, were independently associated with hyponatremia in GBS. Second, we evaluated the independent association of hyponatremia with GBS outcome.
Materials and methods
Patients
We retrospectively evaluated consecutive patients with GBS who visited Osaka Medical and Pharmaceutical University Hospital from January 2005 to November 2021. In our department, we generate our own list of diagnoses for all patients admitted to the hospital. Therefore, we utilize not only ICD codes but also our internal list of diagnoses when searching for patients. All patients met the clinical criteria for GBS13, except those for areflexia or hyporeflexia. We enrolled these patients because the presence of GBS with normal or exaggerated tendon reflexes has been reported previously14,15.
Disability was evaluated using the Hughes grading scale: 0, healthy; 1, minor signs and symptoms, able to run; 2, able to walk independently; 3, able to walk with a walker or support; 4, bed or chair bound; 5, assisted respiration required for at least part of the day; and 6, dead16. As indicators of poor outcome, we used a hospitalization length of stay ≥ 60 days and an inability to walk 10 m independently at 6 months. We also focused on urinary retention as an indicator of autonomic dysfunction.
Electrophysiological examinations and motor and sensory nerve conduction examinations were performed using the MEB-9104 Neuropack mu® electrodiagnostic system (Nihon Kohden, Tokyo, Japan) according to the methods and reference values described by Kimura et al.17. Based on these results, patients were classified into acute inflammatory demyelinating polyneuropathy (AIDP) or acute motor axonal neuropathy (AMAN) according to the criteria reported by Rajabally et al.18. When the electrophysiological findings did not fulfill the criteria for AIDP or AMAN, the patients were designated as equivocal GBS. Hyponatremia was defined as a serum sodium concentration below 135 mmol/L at nadir during hospitalization.
This study was conducted in accordance with the tenets of the 2013 Helsinki Declaration. The Osaka Medical and Pharmaceutical University Ethics Committee approved the study protocol. The need for informed consent was waived because this was a retrospective study, and the data were collected without individual patient identifiers (Approval number #2020-189).
Statistical analyses
The Mann–Whitney U test was used to assess differences in continuous variables, and Fisher’s exact test was applied to examine categorical variables. We analyzed the association between candidate risk factors and hyponatremia in GBS using univariate and subsequent multivariate logistic regression models. We also analyzed the association between hyponatremia and poor outcome in GBS using univariate and subsequent multivariate logistic regression models, considering patients’ prognostic factors of GBS other than hyponatremia. Multivariate analysis was performed using the variables that showed a significant difference (p < 0.05) in the univariate analysis. The values are expressed as mean ± standard deviation, and a P-value < 0.05 was considered statistically significant. All analyses were performed using JMP software version 15.0 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical and laboratory features of patients with GBS and hyponatremia
Eighty patients (47 men, 33 women; mean age; 48.7 years range 16–90 years) were included in this study. Table 1 shows a comparison of the clinical and laboratory findings in patients with GBS with and without hyponatremia. Of 80 patients, 18 (23%) showed hyponatremia. None of the 18 patients with hyponatremia were utilizing diuretics or ACE inhibitors, while four of them had undergone intravenous immunoglobulin (IVIG) treatment before the onset of hyponatremia. Patients with hyponatremia were significantly older than those without hyponatremia. None of the 15 patients with urinary retention exhibited indications of difficulty urinating or further urinary retention before the onset of GBS symptoms, and among the 8 male patients with urinary retention, there were no documented cases of prostate enlargement. Patients with hyponatremia exhibited a higher incidence of urinary retention compared with patients without hyponatremia. Regarding the Hugh grades at admission and nadir, we divided the patients into two groups based on their scores: 0–3 and 4 or higher. Then, we compared the frequency of these groups between those with and without hyponatremia. The proportion of Hugh grade ≥ 4 in patients with hyponatremia were significantly higher than those in patients without hyponatremia both at admission and nadir. The proportions of GBS subtypes in patients with hyponatremia differed significantly from those in patients without hyponatremia. The number of AIDP cases in patients with hyponatremia was significantly higher than that in patients without hyponatremia. Conversely, the number of equivocal cases in patients with hyponatremia was lower than those in patients without hyponatremia, though not significantly. The proportion of development of sepsis in patients with hyponatremia were significantly higher than those in patients without hyponatremia. In patients with hyponatremia, 6 out of 18 required mechanical ventilator support, with 2 of them developing hyponatremia for the first time after starting mechanical ventilator support. On the other hand, in patients without hyponatremia, only 5 out of 62 required mechanical ventilator support. The proportion requiring mechanical ventilator support in patients with hyponatremia were significantly higher than those in patients without hyponatremia. Hospitalization length of stay in patients with hyponatremia were significantly longer than those without hyponatremia. To evaluate whether patients unable to walk independently at 6 months, we followed patients either until they were able to walk or for a period of 6 months at least. Regrettably, one patient could not be followed up, necessitating their exclusion from the analyses. The rate of inability to walk independently at 6 months in patients with hyponatremia was significantly higher than that in patients without hyponatremia.
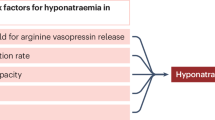
Assessment of risk factors for hyponatremia in patients with GBS
To analyze the risk factors associated with hyponatremia in GBS, we performed univariate and subsequent multivariate analyses (Table 2). The candidate risk factors of hyponatremia were selected mainly based on the findings of previous reports6,8,9. In the univariate analysis, age ≥ 50 years, Hughes grade at admission ≥ 4, AIDP subtype, urinary retention, sepsis, and mechanical ventilator support were significantly associated with hyponatremia. In the multivariate analysis, urinary retention and mechanical ventilator support were significantly associated with hyponatremia.
Association between hyponatremia and outcomes in patients with GBS
To analyze whether hyponatremia was associated with poor outcome, we performed univariate and subsequent multivariate analyses (Table 3). The candidate risk factors of poor outcome, aside from hyponatremia, were selected mainly based on the findings of previous reports19,20,21,22,23. Among patients with hyponatremia, 11 out of 18 experienced a hospitalization length of stay of ≥ 60 days, compared to only 4 out of 62 patients without hyponatremia. Univariate analyses showed a significant association between hyponatremia and hospitalization length of stay ≥ 60 days (OR 22.7, 95% CI 5.7–91.2; P < 0.001), which also remained significant in multivariate analyses (OR 13.2, 95% CI 2.1–83.7; P = 0.006). Furthermore, in patients with hyponatremia, all 18 out of 18 were unable to walk 10 m independently at nadir, whereas 30 out of 62 patients without hyponatremia were unable to do so. There was a significant difference in the proportion of patients with the inability to walk 10 m independently at nadir. Furthermore, 8 out of 18 patients with hyponatremia were unable to walk 10 m independently at 6 months after onset, compared to only 3 out of 61 patients without hyponatremia. Univariate analyses showed a significant association between hyponatremia and the inability to walk 10 m independently at 6 months after onset (OR 15.5, 95% CI 3.5–68.4; P < 0.001), which remained significant in multivariate analyses (OR 19.3, 95% CI 1.5–245.5; P = 0.023).
Discussion
In this study, we showed tha urinary retention was associated with hyponatremia in GBS. We could not find any previous studies about the association between urinary retention itself and hyponatremia. However, as we consider urinary retention to reflect autonomic dysfunction, we could find several previous studies about the association between autonomic dysfunction and hyponatremia. The results of previous studies on the association between autonomic dysfunction and hyponatremia in GBS were controversial. However, while the findings of some previous reports were in agreement with ours10,24,25, to the best of our knowledge, only Chakraborty et al.10 reported a statistically significant association between autonomic dysfunction and hyponatremia in GBS. Nevertheless, they could not demonstrate an independent association between autonomic dysfunction and hyponatremia. That is, our study is the first to demonstrate an independent association between autonomic dysfunction and hyponatremia. Other studies have failed to demonstrate the significant association between autonomic dysfunction and hyponatremia7,12. This inconsistency between our study and those previous ones may be partly attributable to the fact that our study focuses solely on urinary retention, whereas previous studies have dealt with a variety of autonomic nervous system symptoms.
We focused solely on urinary retention as a symptom of autonomic dysfunction. On the other hand, autonomic dysfunction contributed to various other symptoms, such as ileus, hypertension, hypotension, blood pressure fluctuations, tachycardia, bradycardia, heart rate fluctuation, arrhythmias, and sudomotor dysfunction in GBS10,26,27,28. Compared with urinary retention, these symptoms have large individual differences, even among healthy subjects, and large temporal differences, even within the same individual. Thus, abnormalities in these symptoms are more difficult to diagnose than urinary retention10,26. Moreover, compared with urinary retention, these symptoms are more likely to be affected by many factors other than autonomic dysfunction, such as preexisting disease, complications, and even other GBS symptoms10,26,29. Therefore, our approach of assessing the presence of autonomic dysfunction solely through urinary retention has the following characteristics when compared to methods that assess the presence of autonomic dysfunction through various symptoms. Our approach was more exclusive and showed higher specificity for detecting autonomic dysfunction. This might be the reason why we were able to establish an association between autonomic dysfunction and hyponatremia. Conversely, our approach may have excluded more patients with autonomic dysfunction and might have lower sensitivity. To accurately clarify the association of various autonomic symptoms and underlying autonomic mechanisms with hyponatremia in GBS, further studies are needed where researchers prospectively define autonomic dysfunction using the presence of various autonomic symptoms and reasonable diagnostic criteria with reasonable cutoff values and observation periods.
Hyponatremia has various causative factors such as drug-induced hyponatremia, hyponatremia due to inadequate intake, syndrome of inappropriate antidiuretic hormone secretion (SIADH), and renal salt wasting (RSW). We hypothesize that drug-induced factors are unlikely to be the primary cause of hyponatremia in this study. None of the patients with hyponatremia were using diuretics or ACE inhibitors, both of which are commonly associated with hyponatremia. Pseudohypnatremia can occur in GBS as a laboratory artifact following IVIG administration30, with four patients experiencing it prior to the onset of hyponatremia. However, it is improbable for IVIG-induced pesudophyonatremia is the main cause of hyponatremia in this study, given the similar frequency of IVIG use between groups with and without hyponatremia. We postulate that factors related to inadequate intake are also unlikely to be the primary cause of hyponatremia in this study. All patients were receiving nutrition via either oral and/or intravenous routes. After all, we speculate that hyponatremia in this study primarily resulted from SIADH or RSW. Consistent with our speculation, it has been frequently reported in several previous studies that SIADH or RSW are the main causes of hyponatremia in GBS31.
The complete elucidation of the mechanism through which autonomic dysfunction precipitates SIADH or RSW and subsequently induces hyponatremia in GBS remains elusive. Nonetheless, various hypotheses have been proposed. One such hypothesized mechanism suggests that the abnormality of peripheral autonomic afferent fibers originating from vascular stretch receptors, as a component of autonomic dysfunction in GBS, could instigate hyponatremia mainly through SIADH32. Another proposed mechanism posits that sympathoadrenal dysregulation, as a facet of autonomic dysfunction in GBS, might trigger excessive secretion of brain natriuretic peptide, culminating in disproportionate renal sodium excretion, thereby engendering hyponatremia mainly through RSW24.
We also demonstrated an independent association between mechanical ventilator support and hyponatremia in patients with GBS. Previous reports have also indicated an association between mechanical ventilator support and hyponatremia6,8, with some suggesting an independent relationship9. One proposed mechanism is that positive pressure ventilation induces the secretion of antidiuretic hormone (ADH), leading to SIADH33. However, consistent with previous findings, our study observed that more than half of the patients with hyponatremia requiring mechanical ventilator support already had hyponatremia before the initiation of mechanical ventilator support. This implies that additional, undefined mechanisms might be involved.
Another noteworthy finding of our study is that hyponatremia was associated with poor outcome in patients with Guillain-Barré syndrome. Previous reports have associated hyponatremia with poor outcome6,8,9,11,12, some of which showed this association even when some other prognostic factors, such as age, were included in the multivariate analysis9,11,12. A distinctive and superior aspect of our study, compared to these previous studies, is our use of two indicators of poor outcome: a hospitalization length of stay ≥ 60 days and an inability to walk independently at 6 months after onset.
The former showed a significant association, while the latter was not statistically significant but also suggested a possible association. Additionally, we adjusted for various other poor prognostic factors, including age, preceding diarrhea, disability at admission, mechanical ventilator support, and sepsis. However, similar to previous studies, we couldn't determine the exact mechanism explaining why hyponatremia is linked to a poor outcome in GBS. It's possible that factors related to hyponatremia, such as general dyshomeostatic state, could directly contribute to the poor outcome, rather than hyponatremia itself11. Further research is required to elucidate the relationship between hyponatremia and poor outcome.
Our study has some limitations. First, as a retrospective study, we had limited clinical and laboratory information for the enrolled patients. We were unable to thoroughly evaluate various autonomic symptoms other than urinary retention, preventing us from including these symptoms in our assessment of autonomic dysfunction as mentioned above. Especially, we could not include cardiovascular manifestations, which could increase mortality and thus impact outcomes. Furthermore, we could not confirm the etiology of hyponatremia. Based on the limited data available, we speculated that the etiology of hyponatremia could be attributed to either SIADH or RSW. However, due to insufficient information, such as the absence of ADH concentration data, we couldn't definitively determine whether it was SIADH or RSW. Other than that, we lack some important information for analyses, such as MRC sum scores. Second, our study had a relatively small sample size, which limited the robustness of our statistical analysis. Specifically, when examining the risk factors for hyponatremia and its association with poor outcome using multivariate analyses, we incorporated many important factors as variables, but we were not able to include all the factors reported in the past.
In conclusion, our findings show that urinary retention, and subsequently autonomic dysfunction, is a risk factor of hyponatremia in GBS. Moreover, we confirm that hyponatremia is associated with poor outcome in GBS. Our findings add new clinical information about GBS and provide new perspectives that could expand current understanding of the mechanisms involved in GBS.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
van Doorn, P. A., Ruts, L. & Jacobs, B. C. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 7, 939–950. https://doi.org/10.1016/S1474-4422(08)70215-1 (2008).
Kaida, K. Guillain-Barre syndrome. Adv. Exp. Med. Biol. 1190, 323–331. https://doi.org/10.1007/978-981-32-9636-7_20 (2019).
Yuki, N. & Hartung, H. P. Guillain-Barre syndrome. N. Engl. J. Med. 366, 2294–2304. https://doi.org/10.1056/NEJMra1114525 (2012).
Kuwabara, S. & Yuki, N. Axonal Guillain-Barre syndrome: Concepts and controversies. Lancet Neurol. 12, 1180–1188. https://doi.org/10.1016/S1474-4422(13)70215-1 (2013).
Kusunoki, S., Willison, H. J. & Jacobs, B. C. Antiglycolipid antibodies in Guillain-Barre and Fisher syndromes: Discovery, current status and future perspective. J. Neurol. Neurosurg. Psychiatry 92, 311–318. https://doi.org/10.1136/jnnp-2020-325053 (2021).
Saifudheen, K., Jose, J., Gafoor, V. A. & Musthafa, M. Guillain-Barre syndrome and SIADH. Neurology 76, 701–704. https://doi.org/10.1212/WNL.0b013e31820d8b40 (2011).
Hiew, F. L., Winer, J. B. & Rajabally, Y. A. Hyponatraemia in Guillain-Barre syndrome revisited. Acta Neurol. Scand. 133, 295–301. https://doi.org/10.1111/ane.12459 (2016).
Rumalla, K., Reddy, A. Y., Letchuman, V. & Mittal, M. K. Hyponatremia in Guillain-Barre syndrome. J. Clin. Neuromuscul. Dis. 18, 207–217. https://doi.org/10.1097/CND.0000000000000157 (2017).
Wang, Y. & Liu, J. Hyponatremia is a predictor for poor outcome in Guillain-Barre syndrome. Neurol. Res. 37, 347–351. https://doi.org/10.1179/1743132814Y.0000000455 (2015).
Chakraborty, T., Kramer, C. L., Wijdicks, E. F. M. & Rabinstein, A. A. Dysautonomia in Guillain-Barre syndrome: Prevalence, clinical spectrum, and outcomes. Neurocrit. Care 32, 113–120. https://doi.org/10.1007/s12028-019-00781-w (2020).
Sipila, J. O., Kauko, T. & Soilu-Hanninen, M. Admission sodium level and prognosis in adult Guillain-Barre syndrome. Int. J. Neurosci. 127, 344–349. https://doi.org/10.3109/00207454.2016.1163551 (2017).
Gagliardi, D. et al. Sodium levels predict disability at discharge in Guillain-Barre syndrome: A retrospective cohort study. Front. Neurol. 12, 729252. https://doi.org/10.3389/fneur.2021.729252 (2021).
Asbury, A. K. & Cornblath, D. R. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann. Neurol. 27(Suppl), S21-24. https://doi.org/10.1002/ana.410270707 (1990).
Yuki, N. et al. Guillain-Barre syndrome associated with normal or exaggerated tendon reflexes. J. Neurol. 259, 1181–1190. https://doi.org/10.1007/s00415-011-6330-4 (2012).
Uncini, A., Notturno, F. & Kuwabara, S. Hyper-reflexia in Guillain-Barre syndrome: Systematic review. J. Neurol. Neurosurg. Psychiatry 91, 278–284. https://doi.org/10.1136/jnnp-2019-321890 (2020).
Hughes, R. A., Newsom-Davis, J. M., Perkin, G. D. & Pierce, J. M. Controlled trial prednisolone in acute polyneuropathy. Lancet 2, 750–753. https://doi.org/10.1016/s0140-6736(78)92644-2 (1978).
Kimura, J. Electrodiagnosis in Deseases of Nerve and Muscle: Principles and Practice 2nd edn. (F.A. Davis, 1989).
Rajabally, Y. A., Durand, M. C., Mitchell, J., Orlikowski, D. & Nicolas, G. Electrophysiological diagnosis of Guillain-Barre syndrome subtype: Could a single study suffice?. J. Neurol. Neurosurg. Psychiatry 86, 115–119. https://doi.org/10.1136/jnnp-2014-307815 (2015).
Rajabally, Y. A. & Uncini, A. Outcome and its predictors in Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatry 83, 711–718. https://doi.org/10.1136/jnnp-2011-301882 (2012).
Walgaard, C. et al. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology 76, 968–975. https://doi.org/10.1212/WNL.0b013e3182104407 (2011).
Khedr, E. M., Mahmoud, D. M., Ahmed, G. K. & Haridy, N. A. Predictors of long-term health-related quality of life in Guillain-Barre syndrome: A hospital-based study. Clin. Neurol. Neurosurg. 235, 108026. https://doi.org/10.1016/j.clineuro.2023.108026 (2023).
Hughes, R. A. et al. Supportive care for patients with Guillain-Barre syndrome. Arch. Neurol. 62, 1194–1198. https://doi.org/10.1001/archneur.62.8.1194 (2005).
Netto, A. B. et al. Complications in mechanically ventilated patients of Guillain-Barre syndrome and their prognostic value. J. Neurosci. Rural Pract. 8, 68–73. https://doi.org/10.4103/0976-3147.193542 (2017).
Lenhard, T., Grimm, C. & Ringleb, P. A. Renal salt wasting as part of dysautonomia in Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatry 82, 1051–1053. https://doi.org/10.1136/jnnp.2009.192369 (2011).
Kumar, M., Kalita, J. & Misra, U. K. Renal salt wasting in Guillain-Barre syndrome. Postgrad. Med. J. 95, 628–629. https://doi.org/10.1136/postgradmedj-2019-136870 (2019).
Zaeem, Z., Siddiqi, Z. A. & Zochodne, D. W. Autonomic involvement in Guillain-Barre syndrome: An update. Clin. Auton. Res. 29, 289–299. https://doi.org/10.1007/s10286-018-0542-y (2019).
Lichtenfeld, P. Autonomic dysfunction in the Guillain-Barre syndrome. Am. J. Med. 50, 772–780. https://doi.org/10.1016/0002-9343(71)90185-9 (1971).
Singh, N. K., Jaiswal, A. K., Misra, S. & Srivastava, P. K. Assessment of autonomic dysfunction in Guillain-Barre syndrome and its prognostic implications. Acta Neurol. Scand. 75, 101–105. https://doi.org/10.1111/j.1600-0404.1987.tb07902.x (1987).
Zochodne, D. W. Autonomic involvement in Guillain-Barre syndrome: A review. Muscle Nerve 17, 1145–1155. https://doi.org/10.1002/mus.880171004 (1994).
Colls, B. M. Guillain-Barre syndrome and hyponatraemia. Intern. Med. J. 33, 5–9. https://doi.org/10.1046/j.1445-5994.2002.00322.x (2003).
Cui, H. et al. Inappropriate antidiuretic hormone secretion and cerebral salt-wasting syndromes in neurological patients. Front. Neurosci. 13, 1170. https://doi.org/10.3389/fnins.2019.01170 (2019).
Posner, J. B., Ertel, N. H., Kossmann, R. J. & Scheinberg, L. C. Hyponatremia in acute polyneuropathy. Four cases with the syndrome of inappropriate secretion of antidiuretic hormone. Arch. Neurol. 17, 530–541. https://doi.org/10.1001/archneur.1967.00470290084011 (1967).
Hemmer, M., Viquerat, C. E., Suter, P. M. & Vallotton, M. B. Urinary antidiuretic hormone excretion during mechanical ventilation and weaning in man. Anesthesiology 52, 395–400. https://doi.org/10.1097/00000542-198005000-00004 (1980).
Author information
Authors and Affiliations
Contributions
S.O. designed the study, carried out the acquisition of data, and wrote the manuscript. T.H. designed the study, carried out the acquisition of data, analyzed the data, and wrote the manuscript. C.H. carried out the acquisition of data. T.S. carried out the acquisition of data. K.K. carried out the acquisition of data. D.N. analyzed the data. Y.Y. carried out the acquisition of data. Y.M. carried out the acquisition of data. Y.K. carried out the acquisition of data. S.O. carried out the acquisition of data. S.A. supervised and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ogawa, S., Hosokawa, T., Hayakawa, C. et al. Risk factors and outcome of hyponatremia in patients with Guillain–Barré syndrome. Sci Rep 14, 16664 (2024). https://doi.org/10.1038/s41598-024-67427-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67427-6