Abstract
The demersal fish orange roughy (Hoplostethus atlanticus) can live for up to 250 years, twenty times more than its congener silver roughy (Hoplostethus mediterraneus). Studies of Hoplostethus have focused mainly on its ecology and conservation due to its vulnerability to commercial fishing. In this work, we present the de novo genomes of orange and silver roughies and explore the genomic mechanisms that could contribute to such differential longevities. Using comparative genomics on a list of more than 400 genes, we identified gene candidates with differential residue changes in Hoplostethus that are related to genomic instability, disabled macroautophagy and intercellular communication. We hypothesized that these mechanisms could have been selected as adaptations to the deep environment and, as an epiphenomenon of these mechanisms, may have contributed to an extension of the lifespan of H. atlanticus.
Similar content being viewed by others
Ageing involves molecular processes that are conserved throughout evolution. According to recent classifications, aging features can be grouped into twelve hallmarks. These hallmarks can be (1) related to primary causes of cellular damage (genome instability, telomere attrition, epigenetic alterations, loss of proteostasis and disabled macroautophagy); (2) involved in a compensatory or antagonistic response to this damage (deregulated nutrient-sensing, mitochondrial dysfunction and cellular senescence); or (3) represent an integrative consequence of the other two categories (stem cell exhaustion, altered intercellular communication, chronic inflammation and dysbiosis)1. Multispecies comparative genomics in different animal taxa has revealed that genomic alterations affecting one or several hallmarks are candidates that contribute to differential interspecific longevity2,3,4,5.
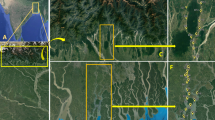
Fishes are a vertebrate taxon with a wide range of lifespans, varying from weeks in pygmy gobies6 to approximately 400 years in Greenland sharks7. Large differences even occur among species within the same genus. For example, the longevity of rockfish ranges from 11 years in Sebastes minor to more than 200 years in Sebastes aleutianus. Similarly, species of the genus Hoplostethus have lifespans that vary between 11 years (Hoplostethus mediterraneus) and 250 years (Hoplostethus atlanticus)8,9,10. In terms of the habitat range of these two Hoplostethus species, both species are demersal: H. atlanticus lives on the slope of the continental shelf and on seamounts from 700 to 1300 m depth11, and H. mediterraneus is mostly distributed in habitats from 500 to 750 m depth12 (Fig. 1). Hoplostethus mediterraneus is often caught as a bycatch species via bottom trawling, while H. atlanticus has been of commercial interest for decades12. Regarding their biology, H. mediterraneus has a maximum length of 198 mm and reaches maturity at sizes greater than 115 mm, which corresponds to an age of approximately 2 years13. In contrast, H. atlanticus can measure up to 500 mm14 and grows slowly until it reaches sexual maturity at approximately 20–30 years, which makes this species highly vulnerable to overfishing11. For this reason, research on Hoplostethus, especially H. atlanticus, has focused exclusively on ecology and conservation, as well as on reporting the state of its populations around the world9, whereas less attention has been given to exploring the extreme differences in their lifespan.
Locations from where samples of (top) H. mediterraneus and (bottom) H. atlanticus were collected. Depth ranges11,12, temperature and salinity of H. mediterraneus depth range81, diets45,46, age at maturity12,13 and longevity8,9,10 were extracted from the literature, whereas the temperature and salinity ranges for H. atlanticus were extracted by plotting depth profiles (Supplementary information Fig. S2) via Argo buoy data82. The drawing of the world map and the specific geographic areas were designed using an image from Freepik (https://www.freepik.es/).
In this work, we sequenced and assembled the genomes of H. atlanticus and H. mediterraneus de novo, performed manually supervised annotation of a set of more than 400 genes related to aging, and used comparative genomics to select gene amplifications and residue changes specific to either of these species that could contribute to the 20-fold difference in longevity of Hoplostethus sp.
Results and discussion
Genome assembly
The assembly of H. atlanticus has a total size of 634 Megabases (Mb), with 1436 contigs with an N50 of 3290 kb. The assembly of Hoplostethus mediterraneus has 552 Mb with 657 contigs and an N50 of 3054 kb. Despite the smaller size and lower number of contigs of the H. mediterraneus assembly, both have a high degree of completeness, with more than 91% Benchmarking Universal Single-Copy Ortholog (BUSCO)15 complete genes and a similar GC content of approximately 43% (Table 1). Automatic annotation led to 74,284 and 73,650 predicted protein-coding genes in H. mediterraneus and H. atlanticus, respectively. The total number of repetitive elements (Supplementary Table S2) spanned 17.77% of the H. atlanticus genome and 13.85% of the H. mediterraneus genome, similar to values reported in other fish genomes, such as Nothobranchius furzeri (15.86%)16. Compared with H. mediterraneus, Hoplostethus atlanticus presented higher percentages of most repetitive element types, which could be explained by its greater genome size17 but slightly lower simple and low-complexity repeats. Simple repeats were the most abundant elements in both Hoplostethus species, and retrotransposons were infrequent compared with those in other species, such as the short-lived N. furzeri16.
Hypothesis-driven manual annotation
Using manually supervised annotation, we compared the status of more than 400 genes linked to aging and longevity between H. mediterraneus and H. atlanticus (Supplementary Table S3) and identified multiple amplified genes and residue changes in comparison to the other species analysed. From these results, we selected those with differential changes between the two Hoplostethus species, resulting in a set of 19 sequence variants (Table 2 and Fig. 2). Notably, all changes in gene amplification and several point variants found in candidate genes were discarded after PCR validation (see Supplementary Tables S4 and S5). This underscores the importance of integrating experimental validation with bioinformatics analysis to yield more reliable and robust results.
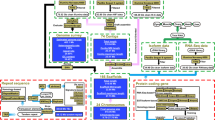
Genomic bases of longevity in Hoplostethus. (A) Candidate genes contributing to differential longevity between H. mediterraneus (left) and H. atlanticus (right), classified by the hallmarks of aging. * Refers to the number of point variants of interest found in each gene. (B) Residue changes are more relevant in H. atlanticus than in other long- and short-lived fishes.
The molecular and cellular mechanisms underlying the process of aging have recently been categorized into twelve hallmarks, which are conserved throughout evolution1. These hallmarks are relevant because they are associated with alterations that increase over time, can be experimentally controlled in models that mimic accelerated aging, and can be used to understand how each specific hallmark contributes to the overall aging process. Finally, they permit the identification of potential targets to decelerate, cease, or reverse aging through therapeutic interventions targeting each specific hallmark1. The genes that we propose as candidates to play a role in the long-lived phenotype shown by H. atlanticus can also be grouped into the following twelve hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis. Importantly, although we classified each candidate gene into a single hallmark for the purpose of organizing the results, some genes can be involved in more than one function or mechanism.
Genomic instability
The accumulation of genetic damage resulting in genomic instability can challenge tissue homeostasis and accelerate aging18, modulating species longevity. DNA integrity can be compromised not only by exogenous factors, including biological, physical19 (such as hydrostatic pressure) or chemical agents20 but also by endogenous factors, such as reactive oxygen species (ROS) or DNA replication errors21. Genomic instability can be prevented by having both efficient replicative mechanisms and DNA repair systems. Consequently, we explored several genes involved in the maintenance of DNA integrity. This analysis revealed that H. atlanticus presents two variants (p.E417Q and p.M427T) in the heterodimer interface of the protein XRCC522. Heterodimerization with XRCC6 is crucial for NHEJ-dependent DNA repair. The residue p.M427 is conserved only in vertebrates, and changes in this position are also found in two other long-lived fishes (Hippoglossus hippoglossus, p.M427A; Anguilla anguilla, p.M427V) and in one short-lived fish (Eucyclogobius newberryi, p.M427T). The residue p.E417 is also conserved within fungi, and the same p.E417Q variant can also be found in the long-lived H. hippoglossus but is absent in all short-lived fishes analysed (Fig. 2B).
A homology model of the XRCC5 protein suggested that the p.M427T variant reduces intramolecular hydrogen bonding (Fig. 3), which could modify flexibility in heterodimerization and therefore modulate the efficiency of XRCC5-dependent DNA repair mechanisms. Deletion of XRCC5 results in early apoptosis of human cells23 and, in mice, leads to deficiencies in the immune system and early senescence23,24. Its heterodimer counterpart, XRCC6, is also involved in aging, since knockout of Xrcc6 in mice results in decreased lifespan22, and high expression of XRCC6 in humans leads to a longer average lifespan25. In fish, Ku80 has been associated with DNA repair during stages of development in zebrafish exposed to irradiation stress26. Additionally, recent comparative analyses of ~ 1000 mammalian species have shown that this gene likely plays a role in both longevity and social organization27. Hence, we propose a moderate effect for these variants, particularly for the change affecting residue p.E417, in XRCC5 function and in Hoplostethus lifespan.
Protein sequence comparison and homology modelling of XRCC5. A. Partial amino acid sequence alignment of both Hoplostethus and other fish, vertebrate and invertebrate species, where the positions p.417 and p.427 are highlighted, and B. Model of the human heterodimer Ku70-Ku80 with interaction with dsDNA83 and the specific changes on p. 417 and p. 427 in Hoplostethus. The dashed blue lines indicate H-bonds.
We also found several residue variations affecting the gene encoding the serine/threonine protein kinase ATM, a protein that acts as a DNA damage sensor and is located in highly conserved positions. First, we discovered four different variants in H. atlanticus affecting protein function: two (p.L8F and p.V1085I) in the N-terminal region, which are predicted to be damaging in humans (Table 2), and another two (p.I2914V and p.V2937M), which belong to the kinase domain28, are predicted to be benign and possibly damaging in humans, respectively (Table 2). These changes were not detected in any other short- or long-lived fishes (Fig. 2B). In H. mediterraneus, we found two variants (p.R184Q and p.Q2177R), both of which are predicted to affect protein function and be probably damaging in humans. All the positions of the preceding point variants in ATM have been associated with cancer predisposition and/or ataxia-telangiectasia syndrome (Table 2). These findings would suggest a relevant role for both positions in ATM evolution.
The gene BRCA2 encodes a protein that is involved in double-strand break repair and homologous recombination. We observed a change in the residues p.S2807 to Cys in H. atlanticus and to Arg in H. mediterraneus, which changed from polar to nonpolar and positively charged, respectively. Changes in this position have been associated with hereditary breast and ovarian cancers (Table 2). This highly conserved residue is in the oligonucleotide binding fold (OB1), which interacts with the exoribonuclease DSS129. Cys is present not only in the long-lived S. aleutianus and Anoplopoma fimbria but also in several other fishes, such as Poeciliopsis occidentalis, Xiphophorus helleri, Poecilia reticulata and Oreochromis niloticus, which have lived for less than 10 years (Fig. 2B, Supplementary Fig. S1). BRCA2 mutations at different positions have been associated with cancer risk in adult zebrafish tissue30. Additionally, we detected residue changes in other components of the Fanconi anaemia complex, including a variant in FANCI at p.R393H in H. mediterraneus and at p.M427V in FANCA in H. atlanticus, both of which are related to Fanconi anaemia (Table 2). These two variants are in highly conserved positions, since the same residue is maintained in more than 80% of the species, even though few fishes, birds and mammals, including two short-lived fishes, show changes in this position (Fig. 2B). Both variants are predicted to be benign in humans (Table 2).
Finally, the variant p.G58S, which is exclusive to H. mediterraneus, lies within the nudix motif of the hydrolase NUDT131, which appears to be important for maintaining the stable structure of the protein32, and changes in other residues of the nudix motif led to loss of activity32. This protein is involved in preventing DNA transversions and therefore plays a relevant role in protection from oxidative stress. Additionally, NUDT1 deficiency leads to increased tumor incidence in mice33. The abovementioned variant is predicted to be deleterious or possibly damaging in humans (Table 2). However, its effect on protein function has yet to be studied.
Disabled macroautophagy
Macroautophagy is a type of autophagy that involves the sequestration of cytoplasmic material into double-membrane vesicles, called autophagosomes, which then fuse with lysosomes for the digestion of their contents. It affects all kinds of macromolecules, entire organelles, and even invading pathogens34, and strong evidence suggests that autophagy is a relevant mechanism that regulates aging1.
In this context, we also discovered the variant p.V412I in the protein SIRT1, which lies in a highly conserved residue involved in substrate binding, in H. atlanticus35. This change results in steric clashes with L418 and H363, which can slightly modify the folding of the substrate-binding domain and is predicted to affect protein function in humans (Fig. 4, Table 2). While macroautophagy downregulates the activity of the deacetylase SIRT1 during aging and senescence, this protein is also a negative regulator of mTOR36, a complex that has direct implications in aging37. Among other relevant functions in the cell, SIRT1 induces insulin sensitivity38,39 and keratinocyte differentiation40. In addition, SIRT1 also stimulates tissue regeneration in zebrafish via modulation of the mitochondrial unfolded protein response (UPRmt)41. The regulation of sirtuin activity through NAD + precursors has many implications for different hallmarks of aging1. Therefore, we hypothesize that the abovementioned variant found in SIRT1 may act through different mechanisms to promote longevity in H. atlanticus. Further experimental approaches may contribute to shed light on the effect of this mutation in SIRT1 protein function.
Protein sequence comparison and homology modelling of SIRT1. (A) Partial amino acid sequence alignment of Hoplostethus and other fish, vertebrate and invertebrate species; position p.412 is highlighted. This position is also represented in a protein model of SIRT1 from (B) H. mediterraneus; (C) H. atlanticus; and (D) a human model with interaction with resveratrol84. The dashed blue lines indicate H-bonds, whereas the dashed purple line indicates steric clashes.
Deregulated nutrient sensing
The regulation of nutrient sensing involves several pathways in the cell, such as the IGF-1 signalling (ISS) pathway, which in turn modulates other pathways associated with longevity, such as the FOXO, sirtuin and mTOR pathways. High nutrient availability activates signalling pathways that promote aging, whereas nutrient scarcity has the opposite effect42. Moreover, integrative transcriptomic analyses of more than 40 mammalian species have recently revealed shared longevity mechanisms that act as biomarkers of longevity and ageing, including downregulation of IGF137. With respect to nutrient sensing, we found a variant exclusive to H. mediterraneus in CETP, a gene that codes for a protein in the plasma that is involved in cholesterol and triglyceride transport43. This variant p.V29A is in a highly conserved position within the BPI dimerization interface44; however, the same residue change is present in some birds and fishes, such as Latimeria chalumnae, which presents a longevity of 48 years. This variant could be associated with the different diets of the two species, as H. mediterraneus preys mostly on crustaceans45, whereas H. atlanticus feeds on crustaceans only in the early stages and mostly on fish when adults46.
Altered intercellular communication
Alterations in intercellular communication, including endocrine, neuroendocrine or neuronal pathways, can also promote aging1. In this context, we found a residue change (p.R509H) exclusive to H. atlanticus in the ACE gene, which codes for a protein that catalyzes the conversion of angiotensin I into angiotensin II, modulating its vasoconstrictor activity and osmoregulation in fish47. This variant has been associated with autosomal recessive renal tubular dysgenesis (RTD), a disease with symptoms of arterial hypotension, probably by affecting the renin–angiotensin system (RAS)48. p.R509 is a highly conserved position, and the change of Arg to His would modify H-bonds with p.D515 and p.N114 (Fig. 5, Table 2). ACE inhibition reduces angiotensin II production, which reduces arterial pressure49 and impairs mitochondrial dysfunction48, contributing to increased longevity. Moreover, high levels of angiotensin II increase blood pressure through the release of catecholamines50 and affect renal function by reducing parameters such as the glomerular filtration rate or urine flow in bony fishes51. Thus, we hypothesize that this residue change in ACE could modulate the RAS system and help control blood pressure, the antioxidant environment and osmoregulation.
Protein sequence comparison and homology modelling of ACE. (A) Partial amino acid sequence alignment of Hoplostethus and other fish, vertebrate and invertebrate species, with the position p.509 highlighted. (B) Details of the amino acid interactions at this position are shown for H. atlanticus and H. mediterraneus in the protein human model85. The dashed blue lines indicate H-bonds.
Chronic inflammation
Inflammation is known to be closely related to aging, a trait known as “inflammaging”5. Given that inflammation and circulating concentrations of inflammatory cytokines increase with age, while the function of the immune system decreases, we explored several genes related to these two processes. We identified the p.R393H variant in the DOCK8 protein of H. mediterraneus, which is involved in T-cell and dendritic cell migration during immune responses. This variant is predicted to affect protein function and to be probably damaging in humans (Table 2), and it has been associated with autosomal recessive hyper-IgE syndrome (Table 2). Despite this variant being in a highly conserved position, the same residue change occurs in one of the three copies of the corresponding genes in Salmo salar, which suggests a case of parallel evolution52. In addition, we explored members of the butyrophilin gene family (BTN and BTNL genes), which are immune regulators involved in human inflammatory disease and are associated with depth components of lifespan in rockfish3. However, we did not find differences in the gene amplification of any specific gene within this family (Supplementary Table S6).
Deep-sea environment and aging
Deep-sea marine environments are characterized by low temperatures, low food availability and high hydrostatic pressures, which can induce DNA damage and affect protein function53,54. Thus, maintenance of genomic stability could be relevant to address this source of damage. Accordingly, genes related to DNA repair (ATM, BRCA2, FANCA, FANCI, NUDT1 and XRCC5) were relevant in both Hoplostethus species—with a slightly greater number of variants in H. atlanticus—which also reinforces the fact that this primary hallmark is a common denominator highlighted in many comparative genomic studies of aging, such as bowhead whale55, giant tortoise2, tardigrade56, immortal jellyfish4, killifish16 or rockfish3. Moreover, the upregulation of genes related to DNA repair was linked to longevity in mammals57. Interestingly, variants and amplifications in the heterodimer Ku70-Ku80 (encoded by XRCC5 and XRCC6) have also been reported in other comparative genomic studies of long- and short-lived species, such as killifish, immortal jellyfish, and giant tortoise4,16,22. The importance of the variants found in XRCC5 in H. atlanticus is reinforced by the presence of the same amino acid change (p.E417Q) in another long-lived fish, H. hippoglossus, and by the finding of different changes in residue p.M427 in two other long-lived species (H. hippoglossus and A. anguilla) and in one short-lived fish (E. newberryi), which suggests a case of parallel evolution52. This result may indicate that this heterodimer could act as a node to modulate aging in both directions depending on the position of the residue, which is changed in different species16.
Hydrostatic pressure damages DNA, alters membrane fluidity, and affects proteins directly. Under high hydrostatic pressure, proteins may experience functional changes due to mechanisms such as decreased molar volume, hydration of uncharged regions, unfolding, and denaturation, while also dissociating multimeric proteins and affecting enzymatic efficiency. Deep-sea species have adapted to these conditions, including modifications in membrane lipids to maintain fluidity and changes in protein amino acid residues, which are sufficient to mitigate the negative effects of high pressure on protein function58. Additionally, many of these species produce protein-stabilizing osmolytes such as trimethylamine-N-oxide (TMAO) to create an intracellular environment that protects against protein instability53,59. TMAO levels increase in bony fishes and invertebrates as they inhabit greater depths60 and are significantly greater in H. atlanticus than in other fishes61. Since TMAO is synthesized by hepatic flavin monooxygenase (FMO) through the oxidation of trimethylamine (TMA)62, we manually annotated FMO family genes in Hoplostethus and other fishes and found that all of these fishes contain FMO2 and FMO5 without any differential gene amplification in H. atlanticus (Supplementary Table S7). When protein aggregates are formed, macroautophagy removes them along with other intracellular macromolecules, organelles, and pathogens1. In this context, we observed the abovementioned residue change in the protein SIRT1, an epigenetic regulator related to macroautophagy. SIRT1 is also involved in mitochondrial proteostasis in zebrafish through the mitochondrial unfolded protein response (UPRmt)41. Considering that loss of proteostasis is a primary hallmark of aging, this protective mechanism, which may help maintain functional proteins at pressures up to 100 atm (at 1000 m), could also contribute to increasing the longevity of H. atlanticus.
Fishes, as ectotherms, are especially affected by changes in water temperature, which affects their metabolic and growth rates63. At great depths, low temperatures can act in synergy with high pressures to reduce the fluidity of the cellular membrane and can also affect protein stability58. The temperature values within the depth range distribution of H. atlanticus on the Challenger Plateau were much lower than those of H. mediterraneus in the Cantabrian Sea. In contrast, salinity variation within the depth range was almost negligible for both species and presented a small difference among habitats (Fig. 1). Thus, the influences of low temperature and high pressure are likely more relevant than salinity in explaining these two species differences. A reduction in the fluidity of the cellular membrane will modify the diffusion of membrane molecules, including ions. Therefore, low temperatures and high pressures can affect fish osmoregulation in a complex manner47. Angiotensin II plays a relevant role in osmoregulation and affects blood pressure in fishes47,50. Thus, we hypothesize that ACE could help to modulate angiotensin II production, stabilize blood pressure under changing temperature conditions, reinforce homeostatic resilience and maintain a healthy state, which could contribute to increased longevity in H. atlanticus64.
Food availability can be low at greater depths, and the area where H. atlanticus was collected (Challenger Plateau) is characterized by oligotrophic waters65. In contrast, primary production in the Cantabrian Sea, where H. mediterraneus is fished, is generally high66. These differences in habitat conditions could shape the adaptation of H. atlanticus to food limitations. As an epigenetic regulator, SIRT1 is also a relevant player in nutrient sensing via the mTOR pathway36, which can regulate nutrient input during a restricted diet and changes in metabolism. Accordingly, genes related to nutrient sensing were positively selected in both long-living killifish16 and rockfish3 (Fig. 6). Compared with H. mediterraneus, H. atlanticus, especially adults, has low metabolic rates67,68, which is evidenced by its larger size and later reproductive age. Although low metabolism is intuitively associated with dietary restriction, there is no evidence of an association between low food availability and low metabolic rates. This exponential decrease at greater depths could also be explained by the temperature changes and lack of visual predation, which relaxed the selective pressure for locomotory activity due to the lower number of predators67,68.
Deep-sea fishes are adapted to extreme environments characterized by high pressures and low light levels, temperatures, and food availability. At the same time, these environmental factors play a significant role in shaping the continuous increase in longevity with depth69. While this seems to be the general trend, there are also few exceptions, such as the hadal fish Pseudoliparis swirei70. In accordance with this trend and with the “adaptation hitch-hike model”71, we hypothesize that residue changes found in H. atlanticus could have been positively selected because they would entail adaptation to the deep-sea environment (namely, high pressures, low food availability and salinity changes), with increased longevity being an epiphenomenon of these adaptations. Nevertheless, further evolutionary analyses are needed to confirm this hypothesis.
Conclusion
In this study, we present two de novo fish genomes, those of H. mediterraneus and H. atlanticus, and identify several point variants in relevant genes associated with the maintenance of genome stability, nutrient sensing, intercellular communication, and macroautophagy via manual annotation. Accordingly, we propose specific gene candidates that could contribute to the remarkable lifespan of H. atlanticus in comparison with H. mediterraneus. Nevertheless, further functional experiments are needed to assess the role of these variants in fish aging.
On the basis of our findings, we hypothesize that enhanced DNA repair, together with the modulation of intercellular communication, nutrient sensing, proteostasis, and macroautophagy pathways, could contribute to the adaptation of orange roughy to maintain cellular and organismal functions at great depths. At the same time, these adaptations would allow this species to live longer than H. mediterraneus. Overall, this work provides novel insights into the molecular mechanisms that confer an extraordinarily long lifespan on orange roughy.
Methods
Sample collection, processing and DNA/RNA isolation
No experiments were conducted in this study, and all methods were carried out in accordance with relevant guidelines and regulations. Hoplostethus atlanticus samples were collected via Thalassa Fisheries Support at the Challenger Plateau (quota management area ORH7A), west of New Zealand at a depth of 870 m in June 2020 (Fig. 1), under the commercial fishing permit of Talley’s Group Management Limited (Fisheries New Zealand Client Number 9760117). Immediately after collection, the samples were fixed with RNAlater at 4 °C overnight and stored at − 80 °C until they were sent to our laboratory. H. mediterraneus was kindly gifted by fishermen of the Avilés fish market, Asturias, Spain, in October 2019 after being stored at − 20 °C for 3 days. Afterward, we maintained frozen individuals at − 80 °C in our laboratory until extraction. Genomic DNA was isolated from muscular tissue via a standard phenol protocol. RNA was extracted from the muscle, gill and brain of H. atlanticus and from the muscle of H. mediterraneus via TRIzol reagent (Invitrogen). Prior to gDNA and RNA sequencing, 16S rDNA was amplified via polymerase chain reaction (PCR) (forward primer: 5′-CCGGTCTGAACTCAGATCACG-3′ and reverse primer: 5′-CGCCTGTTTAACAAAAACAT-3′) and checked for similarity in the NCBI database to confirm species identification.
Genome sequencing, assembly and automatic annotation
The genomes of both Hoplostethus species were sequenced via a combination of PacBio Sequel 20 kb SMRTbell templates and Illumina TruSeq DNA PCR-free (350 bp insert) libraries. A consensus sequence was generated by assembling PacBio reads with the wtdbg2 (v2.3) assembler and mapping Illumina reads via Pilon (v1.21) to correct for base errors, misassemblies and gaps. The length of the resulting assembly was estimated by k-mer analysis, and its completeness was assessed with Benchmarking Universal Single-Copy Ortholog (BUSCO)15 analysis, using bacteria and eukaryote database. Protein-coding genes were automatically annotated using Maker (v2.31.8), and their functions were inferred on the basis of their homology to the protein sequence database (UniProt Swiss-Prot72) with Protein BLAST + (v2.7.1 +). The RNA was sequenced on the Illumina platform using Illumina TruSeq Stranded Total RNA (150 bp insert) libraries, and the reads were aligned to their respective genomes using STAR73. Genomic sequencing, assembly, automatic annotation and RNA sequencing were carried out by Macrogen commercial services (Seoul, Korea).
Manual genome annotation
In parallel with this automatic analysis, we performed manually supervised annotation of the genomes of H. mediterraneus and H. atlanticus using BATI (Blast, Annotate, Tune, Iterate), a pipeline developed by our group74. Briefly, we curated a list of more than 400 genes selected a priori because of their involvement in aging, cancer, the stress response, telomere maintenance and DNA repair (Table 1). Each gene was selected on the basis of its role in age-related molecular processes1,75 and human conditions76, on the basis of the experience of our laboratory in these fields2,4,56, and following a detailed review of the available publications on each subject. Then, we used BATI to precisely define the intron/exon boundaries of the TBLASTN results. This procedure also helps identify novel homologues. In addition to each genome, the pipeline was fed reference protein sequences from Danio rerio and S. salar obtained from the NCBI database since they are phylogenetically closer to the targeted species, together with Homo sapiens proteins. In particularly difficult cases, we used the corresponding sequence from the automatic annotation of H. mediterraneus or H. atlanticus as a reference protein when available. All final gene sequences were compared to H. sapiens genes using the BLAST tool77 to validate the annotation (we considered only hits whose identity with H. sapiens homolog had an e value lower than 0.001).
Genome comparison and candidate gene selection
After both annotations were complete, we performed multiple protein sequence alignments between H. mediterraneus, H. atlanticus and several other species (H. sapiens, Pan troglodytes, Mus musculus, Heterocephalus glaber, Canis lupus familiaris, Gallus gallus, D. rerio, Oryzias latipes, Oncorhynchus mykiss, S. salar and N. furzeri) for all the genes that presented only one copy in both H. mediterraneus and H. atlanticus. Within this list of single-copy genes, we looked for exclusive truncating variants, variants affecting known motifs according to the Conserved Domains database at the NCBI, UniProt and PubMed databases, and/or variants whose human counterparts are related to known genetic diseases according to the Clinvar database. We set a priori that a relevant point variant had to accomplish (1) being exclusive of one of the two fish species (or both) and absent in the rest of the species that we analysed and (2) being featured in at least one of the abovementioned databases. Additionally, relevant variants found exclusively in H. atlanticus were also annotated in other fishes with extreme lifespans: S. aleutianus, A. fimbria, H. hippoglossus and A. anguilla as long-lived fishes and E. newberryi, P. occidentalis, Nothobranchius kuhntae and Hippocampus zosterae as short-lived fishes. Alignments were performed using in-house software and sequences from the NCBI and Ensembl databases. The effect of each candidate variant in humans (as a proxy of the effect of the variant in Hoplostethus) was predicted via the PolyPhen78 and SIFT79 platforms. Moreover, in some cases, we performed a structural prediction of the specific residue change on the human protein model by using ChimeraX80. With respect to gene amplification analyses, genes with differential gene amplification among Hoplostethus species were further compared with the genomes of D. rerio, O. latipes, O. mykiss, S. salar, and N. furzeri. Only genes with unique gene amplification in one of the two Hoplostethus species were selected and subjected to a validation process.
Validation
To validate the amplification of genes of interest, we performed PCRs with primer pairs that anneal to a target region with a differential variant in each copy (Supplementary Table S1). We tested the success of these reactions by performing electrophoresis of the resulting products in a 1.5% agarose gel. Genes whose amplification could not be validated by PCR or whose copy number differences disappeared because more copies were found in the other Hoplostethus species through PCR analysis were discarded. Additionally, point variants in residues with a relevant role in protein function were first validated with RNA-seq data from H. mediterraneus and H. atlanticus. Then, we performed PCRs aimed at analysing the affected nucleotide in each specific case. These products were sequenced via Sanger sequencing using an ABI PRISM 3130xl Genetic Analyser (Thermo Fisher).
Significance Statement
Orange roughy (H. atlanticus) is one of the longest-lived fish species, with a lifespan of up to 250 years—a striking contrast to its congener, silver roughy (Hoplostethus mediterraneus), which lives for only 11 years. In this study, we present the de novo genomes of both orange and silver roughies and conduct comparative genomics analyses with more than 400 genes related to aging to uncover the genomic mechanisms that could underlie the marked differences in their lifespans. This research contributes to a deeper understanding of the molecular factors that drive longevity in fish.
Data availability
Assemblies have been deposited at the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), under Bioproject PRJNA1013967 and biosamples SAMN37314128 and SAMN37314129. RNAseq data have been deposited in Sequence Read Archive (SRA) under the same Bioproject number. Technical reports of both Hoplostethus species and MAKER2 predicted protein sequences can be downloaded from https://github.com/MariaPascualTorner/Hoplostethus.
Code availability
The scripts for manual annotation (BATI) can be accessed at http://degradome.uniovi.es/downloads.html.
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
Quesada, V. et al. Giant tortoise genomes provide insights into longevity and age-related disease. Nat. Ecol. Evol. 3, 87–95 (2019).
Kolora, S. R. R. et al. Origins and evolution of extreme life span in Pacific Ocean rockfishes. Science 1979(374), 842–847 (2021).
Pascual-Torner, M. et al. Comparative genomics of mortal and immortal cnidarians unveil novel keys behind rejuvenation. Proc. Natl. Acad. Sci. 119, e2118763119 (2022).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Depczynski, M. & Bellwood, D. R. Shortest recorded vertebrate lifespan found in a coral reef fish. Curr. Biol. 15, R288-289 (2005).
Nielsen, J., Christiansen, J. S., Grønkjær, P., Bushnell, P. & Bennett, M. B. Greenland shark (Somniosus microcephalus) stomach contents and stable isotope values reveal an ontogenetic dietary shift. Front. Mar. Sci. 6, 427541 (2019).
Tacutu, R. et al. Human ageing genomic resources: New and updated databases. Nucleic Acids Res. 46, D1083–D1090 (2018).
White, T. A., Stefanni, S., Stamford, J. & Hoelzel, A. R. Unexpected panmixia in a long-lived, deep-sea fish with well-defined spawning habitat and relatively low fecundity. Mol. Ecol. 18, 2563–2573 (2009).
Dipper, F. Human impacts 1: sea fisheries and aquaculture. in Elements of Marine Ecology 389–458 (Elsevier, 2022). https://doi.org/10.1016/b978-0-08-102826-1.00006-5.
Burch, P. et al. Implications of the maximum modelled age on the estimation of natural mortality when using a meta-analytic prior: The example of eastern Australian Orange Roughy (Hoplostethus atlanticus). Fish Res. 258, 106534 (2023).
Gordon, J. D. M. & Duncan, J. A. R. Aspects of the biology of Hoplostethus atlanticus and H. mediterraneus (pisces: berycomorphi) from the slopes of the rockall trough and the Porcupine Sea bight (north-eastern Atlantic). J. Mar. Biol. Ass. UK 67, 119–133 (1987).
D’onghia, G., Tursi, A., Marano, C. A. & Basanisi, M. Life history traits of Hoplostethus mediterraneus (Pisces: Beryciformes) from the north-western Ionian Sea (Mediterranean Sea). Mar. Biol. Ass. UK 78, 321–339 (1998).
Australian Fisheries Management Authority. Orange Roughy (Hoplostethus Atlanticus) Stock Rebuilding Strategy. (2022).
Seppey, M., Manni, M. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 1962, 227–245 (2019).
Valenzano, D. R. et al. The African turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell 163, 1539–1554 (2015).
Yuan, Z. et al. Comparative genome analysis of 52 fish species suggests differential associations of repetitive elements with their living aquatic environments. BMC Genom. 19, 141 (2018).
Schumacher, B., Pothof, J., Vijg, J. & Hoeijmakers, J. H. J. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Salehi, F., Behboudi, H., Kavoosi, G. & Ardestani, S. K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 8, 13902 (2018).
Pogribny, I. et al. Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol. Cancer Res. 3, 553–561 (2005).
Chabosseau, P. et al. Pyrimidine pool imbalance induced by BLM helicase deficiency contributes to genetic instability in Bloom syndrome. Nat. Commun. 2, 368 (2011).
Li, H., Vogel, H., Holcomb, V. B., Gu, Y. & Hasty, P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol. Cell Biol. 27, 8205–8214 (2007).
Ghosh, G., Li, G., Myung, K. & Hendrickson, E. A. The lethality of Ku86 (XRCC5) loss-of-function mutations in human cells is independent of p53 (TP53). Radiat. Res. 167, 66–79 (2007).
Vogel, H., Lim, D. S., Karsenty, G., Finegold, M. & Hasty, P. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl. Acad. Sci. USA 96, 10770–10775 (1999).
Ju, Y.-J. et al. Decreased expression of DNA repair proteins Ku70 and Mre11 is associated with aging and may contribute to the cellular senescence. Exp. Mol. Med. 38, 686–693 (2006).
Bladen, C. L., Lam, W. K., Dynan, W. S. & Kozlowski, D. J. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 33, 3002–3010 (2005).
Zhu, P. et al. Correlated evolution of social organization and lifespan in mammals. Nat. Commun. 14, 372 (2023).
Lau, W. C. Y. et al. Structure of the human dimeric ATM kinase. Cell Cycle 15, 1117–1124 (2016).
Yang, H. et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 1979(297), 1837–1848 (2002).
Kouprianov, V. A., Selmek, A. A., Ferguson, J. L., Mo, X. & Shive, H. R. Brca2-mutant zebrafish exhibit context- and tissue-dependent alterations in cell phenotypes and response to injury. Sci. Rep. 12, 883 (2022).
Mildvan, A. S. et al. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 433, 129–143 (2005).
Fujii, Y., Shimokawa, H., Sekiguchi, M. & Nakabeppu, Y. Functional significance of the conserved residues for the 23-residue module among MTH1 and MutT family proteins*. J. Biol. Chem. 274, 38251–38259 (1999).
Tsuzuki, T. et al. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proc. Natl. Acad. Sci. 98, 11456–11461 (2001).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 176, 11–42 (2019).
Avalos, J. L. et al. Structure of a Sir2 enzyme bound to an acetylated p53 peptide. Mol. Cell 10, 523–535 (2002).
Ghosh, H. S., McBurney, M. & Robbins, P. D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5, e9199 (2010).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 4, 210–220 (2006).
Zhang, H. H. et al. SIRT1 overexpression in skeletal muscle in vivo induces increased insulin sensitivity and enhanced complex I but not complex II–V functions in individual subsarcolemmal and intermyofibrillar mitochondria. J. Physiol. Biochem. 71, 177–190 (2015).
Blander, G. et al. SIRT1 promotes differentiation of normal human keratinocytes. J. Invest. Dermatol. 129, 41–49 (2009).
Lin, Y. F., Sam, J. & Evans, T. Sirt1 promotes tissue regeneration in zebrafish through regulating the mitochondrial unfolded protein response. iScience 24, 103118 (2021).
López-Otín, C., Galluzzi, L., Freije, J. M. P., Madeo, F. & Kroemer, G. Metabolic control of longevity. Cell 166, 802–821 (2016).
De Grooth, G. J. et al. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 45, 1967–1974 (2004).
Beamer, L. J., Carroll, S. F. & Eisenberg, D. Crystal structure of human BPI and two bound phospholipids at 2.4 Angstrom resolution. Science 1979(276), 1857–1860 (1997).
Pais, C. Diet of a deep-sea fish, Hoplostethus mediterraneus, from the south coast of Portugal. J. Mar. Biol. Ass. UK 82, 351–352 (2002).
Dunn, M. R. & Forman, J. S. Hypotheses of spatial stock structure in orange roughy Hoplostethus atlanticus inferred from diet, feeding, condition, and reproductive activity. PLoS One 6, e26704 (2011).
Baldisserotto, B. Fish osmoregulation (Science Publishers, 2007).
Gribouval, O. et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum. Mutat. 33, 316–326 (2012).
Cole, J., Ertoy, D. & Bernstein, K. E. Insights derived from ACE knockout mice. J. Renin Angiotensin Aldosterone Syst. 1, 137–141 (2000).
Bernier, N. J. & Perry, S. F. Cardiovascular effects of angiotensin-II-mediated adrenaline release in rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 202, 55–66 (1999).
Brown, J. A., Oliver, J. A., Henderson, I. W. & Jackson, B. A. Angiotensin and single nephron glomerular function in the trout Salmo gairdneri. Am. J. Physiol. 239, R509-514 (1980).
Storz, J. F. Causes of molecular convergence and parallelism in protein evolution. Nat. Rev. Genet. 17, 239–250 (2016).
Wang, K. et al. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nat. Ecol. Evol. 3, 823–833 (2019).
Lan, Y. et al. De novo transcriptome assembly and positive selection analysis of an individual deep-sea fish. BMC Genom. 19, 394 (2018).
Keane, M. et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 10, 112–122 (2015).
Carrero, D., Pérez-Silva, J. G., Quesada, V. & López-Otín, C. Differential mechanisms of tolerance to extreme environmental conditions in tardigrades. Sci. Rep. 9, 14938 (2019).
Lu, J. Y. et al. Comparative transcriptomics reveals circadian and pluripotency networks as two pillars of longevity regulation. Cell Metab. 34, 836-856.e5 (2022).
Macdonald, A. Life at high pressure in the deep sea and other environments (Springer International Publishing, 2021). https://doi.org/10.1007/978-3-030-67587-5.
Somero, G. N. Protein adaptations to temperature and pressure: Complementary roles of adaptive changes in amino acid sequence and internal milieu. Comp. Biochem. Physiol. 136, 577–591 (2003).
Yancey, P. H., Gerringer, M. E., Drazen, J. C., Rowden, A. A. & Jamieson, A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc. Natl. Acad. Sci. USA 111, 4461–4465 (2014).
Summers, G., Wibisono, R. D., Hedderley, D. I. & Fletcher, G. C. Trimethylamine oxide content and spoilage potential of New Zealand commercial fish species. N Z J. Mar. Freshw. Res. 51, 393–405 (2017).
Gawrys-Kopczynska, M. et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife 9, 1–31 (2020).
Black, J. A., Neuheimer, A. B., Horn, P. L., Tracey, D. M. & Drazen, J. C. Environmental, evolutionary, and ecological drivers of slow growth in deep-sea demersal teleosts. Mar. Ecol. Prog. Ser. 658, 1–26 (2021).
Lopez-Otín, C. & Kroemer, G. Hallmarks of health. Cell 184, 1929–1939 (2021).
Pilditch, C. A., Leduc, D., Nodder, S. D., Probert, P. K. & Bowden, D. A. Spatial patterns and environmental drivers of benthic infaunal community structure and ecosystem function on the New Zealand continental margin. N Z J Mar Freshw. Res. 49, 224–246 (2015).
González-Quirós, R., Cabal, J., Álvarez-Marqués, F. & Isla, A. Ichthyoplankton distribution and plankton production related to the shelf break front at the Avilés Canyon. ICES J. Mar. Sci. 60, 198–210 (2003).
Trueman, C. N., Rickaby, R. E. M. & Shephard, S. Thermal, trophic and metabolic life histories of inaccessible fishes revealed from stable-isotope analyses: A case study using orange roughy Hoplostethus atlanticus. J. Fish Biol. 83, 1613–1636 (2013).
Drazen, J. C. & Seibel, B. A. S. Depth-related trends in metabolism of benthic and benthopelagic deep-sea fishes. Limnol. Oceanogr. 52, 2306–2316 (2007).
Drazen, J. C. & Haedrich, R. L. A continuum of life histories in deep-sea demersal fishes. Deep Sea Res. 1 Oceanogr. Res. Pap. 61, 34–42 (2012).
Gerringer, M. E. et al. Life history of abyssal and hadal fishes from otolith growth zones and oxygen isotopic compositions. Deep Sea Res 1 Oceanogr. Res. Pap. 132, 37–50 (2018).
Omotoso, O., Gladyshev, V. N. & Zhou, X. Lifespan extension in long-lived vertebrates rooted in ecological adaptation. Front. Cell Dev. Biol. 9, 704966 (2021).
Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Quesada, V., Velasco, G., Puente, X. S., Warren, W. C. & López-Otín, C. Comparative genomic analysis of the zebra finch degradome provides new insights into evolution of proteases in birds and mammals. BMC Genom. 11, 220 (2010).
Johnson, S. C., Dong, X., Vijg, J. & Suh, Y. Genetic evidence for common pathways in human age-related diseases. Aging Cell 14, 809–817 (2015).
Guo, J. et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target Ther. 7, 391 (2022).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Sim, N. L. et al. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 40, W452–W457 (2012).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Sánchez, F., Serrano, A., Parra, S., Ballesteros, M. & Cartes, J. E. Habitat characteristics as determinant of the structure and spatial distribution of epibenthic and demersal communities of Le Danois Bank (Cantabrian Sea, N. Spain). J. Mar. Syst. 72, 64–86 (2008).
Wong, A. P. S. et al. Argo data 1999–2019: Two million temperature-salinity profiles and subsurface velocity observations from a global array of profiling floats. Front. Mar. Sci. 7, 1–210 (2020).
Walker, J. R., Corpina, R. A. & Goldberg, J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412, 607–614 (2001).
Cao, D. et al. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 29, 1316–1325 (2015).
Corradi, H. R., Schwager, S. L. U., Nchinda, A. T., Sturrock, E. D. & Acharya, K. R. Crystal structure of the N domain of human somatic angiotensin I-converting enzyme provides a structural basis for domain-specific inhibitor design. J. Mol. Biol. 357, 964–974 (2006).
Acknowledgements
We specially thank Richard O’Driscoll from the National Institute of Water & Atmospheric Research Ltd (NIWA) and Rob Tilney from the Thalassa Fisheries Support for providing Orange roughy samples. We also thank Lucia García Flórez from Centro de Experimentación Pesquera (CEP) and Avilés fish Market for providing Silver roughy individuals. We finally thank David Roiz del Valle, David Rodríguez, José Luis Acuña and José M. P. Freije for their helpful comments and advice. This work was supported by European Research Council (DeAge, ERC Advanced Grant, 742067) and the Ministerio de Ciencia e Innovación-FEDER (SAF2017-87655-R). This work was also a contribution of the Observatorio Marino de Asturias.
Author information
Authors and Affiliations
Contributions
M.P-T isolated gDNA and RNA, set the genomes for their manual annotation and performed residue change prediction in protein models. M.P-T and V.Q carried out the bioinformatic analyses. D.C and M.P-T performed the manual supervised annotation and identification of gene amplifications and variants. D.A-P validated the identified variants and copy number variation. D.C and M.P-T wrote the manuscript and set up figures and tables. C.G-G also contributed to figure preparation. C.L-O supervised research, results interpretation, and construction of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carrero, D., Pascual-Torner, M., Álvarez-Puente, D. et al. Insights into aging mechanisms from comparative genomics in orange and silver roughies. Sci Rep 14, 19748 (2024). https://doi.org/10.1038/s41598-024-70642-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70642-w