Abstract
The influence of pulmonary dysfunction on postoperative outcomes in older patients with gastric cancer was assessed. In this retrospective study, 352 older patients (age ≥ 75 years) with gastric cancer who underwent preoperative spirometry and curative gastrectomy were enrolled. Of these patients, 200 underwent laparoscopic gastrectomy. Restrictive and obstructive pulmonary dysfunction were defined as percentage of vital capacity (%VC) < 80% and percent of forced expiratory volume in one second (FEV1.0%) < 70%, respectively. Twenty-six (7.3%) and 123 (34.9%) exhibited restrictive and obstructive pulmonary dysfunction, respectively. The low-%VC group showed a higher incidence of postoperative pneumonia (p = 0.018) while the low-FEV1.0% group did not (p = 0.677). Multivariate analysis identified a decreased %VC as a significant risk factor for postoperative pneumonia. However, this association was not observed in patients who underwent laparoscopic gastrectomy. Concerning the long-term outcomes, restrictive dysfunction was a significant prognostic factor in older patients with gastric cancer who underwent either laparotomy or laparoscopy, whereas obstructive dysfunction did not. Restrictive pulmonary dysfunction increased the risk of postoperative pneumonia and had a negative prognostic effect in older patients with gastric cancer, whereas obstructive pulmonary dysfunction did not.
Similar content being viewed by others
Introduction
The rise in life expectancy in developed countries has led to an increased number of older patients, particularly dominating the demographics of gastric cancer cases in East Asia, owing to the higher prevalence of Helicobacter pylori infection in the older population1. Older patients are generally frail and have a broad range of physiological dysfunctions and comorbidities that are highly correlated with aging. Pulmonary disease, which involves pneumonia and subsequent death, is one such major comorbidity.
The presence of pulmonary comorbidities increases the likelihood of postoperative pulmonary complications2. Therefore, preoperative pulmonary function testing is widely used to select surgical candidates and predict the incidence of postoperative pulmonary complications. Several recent studies have investigated the effect of preoperative pulmonary dysfunction on postoperative pulmonary complications after gastrectomy3,4. Furthermore, pulmonary impairment can influence the long-term prognosis of patients with gastric cancer4,5.
In recent years, laparoscopic gastrectomy has gained widespread acceptance due to its proven safety and efficacy6,7,8,9. Although its safety in older patients with gastric cancer has been established10, the safety and efficacy of laparoscopic gastrectomy in older patients with pulmonary dysfunction remain inadequately discussed. The current study aimed to assess the postoperative complications and prognosis in older patients with gastric cancer and pulmonary dysfunction, with a specific focus on those who underwent laparoscopic gastrectomy.
Methods
Study population
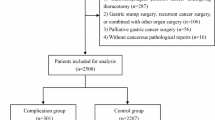
This study included a total of 352 consecutive patients aged ≥ 75 years who underwent curative gastrectomy for gastric cancer and preoperative pulmonary function tests between 2013 and 2018 at Saitama Medical University International Medical Center. Among these patients, 200 underwent laparoscopic gastrectomy. Prognosis and postoperative complications were compared between patients with and without pulmonary dysfunction. Patients’ clinicopathological data and clinical courses were retrospectively collected from hospital records.
Ethical statements
The procedures conducted in this study adhered to the ethical standards of the Institutional Research Committee and the 1964 Declaration of Helsinki, along with its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the Independent Ethics Committee of the Saitama Medical University International Medical Center (approval number: 19226). Informed consent was obtained from all participants.
Study criteria
Pulmonary function, measured using spirometry, was assessed regardless of past pulmonary disease. Restrictive ventilatory impairment was defined as a percentage of vital capacity (%VC) < 80% of the predicted value, whereas obstructive ventilatory impairment was defined as a percent forced expiratory volume in one second (FEV1.0%) < 70% of the forced vital capacity11. Tumor staging followed the Union for International Cancer Control / Tumour-Node-Metastasis (UICC/TNM) staging system, 8th edition12. Preoperative comorbidities were categorized using a Charlson comorbidity index (CCI) score13. Postoperative complications were assessed using the Clavien–Dindo (CD) classification14, with complications of grade 2 or higher within 30 days after the operation defined as postoperative complications in this study. Postoperative pneumonia was diagnosed based on symptoms (cough, dyspnea, fever, etc.), blood tests, and radiographic findings (newly developed infiltrates, etc.)15,16 and defined as grade 2 or higher pneumonia requiring antibiotic therapy14. Postoperative surveillance adhered to the Japanese Gastric Cancer Treatment Guidelines17, involving blood tests and computed tomography (CT) every 6 months, and annual esophagogastroduodenoscopy. Fundamentally, laparoscopic gastrectomy was performed for early gastric cancer according to Japanese Gastric Cancer Treatment Guidelines17. Additionally, laparoscopic surgery was also performed on patients with advanced gastric cancer who participated in the clinical trial6,9. For patients with stage II/III gastric cancer, tegafur/gimeracil/oteracil monotherapy was administered as postoperative adjuvant chemotherapy based on the patient’s consent and general condition17. The median follow-up period was 4.9 years.
Statistical analysis
Data are expressed as medians (interquartile ranges), or frequencies (percentages). Statistical analyses were performed using JMP version 14 (SAS Institute, Cary, NC, USA). Fisher’s exact test or the chi-square test was used to evaluate differences in proportions, and the Wilcoxon test was used to evaluate continuous variables. Survival outcomes were analyzed using the Kaplan–Meier method, and differences were assessed using the log-rank test. Multivariate analyses using covariates with p < 0.10 were performed using Cox and logistic regression analyses. In all analyses, p < 0.05 was considered statistically significant.
Results
Clinical characteristics according to presence or absence of pulmonary dysfunction
Among the 352 patients, 26 (7.3%) showed %VC < 80% (low-%VC group), and 123 (34.9%) showed FEV1.0% < 70% (low-FEV1.0% group). The clinical characteristics of the patients are summarized in Table 1. Regarding the %VC, the low-%VC group had a higher age, lower preoperative body mass index (BMI), and a greater number of patients with a history of pulmonary disease than the normal-%VC group. In contrast, when comparing the FEV1.0%, the low-FEV1.0% group had a greater number of men and a history of smoking. Regarding postoperative complications, the incidence of postoperative pneumonia was higher in the low-%VC group than in the normal-%VC group (low-%VC group, 15.4% vs. normal-%VC group, 3.4%; p = 0.018). However, there was no statistically significant difference in the incidence of postoperative complications and pneumonia between the low- and normal-FEV1.0% groups. Moreover, a low %VC was a significant risk factor for postoperative pneumonia in older patients with gastric cancer (Table 2).
Survival outcomes and prognostic impact of pulmonary dysfunction
The 5-year overall survival (OS) curves for these four groups are shown in Fig. 1; the 5-year OS of the low-%VC and normal-%VC groups were 37.2% and 74.4%, respectively, with the low-%VC group having a significantly worse prognosis (p < 0.001) than the normal-%VC group (Fig. 1a), whereas the 5-year OS of the low-FEV1.0% and normal-FEV1.0% groups were 73.9% and 70.9%, respectively (p = 0.568) (Fig. 1b). Additionally, the prognostic factors influencing the OS were evaluated in older patients with gastric cancer (Table 3). Univariate analysis revealed that low BMI, high CCI scores, history of pulmonary disease, low %VC, laparotomy, postoperative pneumonia, and advanced pathological stage were significant prognostic factors for poorer OS. Furthermore, multivariate analysis revealed that high CCI scores, low %VC, postoperative pneumonia, and advanced pathological stage were independent prognostic factors for poorer OS (high CCI scores, HR: 1.58, p = 0.044; low %VC, HR: 2.08, p = 0.041; postoperative pneumonia, HR: 5.53, p < 0.001; and advanced pathological stage, HR: 3.44, p < 0.001).
Clinical characteristics according to presence or absence of pulmonary dysfunction in patients undergoing laparoscopic gastrectomy
Among the 200 patients who underwent laparoscopic gastrectomy, 11 (5.5%) showed %VC < 80% and 73 (36.5%) showed FEV1.0% < 70%. The clinical characteristics of the patients are summarized in Table 4. In the comparison of %VC, the low-%VC group had a lower BMI and advanced pathological stage, whereas in the comparison of FEV1.0%, the low-FEV1.0% group had a greater number of men. No statistically significant difference in the incidence of postoperative complications and pneumonia was observed between the low- and normal-%VC groups and the low- and normal-FEV1.0% groups.
Survival outcomes and prognostic impact of pulmonary dysfunction undergoing laparoscopic gastrectomy
In patients who underwent laparoscopic surgery, the 5-year OS curves are shown in Fig. 2; the 5-year OS of the low-%VC and normal-%VC groups were 27.2% and 80.1%, respectively (p < 0.001) (Fig. 2a), whereas the 5-year OS of the low-FEV1.0% and normal-FEV1.0% groups were 82.0% and 75.1%, respectively (p = 0.256) (Fig. 2b). Moreover, univariate analysis revealed that high CCI scores, low %VC, and advanced pathological stage were significant prognostic factors for poor OS. Multivariate analysis revealed that high CCI scores, low %VC, and advanced pathological stage were independent prognostic factors for poorer OS (high CCI scores, hazard ratio (HR): 2.26, p = 0.019; low %VC, HR: 3.05, p = 0.036; and advanced pathological stage, HR: 4.29, p < 0.001) (Table 5).
Discussion
This study demonstrated that low %VC increased the risk of postoperative pneumonia, whereas low FEV1.0% did not elevate the risk of postoperative complications including pneumonia. In patients who underwent laparoscopic gastrectomy, both low %VC and FEV1.0% were not associated with an increased risk of postoperative complications. Regarding the long-term prognosis, low %VC emerged as a significant risk factor for poor outcomes, along with high CCI scores and advanced pathological stage, irrespective of the surgical approach (laparotomy or laparoscopic surgery).
In this study, restrictive pulmonary impairment increased the incidence of postoperative pulmonary complications, which is consistent with the results of previous research. Several studies have highlighted that restrictive pulmonary dysfunction is a risk factor for postoperative pneumonia in patients with gastric cancer3,4,18, particularly the older19. These studies suggested that the reason for the increase in postoperative pneumonia is that restrictive dysfunction reflects reduced systemic function3,4,5,19. In fact, our results showed that lower BMI, higher CCI scores, and a higher number of patients with a history of pulmonary disease were observed in the low-%VC group. Conversely, obstructive pulmonary impairment did not pose a risk of postoperative complications and pneumonia, consistent with previous findings19, possibly because obstructive dysfunction was not associated with frailty20,21. Consequently, patients with a low %VC should be considered more vulnerable, necessitating increased perioperative care.
Similar to the short-term outcomes, although obstructive pulmonary impairments did not influence long-term outcomes, restrictive pulmonary impairments negatively affected long-term survival. Previous studies have reported that decreased %VC is associated with poor prognosis in patients with gastric cancer4. Particularly in the older, a greater impact on non-gastric cancer-related deaths has been reported19. Our results indicated that the low-%VC group exhibited worse noncancer-specific survival than the normal-%VC group (5-year OS: 59.0% vs. 89.1%, p < 0.001), with no significant difference in cancer-specific survival (5-year OS: 63.0% vs. 83.5%, p = 0.152) (Supplementary Fig. 1). These results may be attributed to the vulnerability of patients with restrictive pulmonary impairments, as mentioned earlier. Hence, they require vigilant long-term care, particularly for non-cancer-related conditions.
Previous studies have suggested that the laparoscopic approach can benefit older patients with pulmonary dysfunction4,19. In fact, our results revealed that restrictive pulmonary impairment did not increase the rate of postoperative complications including pneumonia in older patients who underwent laparoscopic gastrectomy. Previous studies have reported similar results. Huh et al. showed that preoperative pulmonary impairments were not associated with postoperative pulmonary complications in patients who underwent laparoscopic gastrectomy22. Kimura et al. reported that the presence of preoperative pulmonary disease was not a risk factor for postoperative pneumonia in older patients who underwent laparoscopic surgery23. This could be attributed to the reduced invasiveness of laparoscopic surgery, as evidenced by a large nationwide retrospective analysis indicating a lower rate of postoperative pneumonia in laparoscopy than in laparotomy10. One reason of this fact may be that laparoscopic surgery can avoid the epigastrium incision. Park et al. argued that larger incisions in the epigastrium may cause more pain, limit deep breathing, and decrease pulmonary function24,25. Indeed, postoperative pulmonary complications were more frequently observed after open surgery than after laparoscopic surgery in this cohort. Therefore, the laparoscopic approach may be a favorable option for patients with pulmonary dysfunction to help reduce postoperative pneumonia.
A previous study identified postoperative pneumonia as a prognostic factor for OS26. However, despite the expected improvement in survival with decreased postoperative pneumonia, restrictive pulmonary impairment continues to exert a significant negative influence on long-term prognosis, even in patients undergoing laparoscopic gastrectomy. This is because restrictive dysfunction can reflect the patients’ overall health status and condition, as mentioned above, and even if operative invasiveness is decreased, patients with restrictive pulmonary dysfunction may not tolerate gastrectomy well. In particular, older patients with both restrictive dysfunction and sarcopenia reportedly have the worst prognosis among those with gastric cancer19. In fact, patients with both low %VC and BMI had a worse OS than those with normal %VC (5-year OS: 29.0% vs. 74.4%, p < 0.001), whereas patients with low %VC without low BMI did not show significant prognostic differences compared to those with normal %VC (5-year OS: 51.4% vs. 74.4%, p = 0.401) in this cohort. Recent studies have indicated that preoperative rehabilitation can improve both inspiratory muscle strength27 and sarcopenic conditions28. However, these improvements did not enhance patients’ outcomes. Thus, new approaches are needed to ameliorate the outcomes of patients with restrictive dysfunction.
This study had some limitations. First, we exclusively evaluated spirometric parameters for preoperative pulmonary function. Other tests, such as lung diffusion capacity, cardiopulmonary function tolerance, and blood gas, could have helped to precisely evaluate pulmonary function. Second, patients with a low %VC had advanced pathological stages, potentially affecting their prognosis. However, no significant difference in the 5-year CSS was found in this study. Therefore, the impact of this bias is considered to be relatively small. Third, this was a single-center, retrospective study. Further studies with larger prospective cohorts are required to obtain more compelling results.
In conclusion, the presence of low preoperative %VC increased the risk of postoperative pneumonia in older patients with gastric cancer, but not in those who underwent laparoscopic gastrectomy. However, irrespective of the use of laparoscopy, decreased %VC negatively affected the survival outcomes. Although curative laparoscopic gastrectomy can be safely performed in patients with pulmonary dysfunction, careful postoperative and long-term management are required.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Inoue, M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer 20, 3–7 (2017).
Kanat, F., Golcuk, A., Teke, T. & Golcuk, M. Risk factors for postoperative pulmonary complications in upper abdominal surgery. ANZ J. Surg. 77, 135–141 (2007).
Jeong, O., Ryu, S. Y. & Park, Y. K. The value of preoperative lung spirometry test for predicting the operative risk in patients undergoing gastric cancer surgery. J. Korean Surg. Soc. 84, 18–26 (2013).
Sekimoto, A., Miyake, H., Nagai, H., Yoshioka, Y. & Yuasa, N. Significance of preoperative pulmonary function on short- and long-term outcomes following gastrectomy for gastric cancer. J. Gastrointest Surg. 27, 866–877 (2023).
Feng, F. et al. Low forced vital capacity predicts poor prognosis in gastric cancer patients. Oncotarget. 8, 28897–28905 (2017).
Inaki, N. et al. A Multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J. Surg. 39, 2734–2741 (2015).
Kim, W. et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann. Surg. 263, 28–35 (2016).
Katai, H. et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 20, 699–708 (2017).
Etoh, T. et al. Five-Year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: The JLSSG0901 randomized clinical trial. JAMA Surg. 158, 445–454 (2023).
Honda, M. et al. Surgical risk and benefits of laparoscopic surgery for elderly patients with gastric cancer: a multicenter prospective cohort study. Gastric Cancer 22, 845–852 (2019).
Godfrey, M. S. & Jankowich, M. D. The vital capacity is vital: Epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 149, 238–251 (2016).
Brierley, J. D., Gospodarowicz, M. K. & Wittekind, C. TNM Classification of Malignant Tumours (Wiley, 2016).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 250, 187–196 (2009).
Ntutumu, R. et al. Risk factors for pulmonary complications following laparoscopic gastrectomy: A single-center study. Medicine. 95, e4567 (2016).
Tu, R. H. et al. Prognostic significance of postoperative pneumonia after curative resection for patients with gastric cancer. Cancer Med. 6, 2757–2765 (2017).
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 24, 1–21 (2021).
Miki, Y. et al. Risk factors for postoperative pneumonia after gastrectomy for gastric cancer. Surg. Today. 46, 552–556 (2016).
Sugawara, K. et al. Preoperative restrictive pulmonary dysfunction influences the survival after gastrectomy for elderly patients with gastric carcinoma. Surg. Today. 50, 1065–1073 (2020).
Jeon, Y. K. et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos Int. 26, 2423–2429 (2015).
Shirai, H. et al. Preoperative low muscle mass and low muscle quality negatively impact on pulmonary function in patients undergoing hepatectomy for hepatocellular carcinoma. Liver Cancer. 7, 76–89 (2018).
Huh, J., Sohn, T. S., Kim, J. K., Yoo, Y. K. & Kim, D. K. Is routine preoperative spirometry necessary in elderly patients undergoing laparoscopy-assisted gastrectomy?. J. Int. Med. Res. 41, 1301–1309 (2013).
Kimura, R. et al. Risk factors for postoperative pneumonia after laparoscopic gastrectomy in patients aged 75 years and over with gastric cancer. Asian J. Endosc. Surg. 14, 408–416 (2021).
Park, S. H. et al. Postoperative morbidity and quality of life between totally laparoscopic total gastrectomy and laparoscopy-assisted total gastrectomy: A propensity-score matched analysis. BMC Cancer. 21, 1016 (2021).
Park, S. H. et al. Totally laparoscopic versus laparoscopy-assisted distal gastrectomy: The KLASS-07, a randomized controlled trial. Int. J. Surg. 2, 63 (2024).
Suzuki, S. et al. Long-term impact of postoperative pneumonia after curative gastrectomy for elderly gastric cancer patients. Ann. Gastroenterol. Surg. 2, 72–78 (2018).
Valkenet, K. et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br. J. Surg. 105, 502–511 (2018).
Yamamoto, K. et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. 20, 913–918 (2017).
Author information
Authors and Affiliations
Contributions
Study conception design by K.T., S.S. and K.Y. Data acquisition and data analysis and interpretation by all authors. Drafting the article by K.T. and S.S. Critical revision for intellectual content by all authors. All authors approved of the final manuscript and agreed to be accountable for all aspects of work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takabatake, K., Sakuramoto, S., Kobayashi, R. et al. Prognostic impact of pulmonary dysfunction in older gastric cancer patients. Sci Rep 14, 19605 (2024). https://doi.org/10.1038/s41598-024-68806-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68806-9