Abstract
Eriodictyol, a flavonoid distributed in citrus fruits, has been known to exhibit anti-inflammatory activity. In this study, destabilized medial meniscus (DMM)-induced OA model was used to investigate the protective role of eriodictyol on OA. Meanwhile, we used an IL-1β-stimulated human osteoarthritis chondrocytes model to investigate the anti-inflammatory mechanism of eriodictyol on OA. The production of nitric oxide was detected by Griess reaction. The productions of MMP1, MMP3, and PGE2 were detected by ELISA. The expression of LXRα, ABCA1, PI3K, AKT, and NF-κB were measured by western blot analysis. The results demonstrated that eriodictyol could alleviate DMM-induced OA in mice. In vitro, eriodictyol inhibited IL-1β-induced NO, PGE2, MMP1, and MMP3 production in human osteoarthritis chondrocytes. Eriodictyol also suppressed the phosphorylation of PI3K, AKT, NF-κB p65, and IκBα induced by IL-1β. Meanwhile, eriodictyol significantly increased the expression of LXRα and ABCA1. Furthermore, eriodictyol disrupted lipid rafts formation through reducing the cholesterol content. And cholesterol replenishment experiment showed that adding water-soluble cholesterol could reverse the anti-inflammatory effect of eriodictyol. In conclusion, the results indicated eriodictyol inhibited IL-1β-induced inflammation in human osteoarthritis chondrocytes through suppressing lipid rafts formation, which subsequently inhibiting PI3K/AKT/NF-κB signaling pathway.
Similar content being viewed by others
Introduction
Osteoarthritis is one of the most common diseases in the elderly and an important cause of disability1. The major pathological change of osteoarthritis is cartilage degeneration2. Previous studies demonstrated that cartilage degeneration was closely related to inflammation3. Inflammation can promote the degeneration of cartilage, and cartilage degeneration can stimulate the development of inflammation4. Inflammatory cytokines can induce the destruction of chondrocytes and extracellular matrices5. Furthermore, these inflammatory cytokines could induce the release of matrix metalloproteinases (MMP), which could lead to the destruction of cartilage6. Therefore, inhibition of the inflammation may attenuate the destruction of cartilage and have protective effects of OA.
Eriodictyol (Fig. 1A) is a flavonoid distributed in citrus fruits. Accumulated evidences suggested that eriodictyol has anti-inflammatory effect7. Eriodictyol was found to inhibit LPS-induced acute lung injury in mice8. Eriodictyol also suppressed LPS-induced inflammatory cytokine production in RAW264.7 cells9. Also, eriodictyol was found to reduce liver injury induced by arsenic trioxide10. Furthermore, eriodictyol has been reported to attenuate myocardial ischemia–reperfusion injury11. In addition, eriodictyol was found to inhibit RANKL-induced osteoclast formation12. However, the effects of eriodictyol on IL-1β-induced inflammatory response in human osteoarthritis chondrocytes have not been reported. In this study, we found eriodictyol inhibited IL-1β-induced inflammation through suppressing PI3K/AKT/NF-κB signaling pathway.
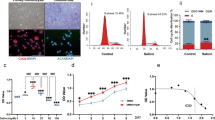
(A) The chemical structure of eriodictyol. (B) Effects of eriodictyol on the cell viability of human osteoarthritis chondrocytes. Cells were cultured with different concentrations of eriodictyol (5, 10, 15, 20, 25 μM) for 24 h. The cell viability was determined by MTT assay. The values presented are the means ± SEM of three independent experiments. **p < 0.01 versus control group.
Experimental section
Reagents
Eriodictyol (purity > 98%) was purchased from Chengdu Plant Standardized Pure Biotechnology Co., Ltd. (Chengdu, China). LXRα, ABCA1, PI3K, AKT, NF-κB p65, and IκBα antibodies were obtained from Cell Signaling Techonology (Beverly, MA, USA). Recombinant human IL-1β and the ELISA kits were purchased from R&D systems (Minneapolis, MN, USA).
In vivo study
This study was approved by the Ethics Committee of Jilin University (number 20220628). All protocols comply with the NIH Guidelines and were reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). The animal experiment was approved by the Ethical Committee of Jilin University. Fifty C57BL/6 mice were divided into five groups: Sham surgery group, DMM model group, and eriodictyol (10, 20, 40 mg/kg) + DMM groups. The OA model was established as previously described13. Eriodictyol (10, 20, 40 mg/kg) was given by intraperitoneal injection. Then, the cartilage tissues were collected and stained with H&E staining, Masson’s trichrome staining, and western blot analysis.
Cell culture and cytotoxicity assay
Human articular cartilage of six osteoarthritis patients (age: 50 ± 6) were collected according to the guidelines of the Declaration of Helsinki and Tokyo. The experiment was approved by Ethics Committee of Jilin University. The human chondrocytes were isolated and cultured as described previously14. Passage 2–4 were used in the present study15.
The cells in logarithmic growth phase were digested seeded in 96-well plates at 5 × 103 cells/well and cultured 24 h. Then, eriodictyol was added to each well for six replicates and subsequently the supernatant was removed and 20 μl MTT (5 mg/ml) was added to each well for 4 h. Then, 200 μl DMSO was added to fully dissolve the crystals. Absorbance was determined at 540 nm on a microplate reader (TECAN, Austria).
Measurement of PGE2 and NO
Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 24 h after IL-1β treatment, the supernatants were collected. The production of NO was measured by detecting the level of accumulated nitrite in the supernatants by the Griess reagent according to the manufacturer’s instructions (Beyotime, Shanghai, China)16,17. The level of PGE2 in the supernatants was measured by the ELISA kit according to the (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Measurement of MMPs
Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 24 h after IL-1β treatment, the supernatants were collected. The supernatants were centrifuged at 1000 rpm/min for 5 min. The production of MMP1 and MMP3 were tested by ELISA using commercial kits (R&D systems, Minneapolis, MN, USA)18. The contents of MMP1 and MMP3 in each sample were calculated according to the standard curve.
Western blot analysis
Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 30 min after IL-1β treatment, the supernatants were collected. Chondrocytes were lysed with RIPA buffer with 1% protease inhibitor to obtain proteins. The protein concentration was detected by BCA method. The proteins were separated by SDS-PAGE and transferred onto PVDF membranes. The blots were cut prior to hybridisation with antibodies. After incubated with primary and secondary antibodies, the membranes were detected by the enhanced chemiluminescence detection agent and the bands were calculated using the Image J software (National Institutes of Health, USA).
Lipid rafts staining and cholesterol replenishment experiment
Human osteoarthritis chondrocytes were fixed in 4% formaldehyde and stained with 5 µg/ml Alexa Fluor 488-conjugated CTxB for 30 min and stained with Hochest for 5 min. Cholesterol content in lipid raft was assayed by gas–liquid chromatography as described previously19. For cholesterol replenishment experiment, the cells were treated with water-soluble cholesterol (84 µg/ml) for 30 min. Subsequently, the levels of inflammatory cytokines were measured.
Statistical analysis
The data are presented as the mean ± S.E.M. and the experiment was carried in triplicate. Comparisons between different groups were analyzed by one-way analysis of variance. P < 0.05 were considered to indicate statistical significance.
Results
Effects of eriodictyol on chondrocyte viability
MTT assay was used to detect the cytotoxicity of eriodictyol. The results showed that eriodictyol at the concentrations of 0–15 μM did not affect the viability of chondrocytes (Fig. 1B). Eriodictyol at the concentrations of 20 μM and 25 μM significantly decreased the viability of chondrocytes (Fig. 1B). The IC50 of eriodictyol is 44.7 μM.
Effects of eriodictyol on IL-1β-induced NO and PGE2 production
NO and PGE2 are important inflammatory mediators of OA. In the present study, we firstly evaluated the effects of eriodictyol on NO and PGE2 production. As shown in Fig. 2, IL-1β significantly increased the production of NO and PGE2 when compared to the control group. However, IL-1β-induced NO and PGE2 production were dose-dependently inhibited by the treatment of eriodictyol (Fig. 2).
Eriodictyol inhibits IL-1β-induced PGE2 and NO production. Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 24 h after IL-1β treatment, the supernatants were collected. PGE2 and NO levels in the supernatants were tested by the detection kits. The data presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Effects of eriodictyol on IL-1β-induced MMP1 and MMP3 production
MMPs play a critical role in the pathogenesis of OA. In the present study, we secondly evaluated the effects of eriodictyol on MMP1 and MMP3 production. As shown in Fig. 3, IL-1β significantly increased the production of MMP1 and MMP3 when compared to the control group. However, IL-1β-induced MMP1 and MMP3 production were dose-dependently inhibited by the treatment of eriodictyol (Fig. 3).
Eriodictyol inhibits IL-1β-induced MMP1 and MMP3 production. Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 24 h after IL-1β treatment, the supernatants were collected. MMP1 and MMP3 levels in the supernatants were tested by the detection kits. The data presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Effects of eriodictyol on IL-1β-induced NF-κB activation
To clarify the anti-inflammatory mechanism of eriodictyol, we evaluated the effects of eriodictyol on NF-κB activation. As shown in Fig. 4, IL-1β significantly increased the levels of phosphorylated NF-κB p65 and IκBα when compared to the control group. However, IL-1β-induced NF-κB activation was dose-dependently inhibited by the treatment of eriodictyol (Fig. 4). Meanwhile, nucleus and cytoplasm p65 expression were tested. As shown in Fig. 4, IL-1β significantly increased the expression of nucleus p65 and decreased cytoplasm p65 expression when compared to the control group. These changes induced by IL-1β were reversed by eriodictyol treatment (Fig. 4).
Eriodictyol inhibits IL-1β-induced NF-κB activation and IκBα degradation. Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 30 min after IL-1β treatment, the cells were collected for western blot analysis. β-actin was used as a control. The values presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Effects of eriodictyol on IL-1β-induced PI3K and AKT expression
To further clarify the anti-inflammatory mechanism of eriodictyol, we evaluated the effects of eriodictyol on PI3K/AKT signaling pathway. The results showed that IL-1β significantly increased the levels of phosphorylated PI3K and AKT. Eriodictyol attenuated IL-1β-induced phosphorylation of PI3K and AKT, which are upstream molecules of NF-κB, in IL-1β-stimulated cells (Fig. 5).
Effects of eriodictyol on IL-1β-induced PI3K and AKT expression. Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 30 min after IL-1β treatment, the cells were collected for western blot analysis. The values presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Effects of eriodictyol on LXRα and ABCA1 expression
LXRα-ABCA1 signaling was related to the regulation of cholesterol. Lipid rafts are plasma membrane microdomains that contain high concentrations of cholesterol and glycosphingolipids. In this study, we evaluated the effects of eriodictyol on LXRα and ABCA1 expression. The results showed that IL-1β significantly decreased the expression of LXRα and ABCA1. Eriodictyol significantly increased the expression of LXRα and ABCA1, which results in disrupting lipid rafts by depleting cholesterol (Fig. 6).
Effects of eriodictyol on LXRα-ABCA1 expression. Chondrocytes were pretreated with eriodictyol (5, 10, 15 μM) 12 h before IL-1β (10 ng/ml) treatment. 30 min after IL-1β treatment, the cells were collected for western blot analysis. The values presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Eriodictyol exhibits anti-inflammatory effects through disrupting the formation of lipid rafts
Lipid rafts has been known to play an important role in PI3K/AKT signaling pathway. The results of this study showed that eriodictyol could disrupt lipid rafts formation (Fig. 7) by decreased cholesterol level in lipid rafts (Fig. 8). And cholesterol replenishment experiment demonstrated that adding cholesterol to replenish lipid rafts could reverse the anti-inflammatory effects of eriodictyol (Fig. 9).
Effects of eriodictyol on the formation of lipid rafts. (A) Control group; (B) IL-1β group; (C) IL-1β + eriodictyol (5 μM) group; (D) IL-1β + eriodictyol (10 μM) group; (E) IL-1β + eriodictyol (15 μM) group. The values presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus IL-1β group.
Eriodictyol inhibits OA development in an OA model
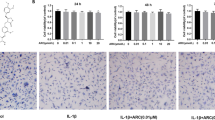
H&E and Masson’s trichrome staining showed that the control group’s joint cartilage tissue exhibited a smooth articular surface, rich matrix, no defect site, and a regular arrangement of cells in each layer of the joint cartilage, with visible tidal lines. The experimental group experienced cartilage degeneration, experienced matrix loss. The defect was located in the superficial layer of the joint surface, crossing the tidal line and invading the calcified cartilage area. The tidal line was damaged or even disappeared. However, these changes were significantly inhibited by eriodictyol (Fig. 10). Meanwhile, the expression of MMP1, MMP3, iNOS, and COX-2 in cartilage tissues were detected. The results showed that the expression of MMP1, MMP3, iNOS, and COX-2 in cartilage tissues increased significantly in OA group. However, eriodictyol (10, 20, 40 mg/kg) treatment significantly inhibited MMP1, MMP3, iNOS, and COX-2 expression in OA model (Fig. 10F).
Eriodictyol inhibits OA development in a DMM model. (A,a) Sham surgery group, (B,b) DMM model group, and (C–E,c–e) eriodictyol (10, 20, 40 mg/kg) + DMM groups. (F) The effects of eriodictyol (10, 20, 40 mg/kg) on MMP1, MMP3, iNOS, and COX-2 expression. The values presented are the means ± SEM of three independent experiments. #p < 0.01 versus control group; **p < 0.01 versus OA group.
Discussion
Flavonoids are secondary metabolites found in fruits and plants, with varied polyphenolic structures. Generally, these polyphenolic compounds have a basic 15-carbon backbone, consisting of two benzene rings (C6) joined by a linear-3-carbon chain (C3), which may be represented by C6–C3–C620. Eriodictyol is a flavonoid distributed in citrus fruits that has been reported to have anti-inflammatory effects9. In a detailed study of the volumes of distributions of eriodictyol, hesperetin, and naringenin metabolites in rats, flavanone metabolites in blood plasma are observed to exhibit preferential binding to tissues and to reside in the body21. Eriodictyol metabolites can enter in the blood and may enter into cartilage tissue through the blood. In this study, we evaluated the anti-inflammatory effects of eriodictyol on osteoarthritis. The results demonstrated that eriodictyol exhibited anti-inflammatory effects on osteoarthritis through inhibiting IL-1β-induced NO and PGE2 production, as well as MMP1 and MMP3 production. The mechanism was through inhibiting PI3K/AKT/NF-κB signaling pathway.
Inflammation was involved in the development of OA22. Secreted inflammatory mediators were involved in the disturbed processes implicated in OA pathophysiology23. IL-1β is one of the most important cytokine that involved in the pathogenesis of OA24. It could induce the production of MMPs which leads to the degeneration of articular cartilage matrix24. Previous studies showed that stimulation of chondrocytes by IL-1β could induce the release of MMP1, MMP3, and MMP1325. These enzymes have the ability to degrade extracellular matrix components. Also, IL-1β could induce the production of inflammatory mediators PGE2 and NO production. The release of NO and PGE2 could suppress the synthesis of collagen and induce apoptosis26. Therefore, inhibition of IL-1β-induced inflammation can be considered potential targets for therapeutic strategies. Our results showed that eriodictyol significantly inhibited IL-1β-induced PGE2, NO, MMP1, and MMP3 production.
NF-κB is an important transcription factor that involved in the regulation of inflammatory mediator and matrix degrading enzymes production27. Activated NF-κB was observed in OA animal model and IL-1β stimulated chondrocytes28. Inhibition of NF-κB activation could reduce the development of OA29. Therefore, targeting of the NF-κB signalling pathway is a promising therapeutic strategy for OA treatment30. The present study we showed that eriodictyol significantly inhibited IL-1β-induced NF-κB activation. PI3K and AKT are upstream molecules of NF-κB signaling pathway. Furthermore, studies showed that inhibition of PI3K/AKT signaling pathway could attenuate the development of OA31. Therefore, we detected the effects of eriodictyol on PI3K and AKT expression. The results showed that eriodictyol significantly attenuated IL-1β-induced phosphorylation of PI3K and AKT. Lipid rafts has been known to play an important role in PI3K/AKT signaling pathway32. A previous study showed that inhibiting the formation of lipid rafts could suppress PI3K/AKT signaling pathway33. In this study, we found eriodictyol could disrupt lipid rafts formation by deleting cholesterol. And cholesterol replenishment experiment demonstrated that adding cholesterol to replenish lipid rafts could reverse the anti-inflammatory effects of eriodictyol.
Liver X receptor (LXR) is a ligand activated nuclear transcription factor that is a member of the nuclear receptor superfamily34. Currently, two subtypes of human LXR have been identified, namely LXRα and LXRβ35. LXRα, as a receptor for cholesterol metabolism and lipid biosynthesis, is activated by the oxidized form of endogenous cholesterol (oxysterols) and is an important regulatory factor in cholesterol and lipid metabolism processes36. ABCA1 is a plasma membrane protein which plays an important role in the movement of cholesterol37. The main function of ABCA1 is to promote the transport of intracellular free cholesterol and phospholipids to extracellular lipoprotein A-I (apoA-I), thereby maintaining cellular cholesterol homeostasis38. ABCA1 is a downstream gene of LXRα, and the use of LXRα agonist T0901317 can promote the expression of ABCA139. Recent studies demonstrated that activating LXRα-ABCA1 signaling pathway could disrupt lipid rafts by depleting cholesterol40,41. Therefore, the effect of eriodictyol on LXRα-ABCA1 signaling pathway was detected. In this study, we found eriodictyol could activate LXRα-ABCA1, which results in disrupting lipid rafts by depleting cholesterol.
Conclusions
In conclusion, this study revealed that eriodictyol inhibited IL-1β-induced inflammation in chondrocytes. Eriodictyol inhibited IL-1β-induced inflammation through activating LXRα-ABCA1 and inhibiting lipid rafts formation, which led to the inhibition of PI3K/AKT/NF-κB signaling pathway. Eriodictyol may be used as an anti-inflammatory agent for the treatment of osteoarthritis.
Data availability
All relevant data of this research can be requested from the corresponding author.
Change history
10 October 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-74713-w
References
Hofman, A., Grobbee, D. E., de Jong, P. T. & van den Ouweland, F. A. Determinants of disease and disability in the elderly: The Rotterdam Elderly Study. Eur. J. Epidemiol. 7, 403–422 (1991).
Goldring, S. R. & Goldring, M. B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal Interact. 6, 376–378 (2006).
Guilak, F. et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin. Orthop. Relat. Res. 423, 17–26 (2004).
Sokolove, J. & Lepus, C. M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 5, 77–94 (2013).
Goldring, S. R. & Goldring, M. B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 427, S27–S36 (2004).
Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 16, 197–205 (2006).
Deng, Z. et al. Pharmacological activity of eriodictyol: The major natural polyphenolic flavanone. Evid. Based Complement. Altern. Med. 2020, 6681352 (2020).
Zhu, G. F., Guo, H. J., Huang, Y., Wu, C. T. & Zhang, X. F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp. Ther. Med. 10, 2259–2266 (2015).
Lee, J. K. Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine macrophages. Arch. Pharm. Res. 34, 671–679 (2011).
Xie, G., Meng, X., Wang, F., Bao, Y. & Huo, J. Eriodictyol attenuates arsenic trioxide-induced liver injury by activation of Nrf2. Oncotarget 8, 68668–68674 (2017).
Li, D. et al. Eriodictyol attenuates myocardial ischemia-reperfusion injury through the activation of JAK2. Front. Pharmacol. 9, 33 (2018).
Song, F. et al. Eriodictyol inhibits RANKL-induced osteoclast formation and function via inhibition of NFATc1 activity. J. Cell. Physiol. 231, 1983–1993 (2016).
Gao, W. et al. CHMP5 attenuates osteoarthritis via inhibiting chondrocyte apoptosis and extracellular matrix degradation: Involvement of NF-kappaB pathway. Mol. Med. 30, 55 (2024).
Cheng, A. W. M., Stabler, T. V., Bolognesi, M. & Kraus, V. B. Selenomethionine inhibits IL-1 beta inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary human chondrocytes. Osteoarthr. Cartil. 19, 118–125 (2011).
Ma, C. et al. The role of PPAR gamma in advanced glycation end products-induced inflammatory response in human chondrocytes. PLoS ONE 10, e0125776 (2015).
Sun, J. Y., Song, X. J., Wang, C. J. & Ruan, Q. Geniposidic acid alleviates osteoarthritis progression through inhibiting inflammation and chondrocytes ferroptosis. J. Cell. Mol. Med. 28, e18228 (2024).
Sun, J. Y., Zhang, Y. F., Wang, C. J. & Ruan, Q. Kukoamine A protects mice against osteoarthritis by inhibiting chondrocyte inflammation and ferroptosis via SIRT1/GPX4 signaling pathway. Life Sci. 332, 122117 (2023).
Wang, C., Zeng, L., Zhang, T., Liu, J. & Wang, W. Tenuigenin prevents IL-1beta-induced inflammation in human osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-kappaB signaling pathway. Inflammation 39, 807–812 (2016).
Fu, Y. H. et al. Cyanidin-3-O-beta-glucoside ameliorates lipopolysaccharide-induced acute lung injury by reducing TLR4 recruitment into lipid rafts. Biochem. Pharmacol. 90, 126–134 (2014).
Rufino, A. T. et al. Rheumatoid arthritis molecular targets and their importance to flavonoid-based therapy. Med. Res. Rev. 44, 497–538 (2024).
Yáñez, J. A. et al. Pharmacokinetics of selected chiral flavonoids: Hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 29, 63–82 (2008).
Wojdasiewicz, P., Poniatowski, L. A. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 561459 (2014).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Daheshia, M. & Yao, J. Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 35, 2306–2312 (2008).
Jia, T., Qiao, J., Guan, D. & Chen, T. Anti-inflammatory effects of licochalcone A on IL-1beta-stimulated human osteoarthritis chondrocytes. Inflammation 40, 1894–1902 (2017).
Henrotin, Y. E., Bruckner, P. & Pujol, J. P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 11, 747–755 (2003).
Panday, A. et al. Transcription factor NF-kappaB: An update on intervention strategies. Arch. Immunol. Ther. Exp. (Warsz) 64, 463–483 (2016).
Wu, D. et al. Sauchinone inhibits IL-1beta induced catabolism and hypertrophy in mouse chondrocytes to attenuate osteoarthritis via Nrf2/HO-1 and NF-kappaB pathways. Int. Immunopharmacol. 62, 181–190 (2018).
Marcu, K. B., Otero, M., Olivotto, E., Borzi, R. M. & Goldring, M. B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 11, 599–613 (2010).
Roman-Blas, J. A. & Jimenez, S. A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 14, 839–848 (2006).
Sun, K. et al. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 28, 400–409 (2020).
Dufour, C. et al. FGFR2-Cbl interaction in lipid rafts triggers attenuation of PI3K/Akt signaling and osteoblast survival. Bone 42, 1032–1039 (2008).
Ediriweera, M. K., Moon, J. Y., Nguyen, Y. T. K. & Cho, S. K. 10-Gingerol targets lipid rafts associated PI3K/Akt signaling in radio-resistant triple negative breast cancer cells. Molecules 25, 3164 (2020).
Hu, X., Li, S. Z., Wu, J., Xia, C. S. & Lala, D. S. Liver X receptors interact with corepressors to regulate gene expression. Mol. Endocrinol. 17, 1019–1026 (2003).
Stenson, B. M. et al. Liver X receptor (LXR) regulates human adipocyte lipolysis. J. Biol. Chem. 286, 370–379 (2011).
Hong, C. & Tontonoz, P. Liver X receptors in lipid metabolism: Opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 (2014).
Chen, L., Zhao, Z. W., Zeng, P. H., Zhou, Y. J. & Yin, W. J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 21, 1121–1139 (2022).
Huang, C. X. & Zhang, Y. L. The target of regulating the ATP-binding cassette A1 protein (ABCA1): Promoting ABCA1-mediated cholesterol efflux in different cells. Curr. Pharm. Biotechnol. 14, 623–631 (2013).
Zhao, L. et al. The roles of liver X receptor α in inflammation and inflammation-associated diseases. J. Cell. Physiol. 236, 4807–4828 (2021).
Fu, Y. H., Hu, X. Y., Cao, Y. G., Zhang, Z. C. & Zhang, N. S. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radic. Biol. Med. 89, 777–785 (2015).
Ito, A. et al. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 4, e08009 (2015).
Author information
Authors and Affiliations
Contributions
Z.J. designed the experiment; K.W., X.Q., D.H., W.W., and H.G. performed the experiment; K.W. analyzed the data; K.W. and Z.J. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article Wenbo Kang and Qinli Xu were omitted as equally contributing authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, W., Xu, Q., Dong, H. et al. Eriodictyol attenuates osteoarthritis progression through inhibiting inflammation via the PI3K/AKT/NF-κB signaling pathway. Sci Rep 14, 18853 (2024). https://doi.org/10.1038/s41598-024-69028-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69028-9