Abstract
We aimed to compare the extent of bone turnover suppression between patients with atypical femoral fractures (AFFs) and osteoporotic hip fractures (typical femur fractures, TFFs) using a one-to-one matching strategy. A single-center retrospective comparison of females aged ≥ 60 years who underwent operative treatment for AFFs and TFFs between January 2010 and March 2021 was conducted. Demographic characteristics and clinical data including fracture site, past medical history, bone mineral density (BMD), bisphosphonate (BP) medication history, and serum bone turnover marker (BTM) levels were examined. Moreover, we performed a logistic regression analysis to determine the risk factors for AFFs and a one-to-one matched-pair analysis to compare various BTMs. Overall, 336 consecutive females were included: 113 with AFFs and 213 with TFFs. The mean age, BMI, and lowest BMD T-score were 78.6 years, 22.8 kg/m2, and −3.3, respectively. Patients with AFF were younger, had lower BMD, higher BMI, higher prevalence of rheumatoid arthritis, a greater proportion with previous steroid or BP use, and a longer history of BP use than patients with TFF. The 48:48 matched-pair analysis revealed higher serum 25(OH) vitamin-D (30.5 vs 18.2 ng/mL, P < 0.001) and calcium levels (8.8 vs 8.3 ng/dL, P = 0.009) and lower serum CTX levels (0.33 vs 0.54 ng/mL, P = 0.010) in the AFF group than in the TFF group, suggesting a more suppressed bone remodeling. No differences in the other BTM levels were found. Despite identical histories and durations of BP use, the AFF group exhibited lower CTX levels, suggesting more suppressed bone remodeling. This observation leads us to infer that more suppressed bone remodeling, indicated by lower CTX levels, could be linked to the occurrence of AFFs.
Similar content being viewed by others
Introduction
When atypical femoral fractures (AFFs) were first described by Odvina et al. in 2005, severe suppression of bone turnover due to long-term bisphosphonate (BP) therapy was suggested as the main cause1. Even though the exact etiology of AFFs remains unclear, many studies have reported possible causes, including lower limb geometry and long-term use of antiresorptive agents such as BPs2,3,4,5,6,7,8,9. Excessive suppression of bone remodeling and reduced natural-repair capacity resulting from prolonged use of antiresorptive agents are the major characteristics of AFFs10,11. As a result, fracture healing is more complicated in AFFs than in typical diaphyseal or subtrochanteric femoral fractures11,12,13,14.
Although bone biopsy is the most accurate method for detecting over-suppression of bone turnover following antiresorptive agent use, its clinical application is limited owing to its invasiveness. Even Jamal et al. reported in 2011 that they found no evidence of suppressed bone turnover in a bone biopsy specimen of AFF with tetracycline labeling from a 55-year-old woman who had been using alendronate for 9 years15. Therefore, measuring serum bone turnover markers (BTMs) is the most commonly used strategy for assessing the state of bone turnover suppression16,17.
To date, only few studies have directly compared the degree of bone turnover suppression, as indicated by BTMs, between fracture patients with AFF and non-AFF. In In 2016, Iizuka et al. compared bone turnover markers between AFFs and typical femur fractures (TFFs), and they reported that the AFF group had significantly suppressed BTMs compared to the TFF group16. However, their study had a limitation in that the AFF group consisted of only 10 patients, while the TFF group included 328 patients, making the number of AFF patients too small.
Herein, we hypothesized that patients with AFF would exhibit lower BTM levels than patients with osteoporotic hip fractures (commonly referred to as TFFs). Hence, this study aimed to identify the distinct characteristics of AFF as compared to TFF and evaluate the degree of bone turnover suppression in patients with AFF using a one-to-one matched comparison with patients diagnosed with TFF.
Materials and methods
Study design and patients
We conducted a single-center retrospective study, which was approved by our institutional review board. We reviewed the medical records from January 2010 to March 2021.
We initially screened consecutive female patients who were diagnosed with a femur fracture. A total of 3965 patients were screened for the femur fracture cohort. Among these patients, we excluded those who were under 60 years of age (13 patients) and those diagnosed with a periprosthetic fracture (26 patients) based on our exclusion criteria. After applying the exclusion criteria, a total of 113 patients were diagnosed with AFF during the study period. All of AFF patients had routinely undergone BTM testing, providing sufficient data. For the TFF group, patients who underwent surgery from January 2019 to March 2021 were included, as BTM testing started in January 2019. Patients from before 2019 were excluded due to insufficient data, resulting in a final inclusion of 213 patients in the TFF group.
We compared the two groups and investigated the risk factors for AFF in the entire femur fracture cohort. Moreover, we performed a one-to-one matching analysis with TFF as a control group to compare demographics and the extent of bone remodeling suppression based on BTM levels.
Diagnosis of atypical femoral fractures (AFFs)
We defined AFFs based on the revised case definition by the f the American Society for Bone and Mineral Research (ASBMR) Task Force in 20145,13,18,19. In alignment with this definition, we diagnosed AFFs by evaluating fracture configurations and AFFs on the contralateral side by radiography. According to the revised ASBMR definition, only cases where AFFs occurred between the lesser trochanter and the supracondylar flare were included, encompassing both complete and incomplete fractures. AFFs were defined as having at least 4 out of the 5 major features (5 major features: fracture occurring with minimal or no trauma, fracture line originates at the lateral cortex and is substantially transverse in its orientation, presence of a medial spike or minimally comminuted in the case of incomplete fractures, periosteal or endosteal thickening of the lateral cortex). We also investigated the injury mechanism and prodromal symptoms such as dull or aching pain in the groin or thigh.
Data collection for analysis
The data we collected included: (1) patient demographic characteristics such as age, sex, and body mass index (BMI); (2) fracture site and configurations; (3) past medical history including smoking, alcohol consumption, steroid use, chronic renal disease, diabetes, rheumatoid arthritis (RA), previous osteoporosis, secondary osteoporosis, and prior history of fragility fractures; (4) results of T-score of bone mineral density in lumbar, total hip, femur neck, and lowest value of them as representative value—dual energy X-ray absorptiometry (BMD-DXA); (5) history of BP use; and (6) levels of BTMs, namely serum Ca (normal range 8.2 ~ 10.8 mg/dL), P (normal range 2.5 ~ 4.5 mg/dL), 25(OH) vitamin-D (Vit. D, normal range 30 ~ 150 ng/mL), parathyroid hormone (PTH, normal range 8 ~ 76 pg/mL), C-terminal telopeptide (CTX, normal range 0.177 ~ 1.015 ng/mL), and osteocalcin (OC, normal range 15 ~ 46 ng/mL).
Statistical analysis
Results are expressed as the mean ± standard deviation for numerical data or proportion (%) for categorical data. Descriptive and comparative analyses were conducted using the \({\chi }^{2}\)-test or Fisher’s exact test for categorical data, and Student’s \(t\)-test or Mann–Whitney test for numerical data.
Risk factors for AFF within the entire femur fracture cohort were determined using binary logistic regression analysis. Multivariate analysis was performed with significant variables in the univariate analysis.
A subgroup analysis was conducted with one-to-one matched-pair samples obtained using the greedy matching technique20,21 to reduce bias and to accurately compare BTMs. The matching criteria included age (± 5 years), BMD T-score (± 0.3), and history and duration of BP use. Overall data were analyzed using the SPSS 21.0 software (SPSS Inc., Chicago, IL), with significance set at P < 0.05.
Ethical approval and consent to participate
This study was approved by the Institutional Review Board of Asan Medical Center and waiver was received for the need to provide written informed consent. All methods were performed in accordance with relevant guidelines and regulations of the committee. Data cannot be shared publicly because it contains potentially identifying information of each patient. Data are available from the Asan Medical Center Institutional Data Access / Ethics Committee (contact via Asan Medical Center Institutional Review Board, Convergence Innovation Bldg. 88, Olympic-ro 43-gil, Songpa-gu, Seoul, Republic of Korea. Website link, http://eirb.amc.seoul.kr/; E-mail, irb@amc.seoul.kr; Phone, + 82-2-3010-7165).
Results
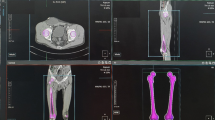
The demographic data and results of the comparative analysis are summarized in Table 1. In the AFF group, the mean age was lower (74.3 vs 80.8 years, P < 0.001), the mean BMI was higher (23.4 vs 22.5 \(kg/{m}^{2}\), P = 0.044), and the lowest BMD T-score was higher (−2.7 vs −3.5, P < 0.001) than the TFF group. In addition, more patients had a history of BP use (66 vs 32%, P < 0.001), with a longer duration (62.5 vs 41.4 months, P = 0.020) and steroid use (13 vs 5%, P = 0.008) in the AFF group than in the TFF group. Moreover, the prevalence of diabetes was lower (14 vs 38%, P < 0.001), whereas that of rheumatoid arthritis was higher (11 vs 4%, P = 0.033) in the AFF group than in the TFF group. The fracture site was most commonly located at the shaft level in the AFF group (56%) and at the intertrochanteric level in the TFF group (89%) (Fig. 1).
In the univariate analysis, age, BMI, BMD, history and duration of BP use, diabetes, steroid use, and rheumatoid arthritis were significant risk factors for AFF (Table 2). In the multivariate analysis, when comparing the AFF group to the entire femur fracture group, age (OR, 0.90; 95% CI, 0.87–0.94), BMD (OR, 1.62; 95% CI, 1.24–2.13), history of BP use (OR, 3.87; 95% CI, 2.15–6.96), and diabetes (OR, 0.19; 95% CI, 0.09–0.39) were significant factors for AFF as compared to TFF (Table 3).
One-to-one matching analysis
In the one-to-one matching analysis, 48 samples in each group were matched. The demographic comparison is shown in Table 4.
After 1:1 matching, there was no difference between the two groups in age, BMI, BMD, history of BP use, and duration of BP use. In addition, the mean CTX level was lower (0.33 vs 0.54 ng/mL, P = 0.010), the mean 25(OH) vitamin-D level was higher (30.5 vs 18.2 ng/mL, P < 0.001), and the mean serum Ca level was higher (8.8 vs 8.3 mg/dL, P = 0.009) (Fig. 2) in the AFF group than in the TFF group.
Comparison of bone turnover markers between the atypical and typical femur fracture groups. Box-and-whisker plots for each bone turnover marker based on a one-to-one matching analysis. The gray boxes in the plots indicate the normal range for each parameter. AFF, atypical femoral fracture; TFF, typical femoral fracture (osteoporotic hip fracture).
Discussion
The principal finding of our study is the lower CTX level in the AFF group, indicating increased suppression of bone turnover in this group. This result, obtained from a comparative analysis adjusted for BP use, duration of BP use, age, and BMD, could suggest a more suppressed state of bone turnover in the AFF group than that in the TFF group.
A previous study proposed a classification model using BTMs such as the N-terminal propeptide of type 1 procollagen (P1NP), beta C-terminal cross-linked telopeptide of type 1 collagen (bCTX), and the P1NP/bCTX ratio17. We could not directly adopt this model in our study due to the use of different BTMs and cut-off values. However, based on a quantitative assessment, it appears that both the AFF and TFF groups in our study align with the low bone formation plus normal bone resorption subtype in the classification model. The authors of the aforementioned study attributed this result to an imbalance between bone formation and resorption17. In the current study, the OC levels in both the AFF and TFF groups were lower than the normal range, with no significant difference between the two groups, indicating decreased bone formation in both groups. Moreover, both groups had CTX levels within the normal range; however, the level in the AFF group was significantly lower than that in the TFF group. Based on these findings and the model from the aforementioned study, it seems likely that the risk of AFF and the balance of bone turnover metabolism are interconnected.
A study explored the causes and risk factors of AFF by determining BTM levels and investigating BP administration status16. Although this study contributes to the understanding of AFFs, its findings should be interpreted with caution owing to its small sample size of only 9 AFF patients and 17 TFF patients. The study noted the role of suppressed bone turnover, lower BTM levels (P1NP, serum tartrate-resistant acid phosphatase-5b [TRACP-5b], undercarboxylated osteocalcin [ucOC]), and BP use in AFF.
Another study provided intriguing insights into the role of BTMs in AFF22. It delved deeper into the variations between different fracture sites, specifically subtrochanteric and mid-shaft AFFs. The study revealed significant differences in bone turnover and bone quality markers among patients with prolonged drug exposure (more than three years). Specifically, the subtrochanteric AFF group exhibited markedly lower levels of TRACP-5b (a bone resorption marker), P1NP and BAP (bone formation markers), and ucOC (a bone quality marker) than the mid-shaft AFF group. Furthermore, histological analysis revealed active bone remodeling or endochondral ossification in the mid-shaft AFF group, implying ongoing bone repair. Contrastingly, the majority of patients in the subtrochanteric AFF group with long-term BP exposure demonstrated a lack of fracture-repair-related biological activity. These findings suggest that the subtrochanteric AFF group exhibits greater bone turnover suppression and diminished bone repair activity compared to the mid-shaft AFF group.
In another study, the impact of long-term BP therapy on the nanomechanical properties of bone was analyzed in 32 patients, half of whom had AFF and the other half did not23. The study revealed that the stiffness (elastic modulus) of cortical bone was greater in patients with AFFs, suggesting a link between increased bone rigidity and atypical fractures. This change was not observed in the cancellous bone or patients without AFFs. These findings suggest that prolonged BP therapy could alter the cortical bone tissue, increasing the risk of atypical fractures. However, further research is needed to fully understand this complex relationship.
These studies corroborate our findings of suppressed bone turnover in patients with AFF despite similar BP usage, with our study benefiting from a larger sample size of 113 AFF and 213 TFF patients. Such a robust sample size not only lends more weight to the findings but also increases the statistical power of the study, enabling more reliable analysis and interpretation. Our study utilized a matched-group analysis to examine differences in BTM levels and other clinical variables between AFF and TFF patients. This methodological approach strengthens the findings by controlling for potential confounding variables and allows for a more direct comparison. However, the reason why BTM levels were lower in the AFF group than in the TFF group, even under identical BP usage conditions, remains unclear. This warrants further investigation from various perspectives, including genetic and ethnic factors, to understand the underlying causes.
In the current study, TFFs, which are osteoporotic hip fractures, were associated with patient characteristics typical of osteoporosis, including (1) older age, (2) lower BMI, and (3) lower BMD. Conversely, patients with AFFs were characterized by (1) a relatively younger age, (2) higher BMI, (3) lower BMD, (4) a more extensive history of BP use, (5) longer duration of BP use, and (6) association with steroid use and RA.
The AFF group in our study had a more extensive history and a longer duration of BP use than TFF group. There have been numerous studies that reported similar findings, indicating that long-term use of BPs influences the risk of AFF1,4,5,6,7,8,13. The risk of AFF increased significantly with longer durations of BP treatment, particularly beyond five years of use, even after multivariate adjustment in this diverse cohort of patients treated with BP8.
Patients with RA and a history of steroid use in the AFF group were more in this study. We included patients who had been exposed to glucocorticoids for longer than 3 months (5 mg prednisone equivalent, or higher, daily) in the group with steroid use24,25. According to the medical records, the causes of using steroids were RA, asthma, chronic obstructive pulmonary disease, ulcerative colitis, autoimmune hepatitis, hypopituitarism, systemic lupus erythematosus, systemic sclerosis, and microscopic polyarteritis. There have been studies indicating an increased incidence of AFF in patients with RA26, as well as the occurrence of AFF in patients with RA during long-term BP treatment27,28. However, we found no studies demonstrating that RA or steroid use are independent risk factors for AFF. Therefore, further investigation is needed regarding the relationship between RA, steroid use, and AFFs.
Regarding the higher (normal range) 25(OH) vitamin-D levels in the AFF group, we interpret this to indicate that there were many patients in the AFF group with normal 25(OH) vitamin-D levels due to osteoporosis treatments, whereas the levels were decreased in the TFF group. The higher Ca levels in the AFF group were considered clinically insignificant due to the difference falling within the normal range. Another possibility is that AFF patients typically have a longer history of osteoporosis medication use, with most having used long-term BP. Incorrect continuous use of long-term BP without a drug holiday is more common in local clinics than in referral university hospitals due to the nature of medical practice in Korea. In these local clinics, due to financial issues, high-dose vitamin D or intravenous vitamin D is used more frequently, which might have caused this bias.
The main strength of our study is that it confirms the decreased CTX level in the AFF group through a matching analysis between the AFF and control groups. Although a few studies have examined bone turnover suppression in patients with AFF, no well-controlled, direct comparison studies have, to date, reported decreased CTX levels in patients with AFF. Our study is the first to confirm a significant decrease in CTX levels in patients with AFF. Furthermore, according to the previous study by Cho et al.29, the average CTX value in Korean postmenopausal women over 60 years of age is 0.389–0.491 ng/mL. Considering this, the CTX value in the AFF group in our study is suppressed and lower than the average value for the same age group in Korea, while the CTX value in the TFF group is within the normal range (AFF: 0.33 ng/mL vs. TFF: 0.43 ng/mL).
Although this study presents meaningful insights, it has several limitations that need to be acknowledged. First, the selection of the TFF group as the control group might not accurately represent the general population, which may limit the generalizability of the findings. To mitigate this issue, we employed a matching technique, although it might not have completely addressed the limitation. Second, being a comparative study with a TFF group, it was challenging to discern and analyze the independent risk factors associated with the occurrence of AFF. This could restrict our understanding on the complete range of risk factors for AFF. Lastly, this study is retrospective and was conducted at a single center, which may lead to potential selection bias and limit the diversity of the study population. Moreover, due to a lack of data, we were unable to analyze AFF caused by medications other than bisphosphonates, such as denosumab. Hence, the conclusions drawn from this study may not apply to all patient populations. However, compared to previous studies, the number of patients in the AFF group in this study is overwhelmingly large, which is a strength of this research. This is because our hospital is one of the largest referral center for other hospitals, and has expert in AFF research, leading to a high number of AFF patients in this study. Therefore, we believe that this study is significant due to the large number of AFF patients included. Future research should focus on expanding these findings with larger studies, ideally multicenter and prospective, to further explore the etiology and risk factors of AFF. Furthermore, prospective monitoring of CTX levels in a broader patient population could help clarify their potential role as an indicator of AFF risk.
Conclusion
The AFF group showed a higher BMI, a greater proportion of patients with a history of steroid use, a higher prevalence of RA, and a longer history of BP use than the TFF group. After adjusting for age, history and duration of BP use, and BMD, the CTX levels were lower in the AFF group than in the TFF group, which might indicate a more suppressed state of bone remodeling.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Odvina, C. V. et al. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 90, 1294–1301. https://doi.org/10.1210/jc.2004-0952 (2005).
Tano, A. et al. Potential bone fragility of mid-shaft atypical femoral fracture: Biomechanical analysis by a CT-based nonlinear finite element method. Injury 50, 1876–1882. https://doi.org/10.1016/j.injury.2019.09.004 (2019).
Haider, I. T., Schneider, P. S. & Edwards, W. B. The role of lower-limb geometry in the pathophysiology of atypical femoral fracture. Curr. Osteoporos. Rep. 17, 281–290. https://doi.org/10.1007/s11914-019-00525-x (2019).
Nieves, J. W. & Cosman, F. Atypical subtrochanteric and femoral shaft fractures and possible association with bisphosphonates. Curr. Osteoporos. Rep. 8, 34–39. https://doi.org/10.1007/s11914-010-0007-2 (2010).
Shane, E. et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 25, 2267–2294. https://doi.org/10.1002/jbmr.253 (2010).
Gedmintas, L., Solomon, D. H. & Kim, S. C. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: A systematic review and meta-analysis. J. Bone Miner. Res. 28, 1729–1737. https://doi.org/10.1002/jbmr.1893 (2013).
Aspenberg, P. & Schilcher, J. Atypical femoral fractures, bisphosphonates, and mechanical stress. Curr. Osteoporos. Rep. 12, 189–193. https://doi.org/10.1007/s11914-014-0200-9 (2014).
Black, D. M. et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 383, 743–753. https://doi.org/10.1056/NEJMoa1916525 (2020).
Saita, Y. et al. The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment. Bone 66, 105–110. https://doi.org/10.1016/j.bone.2014.06.008 (2014).
Kang, J. S. et al. Atypical femoral fractures after anti-osteoporotic medication: A Korean multicenter study. Int. Orthop. 38, 1247–1253. https://doi.org/10.1007/s00264-013-2259-9 (2014).
Lim, S. J. et al. Incidence, risk factors, and fracture healing of atypical femoral fractures: A multicenter case-control study. Osteoporos. Int. 29, 2427–2435. https://doi.org/10.1007/s00198-018-4640-4 (2018).
Giusti, A., Hamdy, N. A. & Papapoulos, S. E. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies. Bone 47, 169–180. https://doi.org/10.1016/j.bone.2010.05.019 (2010).
Shane, E. et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 29, 1–23. https://doi.org/10.1002/jbmr.1998 (2014).
Byun, S. E., Lee, K. J., Shin, W. C., Moon, N. H. & Kim, C. H. The effect of teriparatide on fracture healing after atypical femoral fracture: A systematic review and meta-analysis. Osteoporos. Int. 34, 1323–1334. https://doi.org/10.1007/s00198-023-06768-w (2023).
Jamal, S. A., Dion, N. & Ste-Marie, L. G. Atypical femoral fractures and bone turnover. N. Engl. J. Med. 365, 1261–1262. https://doi.org/10.1056/NEJMc1107029 (2011).
Iizuka, Y. et al. Bone turnover markers and the factors associated with atypical femur fractures among Japanese patients. Injury 47, 2484–2489. https://doi.org/10.1016/j.injury.2016.09.031 (2016).
Fisher, A., Fisher, L., Srikusalanukul, W. & Smith, P. N. Bone turnover status: Classification model and clinical implications. Int. J. Med. Sci. 15, 323–338. https://doi.org/10.7150/ijms.22747 (2018).
Starr, J., Tay, Y. K. D. & Shane, E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr. Osteoporos. Rep. 16, 519–529. https://doi.org/10.1007/s11914-018-0464-6 (2018).
Tile, L. & Cheung, A. M. Atypical femur fractures: Current understanding and approach to management. Ther. Adv. Musculoskelet. Dis. https://doi.org/10.1177/1759720X20916983 (2020).
Stuart, E. A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 25, 1–21. https://doi.org/10.1214/09-STS313 (2010).
Austin, P. C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 33, 1057–1069. https://doi.org/10.1002/sim.6004 (2014).
Oh, Y. et al. Biological activity is not suppressed in mid-shaft stress fracture of the bowed femoral shaft unlike in “typical” atypical subtrochanteric femoral fracture: A proposed theory of atypical femoral fracture subtypes. Bone 137, 115453. https://doi.org/10.1016/j.bone.2020.115453 (2020).
Griffin, L. V. et al. Bone nanomechanical properties and relationship to bone turnover and architecture in patients with atypical femur fractures: A prospective nested case-control study. JBMR Plus 5, e10523. https://doi.org/10.1002/jbm4.10523 (2021).
Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American college of rheumatology Ad Hoc committee on glucocorticoid-induced osteoporosis. Arthr. Rheum. 44, 1496–1503. (2001).
Canalis, E., Mazziotti, G., Giustina, A. & Bilezikian, J. P. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 18, 1319–1328. https://doi.org/10.1007/s00198-007-0394-0 (2007).
Takakubo, Y. et al. The incidence of atypical femoral fractures in patients with rheumatic disease: Yamagata prefectural committee of atypical femoral fractures (YamaCAFe) study. Tohoku J. Exp. Med. 242, 327–334. https://doi.org/10.1620/tjem.242.327 (2017).
Somford, M. P. et al. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: Clues to the mechanism of increased bone fragility. J. Bone Miner. Res. 24, 1736–1740. https://doi.org/10.1359/jbmr.090408 (2009).
Sato, H. et al. Cumulative incidence of femoral localized periosteal thickening (beaking) preceding atypical femoral fractures in patients with rheumatoid arthritis. Osteoporos. Int. 32, 363–375. https://doi.org/10.1007/s00198-020-05601-y (2021).
Cho, D. H., Chung, J. O., Chung, M. Y., Cho, J. R. & Chung, D. J. Reference intervals for bone turnover markers in Korean healthy women. J. Bone Metab. 27, 43–52 (2020).
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C1012972) and by the Shinpoong Research Grant (2021).
Author information
Authors and Affiliations
Contributions
Study concept and design: J.S.A. and J.W.K. Acquisition of the data: C.H.K., Analysis and interpretation of data: J.S.A. and J.W.K. Drafting of the manuscript: J.S.A., C.H.K. and J.W.K. Statistical analysis: J.S.A. Manuscript review and approval: J.S.A., C.H.K., and J.W.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahn, J., Kim, CH. & Kim, J.W. Comparison of bone turnover suppression in atypical femoral fractures and osteoporotic hip fractures. Sci Rep 14, 19974 (2024). https://doi.org/10.1038/s41598-024-71024-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71024-y
Keywords
This article is cited by
-
Association between femoral localized cortical thickening (“beaking” or “flaring”) and bisphosphonate duration in glucocorticoid-induced osteoporosis
Journal of Bone and Mineral Metabolism (2026)