Abstract
This study aimed to examine whether or not aluminum chloride (AlCl3)-induced hepatotoxicity might be mitigated using magnetic water (MW) in rats. This study involved 28 adult male rats randomly assigned into the following 4 groups (7 rats/group): normal control (Cnt), MW, AlCl3, and Al Cl3 + MW. The Cnt group orally received normal saline, the MW group drank MW ad libitum for 2 months, and the AlCl3 and AlCl3 + MW groups were orally administered AlCl3 (40 mg/kg b.w.) alone or in combination with MW for 2 months, respectively. MW reduced AlCl3 toxicity as proved at functional, molecular, and structural levels. Functionally, MW reduced serum levels of liver enzymes (ALT, AST, ALP, GGT), while increased total proteins, and albumin. MW also restored redox balance in the liver (lower MDA levels, higher activities of CAT and SOD enzymes, and upregulated expression of NrF2, HO-1, and GST genes. Molecularly, MW downregulated hepatic expression of the epigenetic (HDAC3), inflammatory (IL1β, TNFα, NFκβ), and endoplasmic reticulum stress (XBP1, BIP, CHOP) genes. Structurally, MW enhanced liver histology. With these results, we could conclude that MW has the potential to ameliorate the hepatotoxic effects of AlCl3 through targeting oxidative stress, inflammation, epigenesis, and endoplasmic reticulum stress.
Similar content being viewed by others
Introduction
Aluminum (Al) is daily used in food additives, cooking, kitchenware, medications, and cosmetics, and its salts are used in water filtration1, however, it possesses deleterious potential on human and animal health and can reach their body through the ingestion, inhalation, and contact with a notable accumulation in liver, brain, and kidney2. Cytotoxicity, mitochondrial malfunction, genetic damage, and carcinogenicity are all possible outcomes of Al interference with cellular structure and macromolecules3. Al-mediated toxicity has been linked to oxidative stress, inflammation, and the induction of endoplasmic reticulum (ER) stress4,5. By these mechanisms, Al can cause toxicity in several cells/organs, including RBCs6, neurons4,7,8, liver and kidney5,8,9,10,11,12,13,14. Al can trigger oxidative stress and inflammatory cytokines production in the liver, kidney, and nervous tissues5,7,8,11,12,13,15,16.
The term "magnetic water" (MW) refers to water that has been filtered by a magnetic field (magnetic tubes). As a result, the oxygen ratio, the velocity of salts, and the solubility of amino acids in water all increase dramatically17. Unlike normal water which has pH of 7, MW has alkaline pH of 9.2 with higher weight and salinity18. A magnetic field has the potential to lower water's surface tension, which in turn makes the liquid more malleable, thinner, and easier to be absorbed19. Furthermore, it was postulated that magnetic fields could influence hydration and protonation of ions, leading to a distortion and weakening of the H-bond19. As a result, water rich in hydrogen could potentially scavenge reactive oxygen species (ROS)20. Lee, Kang21 and Ohsawa, et al.22 have all put forward the theory that magnetized water reduces oxidative stress. Taken together, MW can maintain the oxidant-antioxidant balance23, and decrease serum levels of liver function enzymes (ALT and AST) in goats24. Besides, MW has no delirious effects on liver and bone health25,26. Blood pressure, oxygen content, nutrition delivery, enzyme activation, metabolic waste removal, and libido all benefit from MW's stimulating effects17.
To the best of our knowledge, the role of MW in AlCl3-induced hepatotoxicity has not been investigated yet. Therefore, this study was conducted to address this scientific gap.
Results
Effect of MW on liver function
The impacts of MW and/or AlCl3 on indicators of liver damage and function are shown in Figs. 1 and 2. Administration of AlCl3 in rats initiated liver damage, as revealed by significantly (p < 0.05) elevated AST, ALT, ALP, GGT, total and indirect bilirubin, relative to the two control (Cnt and MW) groups. In contrast, the AlCl3 + MW group had significantly (p < 0.05) decreased levels of these indicators relative to the AlCl3 group. Total protein, and albumin levels significantly (p < 0.05) declined in the serum of rats administered AlCl3 alone when compared with the control groups. While rats co-administrated with MW and AlCl3 exhibited a significant (p < 0.05) elevation of total proteins and albumin, compared with administrated AlCl3 alone. No significant (p > 0.05) changes in all liver function/damage parameters were noticed between the Cnt and MW groups. These findings suggest that MW may mitigate the hepatic dysfunction caused by AlCl3 intoxication.
Influence of MW and/or AlCl3 on serum levels of liver function enzymes (AST, ALT, ALP, and GGT) in rats. Data are expressed as mean ± SEM (n = 7/group). The differences between the groups, represented by the letters a (highest value)–c (lowest value), are statistically significant at the p < 0.05 level. All the groups were compared to each other. Cnt control group, MW magnetic water group, AlCl3 aluminum chloride group, MW + AlCl3 magnetic water and aluminum chloride group.
Effects of MW and/or AlCl3 on serum levels of liver function parameters (total, direct and indirect bilirubin, total proteins, and albumin) in rats. Data are expressed as mean ± SEM (n = 7/group). The differences between the groups, represented by the letters a (highest value)–c (lowest value), are statistically significant at the p < 0.05 level. All the groups were compared to each other.
Effect of MW on Al liver residue and oxidative/antioxidant status
Results presented in Fig. 3 revealed that rats treated with AlCl3 had significantly (p < 0.05) elevated hepatic Al and MDA levels and significantly (p < 0.05) declined activities of SOD and CAT compared to the control groups. However, the MW and AlCl3 co-treated group had significantly reduced concentration of Al in the liver tissue and consequently decreased MDA levels and higher activities of SOD and CAT relative to the group administrated AlCl3 alone. The antioxidant potential of the MW was proved at the molecular levels using qPCR and the results revealed a significant (p < 0.05) upregulation of the antioxidant NrF2, HO-1, GST genes in the MW + AlCl3 group relative to the AlCl3 group (Fig. 3). The two control groups did not vary significantly (p > 0.05) and the MW and AlCl3 co-treated group was unable to restore all evaluated parameters to normal levels.
Effects of MW and/or AlCl3 on hepatic Al residue, lipid peroxide (MDA) levels, activities of antioxidant enzymes (SOD and CAT), and expression of antioxidant NrF2, HO-1, and GST genes. Data are expressed as mean ± SEM (n = 5/group). The differences between the groups, represented by the letters a (highest value)–c (lowest value), are statistically significant at the p < 0.05 level. All the groups were compared to each other.
Influence of MW on expression of inflammation-related genes
In comparison to the two control groups, the livers of rats exposed to AlCl3 exhibited significantly (p < 0.05) upregulated expression of TNFα, ILIβ, and NFκB genes (Fig. 4). In contrast, when MW and AlCl3 were co-administrated to rats, the expression of these inflammatory genes was significantly (p < 0.05) downregulated compared to when AlCl3 was given alone. However, there was no significant (p > 0.05) change between the two control groups and the MW + AlCl3 group continued to have significantly (p < 0.05) increased mRNA levels compared to the Cnt and MW groups (Fig. 4).
Impact of MW and/or AlCl3 on hepatic expression of inflammatory TNFα, ILIβ, and NFκB genes as detected by qPCR. Data are expressed as mean ± SEM (n = 5/group). The differences between the groups, represented by the letters a (highest value)–c (lowest value), are statistically significant at the p < 0.05 level. All the groups were compared to each other.
Impact of MW on expression of epigenesis and endoplasmic reticulum stress genes
The obtained qPCR results (Fig. 5) showed a significant (p < 0.05) upregulation in the expression levels of the epigenetic (HDAC3) and the endoplasmic reticulum stress (XBP1, BIP, CHOP) genes in the rat liver treated with AlCl3 compared to the two control groups. The MW significantly (p < 0.05) reduced this AlCl3-induced rise, while it was still significantly (p < 0.05) greater than the control groups. There was no significant difference in mRNA levels between the Cnt and MW groups.
Effects of MW and/or AlCl3 on hepatic expression of epigenetic (HDAC3) and the endoplasmic reticulum stress (XBP1, BIP, CHOP) genes as detected by qPCR. Data are expressed as mean ± SEM (n = 5/group). The differences between the groups, represented by the letters a (highest value)–c (lowest value), are statistically significant at the p < 0.05 level. All the groups were compared to each other.
Effect of MW on liver histology
Hepatic cells (arrows) extending outward from the central vein and sinusoids were normal in the livers of both control groups (Fig. 6A and B). In contrast, in the AlCl3 group, we found notable hepatic alterations including dilatation of the central vein (CV) and vacuolation (blue arrows, Fig. 6C), degenerative changes (red arrow), necrosis (black arrow in Fig. 6D), and infiltration of mononuclear inflammatory cells (black arrow), and rupture of the endothelial cells of the CV (arrowhead, Fig. 6E). In contrast, the MW + AlCl3 group restored liver histology to normal levels (arrow) as in the Cnt and MW groups (Fig. 6F).
Photomicrographs of liver sections of (A) the normal control group (Cnt); (B) the magnetic water group (MW); (C–E) the aluminum chloride group (AlCl3); (F) the magnetic water and aluminum chloride group (MW + AlCl3). All slides were stained with H&E, scale bars = 40 µm. The text included explanations for all labels including arrows, arrowheads, and abbreviations. central vein (CV).
Discussion
Al cookware is still used in rural areas because it is cheap, even though long-term exposure to it is dangerous. After prolonged exposure, AI may accumulate in the liver, kidneys, and brain, producing cytotoxicity. MW, a hexagonal water that closely resembles the water found within our bodies, is produced by passing regular tap water through a magnetically-enhanced device designed to energize and ionize the water molecules. MW can prevent and treat diseases by enhancing metabolism and bioactivation21. However, little is known regarding its protective role in hepatotoxicity induced by AlCl3. Therefore, this study aimed to check this role. Our results revealed hepatoprotective effects of MW against AlCl3-induced oxidative stress, and epigenetic, molecular, and histopathological changes in rats.
Our data revealed that oral administration of AlCl3 for 2 months was enough for Al to accumulate in the liver. In parallel, several studies reported Al accumulation in the liver and kidney of rodents after a prolonged exposure to AlCl3 for 2 months10,16. In the present study, rats treated with AlCl3 for 2 months showed signs of hepatotoxicity as proved at functional, molecular, and structural levels. At the functional level, these rats had significantly increased serum levels of liver function enzymes (AST, ALT, ALP, and GGT) compared to animals in the normal control group. This elevation is consistent with previous reports and confirms hepatotoxicity induced by AlCl3 and Al nanoparticles5,10,12,13,14,16. AlCl3 induced damage in the cell membrane of hepatocytes leading to the release of these enzymes into the circulation5,10. On the other hand, the MW reduced the AlCl3 hepatotoxicity as revealed by decreased serum levels of these hepatic enzymes. Consistent with our results, Yacout, et al.27 documented that goats drank MW had significantly lower ALT and AST than those who drank non-magnetic water. Similarly, broiler chickens and rabbits who drank MW had significantly lower levels of ALT, AST, ALP, and GGT than those who drank non-magnetic water28,29. Hepatocyte dysfunction, aberrant hemolysis, and biliary obstruction illness can contribute to an elevated bilirubin level30,31,32. The current study showed a significant elevation in the total and indirect bilirubin levels in the AlCl3 group. This was in agreement with Newairy, et al.15 who found that bilirubin levels rose after AlCl3 administration, resulting in liver damage. This elevated bilirubin was attributed to the destructive effect of AlCl3 on erythrocytes33, and free radical overproduction34. In contrast, the use of MW reduced the level of total and indirect bilirubin, which is consistent with Helal, et al.29. Total protein and albumin levels were likewise observed to be significantly reduced in AlCl3-treated rats. Tripathi et al.35 showed a similar decrease in total protein and albumin values. However, Ahmed et al.36 did not find any significant results in total protein and albumin concentrations in rats treated with AlCl3. Again, rats who drank MW exhibited significantly lower total protein and albumin levels than the AlCl3 group. Taken together, hepatic function impairment caused by AlCl3 could be ameliorated by MW.
Although the exact mechanism of Al toxicity is still poorly known, it has been hypothesized that ROS play a major role in Al-induced hepatorenal damage10,13,16. In support, we reported higher MDA and lower CAT and SOD hepatic levels and downregulated expression of NrF2, HO-1, and GST genes in the AlCl3 group. In agreement, several previous studies reported that AlCl3 could induce hepatotoxicity through the disturbance of redox balance as evidenced by increasing oxidative marker (MDA) and decreasing antioxidant enzyme activities of CAT, SOD, and GPx and GST levels and the associated antioxidant NrF2 and HO-1 genes10,12,13,16. Industrial workers with the greatest Al levels in urine also had reduced GST enzymatic activity in erythrocytes37. Downregulated expression of NrF2, HO-1, and GST genes is associated with several hepatic diseases32,38,39,40,41. Thus, the observed increase in free radicals could be partly attributed to the concomitant reduction of CAT, SOD, GPx and GST levels and the associated antioxidant NrF2 and HO-1 genes following AlCl3 treatment. However, the MW group showed significantly lower MDA and significantly higher SOD and CAT and upregulated expression of NrF2, HO-1, and GST genes compared to the AlCl3 control. Consistent with our results, MW increased the activity of the antioxidant enzymes in rodents21,42. The powerful antioxidant properties of MW may account for its hepatoprotective effects23. An H-bond distortion and weakening due to magnetic resonance-induced alterations in hydrogen protons was postulated to influence ion hydration and protonation. Thus, hydrogen-rich water, such as MW, may remove ROS20.
At a molecular level, qPCR results showed a significant upregulation in the hepatic expression levels of the epigenetic HDAC3 and inflammatory (IL1β, TNFα, NFκβ) genes in the AlCl3 group, highlighting the importance of epigenetics and inflammation in AlCl3 toxicity. Similarly, AlCl3 has the potential to induce the accumulation of mononuclear inflammatory cells and subsequent overproduction of inflammatory cytokines (NFκB, IL1β, IL6, and TNFα) in liver, kidney, and nervous tissues of rats7,8,13,43. Inhibitors of HDACs have been shown to suppress the expression of inflammatory genes44. In parallel, our research showed that MW treatment reduced HDAC3 and inflammatory genes (IL1β, TNFα, NFκβ) expression. Liver cancer cell proliferation was slowed and xenograft tumor development was stopped when HDAC3 expression was suppressed45.
In the current work, we described a unique method by which AlCl3 might mediate its hepatotoxic impact. AlCl3 injection was shown to significantly upregulate ER stress-related genes (XBP1, BIP, CHOP) in the liver, a finding we reported for the first time here. Misfolded proteins in the ER set off a cascade of events that relieve stress, restore homeostasis, and promote cell survival by activating ER stress transducers such as XBP1 and BIP46. In contrast, persistent ER stress may trigger apoptotic signaling via CHOP regulation, and its downstream targets Bax, suggesting that the adaptive process has failed, and the cell has switched to the proapoptotic pathway47. In agreement, AlCl3 induced oxidative stress that triggered ER stress, as revealed by higher expression of PERK, EIF2-α, CHOP, and HMGB1, resulting in apoptosis of neurons4,43. The use of chaperones to reduce ER stress as a treatment for liver disease gained popularity over time48. We believe this to be the first research to disclose that MW could ameliorate AlCl3-induced ER stress and the subsequent apoptosis as noticed by the inhibition of the ER stress genes in rat liver. Magnetic fields influence cell proliferation and can alter cellular metabolism, function, and stress response to serve as a self-protective strategy24,49.
Histologically, AlCl3 induced damage in the liver tissue and this agrees with several previous studies which showed distorted liver architecture, degenerative changes, necrosis, dilation of the central vein, and infiltration of mononuclear inflammatory cells following AlCl3 administration9,10,13,16. These histopathological alterations could be attributed to an increase in ROS and accumulation of Al in the liver tissues, overproduction of inflammatory cytokines, and activation of ER stress. Rats co-treated with MW and AlCl3 showed improved liver histology. Research on the histopathological effects of MW on animals, and in particular the liver of rats, seems to be limited. We and Elmoslemany, et al.26 found that rats given MW had normal hepatic histology.
While our study elucidated the protective mechanisms of MW against AlCl3-induced hepatotoxicity through its antioxidant, anti-inflammatory, and ER stress mitigating properties, a limitation lies in the absence of Western blot analysis to directly detect changes in protein expression levels of key markers such as XBP1, BIP, and CHOP. The inclusion of Western blot experiments could have provided more precise insights into the molecular pathways affected by MW treatment, potentially strengthening the mechanistic understanding behind its therapeutic effects. Future studies incorporating Western blot analysis could further validate the observed alterations in protein expression and offer a more comprehensive understanding of the underlying mechanisms involved in the mitigation of hepatotoxicity by MW.
Conclusions
Through their antioxidant, anti-inflammatory, and ER stress mitigating capabilities, MW provides protection against AlCl3-induced hepatotoxicity. This beneficial impact on health may be attributable to the suppression of inflammatory and ER stress markers (XBP1, BIP, and CHOP). Thus, these biomarkers could represent therapeutic targets in AlCl3-induced hepatotoxicity. However, further research is required to determine the specific method of action of MW and to confirm the efficacy and safety of MW therapy in people.
Materials and methods
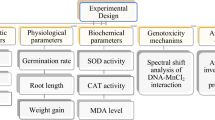
Experimental design
All experimental techniques were approved by the KFS-IACUC ethics committee (KFS-IACUC/146/2023) in Kafrelsheikh University, Egypt. All methods were performed in accordance with ARRIVE guidelines and regulations. All methods were carried out in accordance with relevant guidelines and regulations. Twenty-eight Sprague dawley adult male rats (100 ± 10g) were kept in cages with the usual circumstances (a 12:12 light: dark cycle, a temperature and humidity range of 23 2 °C, and 60% relative humidity). The rats were given pellets to eat and access to food and drink at all times. After the rats had adapted, they were split into four groups of seven at random. Animals of the normal control group (Cnt) were given normal saline. The MW group drank MW ad libitum. By exposing the water to a magnetic field of 14,500 Gauss within a magnetizer, MW was extracted slowly for 5 min from the potable supply26. The AlCl3 group was administered AlCl3 (40 mg/kg b.w. dissolved in saline, Sigma-Aldrich Chemical Co. St. Louis, MO, USA, CAS number 7446-70-0)9,10. The AlCl3 + MW group was co-treated with AlC13 and MW as in the previous groups. All treatments were given orally by gastric lavage for 2 months.
Sampling
By the end of the experiment (2 months after adaptation), blood samples were collected from the medial angle of the eye, and centrifuged (3500 rpm/7 min) to get serum for biochemical assays. After euthanization by exsanguination, the livers were obtained and split into three thirds. The first 1/3 was homogenized in PBS (10,000 rpm/4 °C) and the supernatants were used for oxidant/antioxidant analysis. The second 1/3 was frozen at − 80 °C for the qPCR. The last 1/3 was preserved in 10% formalin for the histology.
Biochemical analysis
AST, ALT, and ALP serum levels were determined using commercially available kits as previously described31,32. GGT, globulin, and bilirubin were also measured using commercially available kits (BioMed-Diagnostics, Egypt) and as previously described50,51. MDA was determined in liver homogenate via the thiobarbituric acid-reactive substances (TBARS)52. SOD and CAT were determined as previously detailed53,54.
Real-time PCR
Trizol reagent (Invitrogen, USA, Cat# 15596026) was used to extract total RNA from liver tissues, as previously described55. Synthesis of cDNA was performed using reverse transcriptase (Thermo Scientific, catalog # 12594100) as previously detailed56. The sequences of all primers used in this study are presented in Table 1. The GAPDH gene was used as an internal reference. The qPCR mixture (cDNA, SYBR Green qPCR, and primers) and thermal conditions were performed as previously reported57. The relative expression of all genes was quantified by the Livak method (2−∆∆Ct).
Histopathology
Fixed (10% formalin) liver specimens were dehydrated in 70–100% ethanol, cleaned in xylene, encapsulated in paraffin, cut 5 µm thick sections, stained with Hematoxylin and Eosin dyes, and examined using a light microscope58.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan post hoc test using GraphPad Prism 8. Statistical results were reported as the mean ± standard error of mean (SEM). Differences between means were considered statistically significant at p < 0.05.
Ethics approval and consent to participate
All experimental techniques were approved by the KFS-IACUC ethics committee (KFS-IACUC/146/2023) at Kafrelsheikh University, Egypt. The study was carried out in compliance with the ARRIVE guidelines and regulations.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AlCl3 :
-
Aluminum chloride
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BIP:
-
Binding immunoglobulin protein
- CAT:
-
Catalase
- CHOP:
-
C/EBP homologous protein
- EIF2-α:
-
α Subunit of eukaryotic initiation factor
- ER:
-
Endoplasmic reticulum
- GGT:
-
Gamma-glutamyl transpeptidase
- GST:
-
Glutathione- S transferase
- HDAC3:
-
Histone deacetylase 3
- HO-1:
-
Heme oxygenase-1
- HMGB1:
-
High-mobility group box 1 protein
- IL1β:
-
Interleukin-1 beta
- MDA:
-
Malondialdehyde
- MW:
-
Magnetic water
- NFκβ:
-
Nuclear factor kappa beta
- NrF2:
-
Nuclear factor erythroid 2–related factor 2
- PERK:
-
PKR-like ER kinase
- SOD:
-
Superoxide dismutase
- TNFα:
-
Tumor necrosis factor alpha
- XBP1:
-
X-box binding protein 1
References
Kumar, V., Bal, A. & Gill, K. D. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology 255, 117–123 (2009).
Bulan, Ö. K., Bayrak, B. B., Sarikaya-Ünal, G. & Yanardağ, R. The influence of melatonin supplementation against aluminum-induced toxicity in brains of male rats. J. Res. Pharm. 23, 275–283 (2019).
Jayamurali, D. et al. An overview of heavy metal toxicity. In Metal, Metal Oxides and Metal Sulphides for Biomedical Applications (eds Rajendran, S. et al.) 323–342 (Springer International Publishing, 2021).
Promyo, K., Iqbal, F., Chaidee, N. & Chetsawang, B. Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem. Toxicol. 146, 111829 (2020).
Jafari-Garageshlaghi, F., Hashtarkhani, F., Soraya, H. & Malekinejad, H. Quercetin protected from aluminum phosphide-induced acute and subacute cardio- and hepatotoxicity in rats. Curr. Pharm. Des. https://doi.org/10.31579/2766-2314/035 (2022).
Algridi, M. A. & Azab, A. E. Ameliorating effects of fenugreek seeds powder against hematotoxicity induced by aluminum chloride in male rabbits. J. Biotechnol. Bioprocess. 2, 2766–2314 (2021).
Sadek, K. M., Lebda, M. A. & Abouzed, T. K. The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and nrf2 signaling apart from metal chelation. Environ. Sci. Pollut. Res. 26, 9174–9183 (2019).
Abu-Elfotuh, K. et al. The protective effects of sesamol and/or the probiotic, Lactobacillus rhamnosus, against aluminum chloride-induced neurotoxicity and hepatotoxicity in rats: Modulation of wnt/β-catenin/gsk-3β, jak-2/stat-3, ppar-γ, inflammatory, and apoptotic pathways. Front. Pharmacol. 14, 1208252 (2023).
Okail, H. A., Ibrahim, A. S. & Badr, A. H. The protective effect of propolis against aluminum chloride-induced hepatorenal toxicity in albino rats. J. Basic Appl. Zool. 81, 1–11 (2020).
Othman, M. S., Fareid, M. A., Abdel Hameed, R. S. & Abdel Moneim, A. E. The protective effects of melatonin on aluminum-induced hepatotoxicity and nephrotoxicity in rats. Oxid. Med. Cell. Longev. 2020, 7375136 (2020).
Wang, X., Gong, J., Gui, Z., Hu, T. & Xu, X. Halloysite nanotubes-induced al accumulation and oxidative damage in liver of mice after 30-day repeated oral administration. Environ. Toxicol. 33, 623–630 (2018).
Karami, E. et al. The aqueous extract of artemisia absinthium l. Stimulates ho-1/mt-1/cyp450 signaling pathway via oxidative stress regulation induced by aluminium oxide nanoparticles (α and γ) animal model. BMC Complement. Med. Ther. 23, 310 (2023).
Sedik, A. A., Hassan, S. A., Shafey, H. I., Khalil, W. K. B. & Mowaad, N. A. Febuxostat attenuates aluminum chloride-induced hepatorenal injury in rats with the impact of nrf2, crat, car3, and mnk-mediated apoptosis. Environ. Sci. Pollut. Res. Int. 30, 83356–83375 (2023).
Abu-Elfotuh, K., Ragab, G. M., Salahuddin, A., Jamil, L. & Abd Al Haleem, E. N. Attenuative effects of fluoxetine and triticum aestivum against aluminum-induced alzheimer’s disease in rats: The possible consequences on hepatotoxicity and nephrotoxicity. Molecules 26, 6752 (2021).
Newairy, A.-S.A., Salama, A. F., Hussien, H. M. & Yousef, M. I. Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem. Toxicol. 47, 1093–1098 (2009).
Al-Kahtani, M., Abdel-Daim, M. M., Sayed, A. A., El-Kott, A. & Morsy, K. Curcumin phytosome modulates aluminum-induced hepatotoxicity via regulation of antioxidant, bcl-2, and caspase-3 in rats. Environ. Sci. Pollut. Res. 27, 21977–21985 (2020).
Al-Nuemi, S. et al. Effect of magnetic water drinking on testis dimension, scrotal circumference and blood parameters of holstein bulls born in iraq. Adv. Anim. Vet. Sci. 3, 413–417 (2015).
Ibrahim, I. Biophysical properties of magnetized distilled water. Egypt. J. Sol 29, 1–7 (2006).
Cho, Y. I. & Lee, S.-H. Reduction in the surface tension of water due to physical water treatment for fouling control in heat exchangers. Int. Commun. Heat Mass Transf. 32, 1–9 (2005).
Xiao, L. & Miwa, N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3d-tissue equivalents, and wound-healing promotion, together with ros-scavenging and relief from glutathione diminishment. Hum. Cell 30, 72–87 (2017).
Lee, H.-J. & Kang, M.-H. Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in stz-induced rats. Nutr. Res. Pract. 7, 34–42 (2013).
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694 (2007).
Hafizi, L. et al. Effects of magnetized water on ovary, pre-implantation stage endometrial and fallopian tube epithelial cells in mice. Iran. J. Reprod. Med. 12, 243 (2014).
Ebrahim, S. A. & Azab, A. E. Biological effects of magnetic water on human and animals. Biomed. Sci. 3, 78–85 (2017).
Aboulfotoh, M., Zedan, A. & Elmoslemany, A. The effect of drinking magnetic water in comparison to water treated by microwave oven on bone minerals changes. Egypt. Dent. J. 68, 1497–1503 (2022).
Elmoslemany, A. M. et al. Liver tissues oxidative status, epigenetic and molecular characteristics in rats administered magnetic and microwave treated water. Sci. Rep. 13, 4406 (2023).
Yacout, M. et al. Effect of magnetic water on the performance of lactating goats. J. Dairy Vet. Anim. Res. 2, 00048 (2015).
Araibi, S. & Dagher, A. Effect of magnetic water on some physiological characters of broiler. Al-Qadisiyah J. Vet. Med. Sci. 13, 181–231 (2014).
Helal, A., Shamardal, E., Hassanein, H. & Mohamed, M. Effect of drinking magnetized water on some production characteristics of rabbits. Egyptian J. Anim. Prod. 59, 79–87 (2022).
Wang, B. et al. Effects of aluminum chloride on serum proteins, bilirubin, and hepatic trace elements in chickens. Toxicol. Ind. Health 32, 1693–1699 (2016).
Abdelhady, D. et al. The ameliorative effect of Aspergillus awamori on aflatoxin b1-induced hepatic damage in rabbits. World Mycotoxin J. 10, 363–373 (2017).
Abdelhady, D. H., El-Magd, M. A., Elbialy, Z. I. & Saleh, A. A. Bromuconazole-induced hepatotoxicity is accompanied by upregulation of pxr/cyp3a1 and downregulation of car/cyp2b1 gene expression. Toxicol. Mech. Methods 27, 544–550 (2017).
Aita, N. A. A. Hepatoprotective effect of spirulina platensis against aluminum chloride induced liver damage in rats. Glob. Vet. 13, 552–559 (2014).
Mangood, S., Kamal, A. & Haggag, A. Propolis protection from toxicity caused by aluminium chloride in male rats. Isotope Radiat. Res. 44, 623–633 (2012).
Tripathi, S. et al. Influence of age on aluminum induced lipid peroxidation and neurolipofuscin in frontal cortex of rat brain: A behavioral, biochemical and ultrastructural study. Brain Res. 1253, 107–116 (2009).
Ahmed, W. M. et al. Amelioration of aluminum-induced hepatic and nephrotoxicity by premna odorata extract is mediated by lowering mmp9 and tgf-β gene alterations in wistar rat. Environ. Sci. Pollut. Res. 29, 72827–72838 (2022).
Halatek, T., Trzcinka-Ochocka, M., Matczak, W. & Gruchala, J. Serum clara cell protein as an indicator of pulmonary impairment in occupational exposure at aluminum foundry. Int. J. Occup. Med. Environ. Health 19, 211–223 (2006).
Prysyazhnyuk, V. et al. Glutathione-s-transferases genes-promising predictors of hepatic dysfunction. World J. Hepatol. 13, 620 (2021).
Attia, A. A. et al. Amygdalin potentiates the anti-cancer effect of sorafenib on ehrlich ascites carcinoma and ameliorates the associated liver damage. Sci. Rep. 12, 6494 (2022).
El-Demerdash, F. M., El-Magd, M. A. & El-Sayed, R. A. Panax ginseng modulates oxidative stress, DNA damage, apoptosis, and inflammations induced by silicon dioxide nanoparticles in rats. Environ. Toxicol. 36, 362–1374 (2021).
El-Magd, M. A. et al. Avocado seeds-mediated alleviation of cyclosporine a-induced hepatotoxicity involves the inhibition of oxidative stress and proapoptotic endoplasmic reticulum stress. Molecules 27, 7859 (2022).
Alhazmi, A. et al. Antioxidant activity of magnetized water in mice injected with nerium oleander ethanolic extract. Fresenius Environ. Bull. 30, 9771–9779 (2021).
Dhivya Bharathi, M. et al. Amelioration of aluminum maltolate-induced inflammation and endoplasmic reticulum stress-mediated apoptosis by tannoid principles of emblica officinalis in neuronal cellular model. Neurotox. Res. 35, 318–330 (2019).
Yu, X., Yu, W., Wu, L., Yang, W. & Lü, Y. Chitotriosidase attenuates brain inflammation via hdac3/nf-κb pathway in d-galactose and aluminum-induced rat model with cognitive impairments. Neurosci. Res. 172, 73–79 (2021).
Lu, X.-F. et al. Histone deacetylase 3 promotes liver regeneration and liver cancer cells proliferation through signal transducer and activator of transcription 3 signaling pathway. Cell Death Dis. 9, 398 (2018).
Ram, B. M. & Ramakrishna, G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine a treated cancer cervix cells is mediated by cyclophilin b inhibition. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1843, 2497–2512 (2014).
Cybulsky, A. V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 13, 681–696 (2017).
Chen, W. T., Ha, D., Kanel, G. & Lee, A. S. Targeted deletion of er chaperone grp94 in the liver results in injury, repopulation of grp94-positive hepatocytes, and spontaneous hepatocellular carcinoma development in aged mice. Neoplasia (New York, NY) 16, 617–626 (2014).
Kataria, S., Rastogi, A., Bele, A. & Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient uv-b stress in soybean. Physiol. Mol. Biol. Plants 26, 931–945 (2020).
Abu Khudir, R., El-Magd, M. A., Salama, A. F., Tousson, E. M. & El-Dsoki, S. M. Curcumin attenuated oxidative stress and inflammation on hepatitis induced by fluvastatin in female albino rats. Alex. J. Vet. Sci. 62, 102–115 (2019).
Alzahrani, F. A. et al. Potential effect of exosomes derived from cancer stem cells and mscs on progression of den-induced hcc in rats. Stem Cells Int. 2018, 17. https://doi.org/10.1155/2018/8058979 (2018).
Uchiyama, M. & Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86, 271–278 (1978).
Paoletti, F., Aldinucci, D., Mocali, A. & Caparrini, A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 154, 536–541 (1986).
Aebi, H. Catalase in vitro. In Methods in Enzymology (ed. Aebi, H.) 121–126 (Academic Press, 1984).
Elmetwalli, A. et al. Modulation of the oxidative damage, inflammation, and apoptosis-related genes by dicinnamoyl-l-tartaric acid in liver cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 396, 3087–3099 (2023).
Zahran, R., Ghozy, A., Elkholy, S. S., El-Taweel, F. & El-Magd, M. A. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia–reperfusion injury in a rat model. Int. J. Urol. 27, 1039–1049 (2020).
Shaban, A. M. et al. Ameliorative effects of camel milk and its exosomes on diabetic nephropathy in rats. Membranes 12, 1060 (2022).
Sallam, A. A. et al. Quercetin alleviated multi-walled carbon nanotubes-induced neurotoxicity in mice through inhibition of oxidation, inflammation, and pyroptosis. Biomed. Pharmacother. 151, 113160 (2022).
Acknowledgements
We express our gratitude to STDF and EKB (Egypt) for their support of our publication.
Funding
The authors did not receive any external fund.
Author information
Authors and Affiliations
Contributions
S. A. E., S.F.M.D., A.M.S., and A.A.M.: Conducted the experiment; A.N.A. and H.K.A.: Analyzed the data; A.M.G.Z., A.A.E., and M.A.E.: Designed the experiment; A.M.G.Z., A.A.E., and M.A.E.: Interpreted the results; S. A. E., S.F.M.D., A.M.S., A.A.M, A.N.A. and H.K.A..: Did the statistical analysis; S. A. E., S.F.M.D., A.M.S., A.A.M, A.N.A. and H.K.A..: Wrote the initial version of the article; A.M.G.Z., A.A.E., and M.A.E.: Revised and edited the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
El-Shazly, S.A., Alhejely, A., Alghibiwi, H.K. et al. Protective effect of magnetic water against AlCl3-induced hepatotoxicity in rats. Sci Rep 14, 24999 (2024). https://doi.org/10.1038/s41598-024-70391-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70391-w
Keywords
This article is cited by
-
Associations of non‑essential metals and their mixture with non-alcoholic fatty liver disease in Chinese older adults
Environmental Geochemistry and Health (2025)