Abstract
The accumulation of monocyte-derived macrophages in the lung tissue during inflammation is important for the pathogenesis of fibrotic lung disease. Deficiencies in chemokine receptors CCR2 and CCR5 and their ligands, which mediate monocyte/macrophage migration, ameliorate bleomycin (BLM)-induced lung fibrosis. Disulfiram (DSF), which is used to treat alcoholism because of its aldehyde dehydrogenase (ALDH)-inhibiting effect, inhibits monocyte/macrophage migration by inhibiting FROUNT, an intracellular regulator of CCR2/CCR5 signalling. Here, we investigated the antifibrotic effect of oral DSF administration in a mouse model of BLM-induced lung fibrosis, focusing on macrophage response and fibrosis progression. The direct inhibitory activity of DSF on monocyte migration was measured using the Boyden chamber assay and compared with that of DSF-related inhibitors with different FROUNT-inhibition activities. Quantitative PCR was used to determine the expression of fibrosis-promoting genes in the lung tissue. DSF significantly suppressed macrophage infiltration into lung tissues and attenuated BLM-induced lung fibrosis. DSF and its metabolites, diethyldithiocarbamate (DDC) and copper diethyldithiocarbamate (Cu(DDC)2), inhibited monocyte migration toward the culture supernatant of primary mouse lung cells mainly comprising CCL2, whereas cyanamide, another ALDH inhibitor, did not. DSF, with higher inhibitory activity against FROUNT than DDC and Cu(DDC)2, inhibited monocyte migration most strongly. In BLM-induced fibrotic lung tissues, profibrotic factors were highly expressed but were reduced by DSF treatment. These results suggest DSF inhibits macrophage infiltration, which might be attributed to its inhibitory effect on FROUNT, and attenuates BLM-induced lung fibrosis. In addition, multiplex immunofluorescence imaging revealed reduced infiltration of S100A4+ macrophages into the lungs in DSF-treated mice and high expression of FROUNT in S100A4+ macrophages in idiopathic pulmonary fibrosis (IPF). These findings underscore the potential of macrophage-targeted therapy with DSF as a promising drug repositioning approach for treating fibrotic lung diseases, including IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common and refractory disease of idiopathic interstitial pneumonia. This fibrosis may result from abnormal wound healing in response to persistent lung injury and is characterised by excessive accumulation of extracellular matrix (ECM) components in the intra-alveolar septum, thereby destroying normal lung architecture and declining lung function. IPF has a poor prognosis, with a mean survival of approximately 3 years after diagnosis. Pirfenidone and nintedanib have emerged as antifibrotic drugs for patients with IPF but are not curative treatments. Hence, the development of novel antifibrotic agents is urgently required.
Among various cells involved in IPF pathogenesis, including alveolar epithelial cells and myofibroblasts, macrophages are crucial in this pathogenesis1. For example, in the bleomycin (BLM)-induced lung fibrosis model, which is frequently used as an animal model of lung fibrosis, deficiencies in monocyte/macrophage-recruiting chemokine receptors CCR2 and CCR5, and their respective ligands CCL2 and CCL3, reduce the infiltration of macrophages into the lung tissues and improve lung fibrosis2,3,4. Similarly, deletion of the CD11b-expressing monocyte-derived macrophages, which migrate from the bone marrow into the lesion as lung fibrosis progresses, significantly reduces BLM-induced lung fibrosis5. Thus, monocytes/macrophages and their precursor monocytes released from the bone marrow and migrated to tissues are important for lung fibrosis.
FROUNT, a cytoplasmic molecule, binds to the intracellular region of the chemokine receptors CCR2 and CCR5 that are expressed on the cell surface of monocytes/macrophages, and promote their migration signals6,7. In mice, FROUNT deficiency inhibits tumour-associated macrophage infiltration and tumour growth. Furthermore, through multistep screening of a library of 131,200 low-molecular-weight compounds, we found that disulfiram (DSF) exerts potent inhibitory activity against FROUNT by directly binding to it8.
DSF inhibits aldehyde dehydrogenase (ALDH), thereby used as a drug for alcoholism for over 70 years9. DSF is metabolised to diethyldithiocarbamate (DDC) and other metabolites, including copper diethyldithiocarbamate (Cu(DDC)2), and some of these metabolites inhibit liver ALDH10. DSF has multiple biological properties, including antitumor11,12,13, antimicrobial14, and anti-inflammatory properties15,16,17. Although DSF has never been known to inhibit macrophage migration, in mouse tumour models, we reported that DSF does indeed inhibit macrophages from migrating into the tumour tissues, exerting an antitumor effect8.
Considering that macrophages have been widely reported as a potential therapeutic target in IPF18,19,20 and that DSF decreases macrophage infiltration into lung tissues8, DSF could be a potential novel drug for fibrotic lung disorders, including IPF. In this study, we investigated the effects of oral DSF administration on BLM-induced lung fibrosis in mice.
Materials and methods
Detailed methods are described in the Supplementary Methods submitted online.
Mice
Wild-type male C57BL/6J mice 5–7 weeks old and weighing 20–25 g were purchased from Sankyo Labo Service (Tokyo, Japan). BLM (Nippon Kayaku Co., Tokyo, Japan) was dissolved in 200 μL of saline at a dose of 85 mg/kg concentration and administered to the mice using an osmotic pump (Alzet model 2002; DURECT Corporation, Cupertino, CA, USA) containing 200 μL of BLM solution. The mice were subcutaneously implanted with the osmotic pump releasing BLM at a continuous infusion rate of 0.5 µL/h for 14 days. Moreover, we orally administered DSF (Mitsubishi Tanabe Pharma Co., Osaka, Japan) to the mice daily by feeding them a diet supplemented with 0.16% DSF from 10 days before to 28 days after implantation of the BLM-containing osmotic pump. Subsequently, these mice were euthanised under isoflurane anaesthesia before and on days 14 and 28 after osmotic pump implantation (hereafter referred to as days 0, 14, and 28). Three randomly assigned groups of mice were analysed: mice receiving neither BLM nor DSF (untreated group), mice receiving BLM but not DSF (DSF non-treated group), and mice receiving BLM and DSF (DSF-treated group). The Ethics Committee for Animal Experiments of Nippon Medical School approved all procedures (approval number: 2020–023, 2021). All experiments were performed in accordance with relevant regulations and guidelines, including the ARRIVE guidelines.
Micro-computed tomography (micro-CT)
A Latheta LCT-200 (Aloka, Tokyo, Japan) X-ray CT system for laboratory animals was used. The CT images with a slice thickness of 192 μm were obtained using an X-ray generator with a tube voltage of 50 kV and a maximum tube current of 0.5 mA. An area of CT values between − 400 and − 200 Hounsfield units was defined as an area of abnormally high absorption in the lungs. Using LCT-200 micro-CT System software, we measured the percentage of abnormally high-density areas in the whole lung field of each slice and calculated the average of all slices.
Measurement of chemokine levels in mouse lung cell culture supernatants
Both lungs of an 11-week-old male C57BL/6J mouse purchased from Sankyo Lab Services (Tokyo, Japan) were removed, chopped with a razor, placed in 10% FBS-DMEM supplemented with penicillin–streptomycin, seeded on 10 cm dishes (surface-treated, CORNING), and cultured for seven days at 37 °C and 5% CO2. Cells grown from the lung tissues were harvested and replated with fresh media in 10 cm dishes. After 72 h of culture, supernatants were collected. The Mouse Proinflammatory Chemokine Panel of the LEGENDplex (BioLegend) was used to measure 13 chemokines (CXCL1, CXCL5, CXCL9, CXCL10, CXCL13, CCL2, CCL3, CCL4, CCL5, CCL11, CCL17, CCL20, and CCL22) in the culture supernatant. The culture supernatant was used as the migration factor in the cell migration experiments described in the next section.
Chemotaxis assay
We used 9–14-week-old male C57BL/6J mice purchased from Sankyo Lab Service Co. The bone marrow cells were flushed from the femur and tibia using 2% FBS-PBS. The bone marrow cells were subsequently incubated in an ammonium-chloride-potassium (ACK) lysing buffer to remove red blood cells and adjusted to 2 × 107 cells/mL concentration. Thereafter, we incubated these cells at 25 °C for 90 min with or without various inhibitors such as CCR2 inhibitors BMS CCR2 22 (Tocris), DSF, DDC (Tokyo Chemical Industry), Cu(DDC)2 (Tokyo Chemical Industry), and cyanamide (SIGMA), an ALDH inhibitor similar to DSF. DDC and Cu(DDC)2 are metabolites of DSF. Each inhibitor was used at 5–50 µM concentrations, whereas cyanamide was used at 50 µM concentration. As a control, dimethyl sulfoxide (DMSO), which was used to dissolve the compounds, was added at the same concentration. The bone marrow cell suspension containing 10% FBS was then added to the upper compartment of a 96-well chemotaxis chamber (Neuro probe) with 5 μm pore size filters. Furthermore, the 72-h culture supernatant of cells derived from mouse lung tissues containing 10% FBS was added to the lower compartment as a migration factor and incubated for 90 min at 37 °C and 5% CO2. “Input cells” were the premigration cells, whereas “migrated cells” were those that migrated to the lower compartment of the chemotaxis chamber. Both input and migrated cells underwent dead cell staining for 10 min at 4 °C, and Fc receptors were blocked by adding unlabelled anti-CD16/32 antibody (Bio X Cell) for 10 min at 4 °C. Subsequently, we stained the cells with fluorescein-conjugated antibodies for 20 min at 4 °C. The following antibodies were used: Ly-6C-Pacific Blue (1:600; BioLegend), CD45-FITC (1:400; BioLegend), CD11b-PE (1:400; BioLegend), Siglec-F-PE-CF594 (1:200; BD Biosciences), CD11c-PE-Cy7 (1:400; BioLegend), and Ly-6G-A700 (1:200; BioLegend). We measured the number of CD45+CD11c-Siglec-F-CD11b+Ly-6G-Ly-6Chi monocytes in the input and migrated cells by flow cytometry using a FACS LSR FotessanX-20. We also calculated the migration efficiency (migrated cells/input cells [%]), which represents the chemotaxis of monocytes, and determined the ratio of migration efficiency with an inhibitor to migration efficiency without an inhibitor (%). Additionally, the percentage of viable cells among the input cells was calculated using dead cell staining. The cell viability (%) in the absence of inhibitors was calculated as a reference (100%).
Multi-fluorescent staining analysis with patient lungs
Lung specimens were obtained from six patients pathologically diagnosed with IPF and five pathologically diagnosed with normal lungs. Detailed clinical information is provided in Supplementary Table 1. Formalin-fixed and paraffin-embedded tissues were analysed. Multi-fluorescent staining was performed according to the Opal staining protocol (Akoya Biosciences). We used the following primary antibodies: anti-human α-smooth muscle actin (α-SMA) mouse monoclonal antibody (1:250 dilution, Dako, Glostrup, Denmark), anti-human CD34 mouse monoclonal antibody (1:100 dilution, Nichirei Bioscience, Tokyo, Japan), anti-human CD68 mouse monoclonal antibody (1:200 dilution, Dako, Glostrup, Denmark), anti-human/mouse S100A4 rabbit monoclonal antibody (1:100 dilution, Abcam, Cambridge, UK), and anti-human FROUNT goat polyclonal antibody (1:150 dilution, Everest biotech, Oxfordshire, UK), followed by peroxidase-labelled secondary antibodies, and incubated with Opal fluorophore Opal 690, Opal 540, Opal 570, Opal 620, Opal 520, respectively. Antigen retrieval and microwave treatment between each staining were performed in an AR6 buffer. Microscopic image data were captured using PhenoImager Mantra2™ quantitative pathology workstation (Akoya Biosciences), and the fluorophores and autofluorescence were spectrally unmixed and quantified using inForm® image analysis software (Akoya Biosciences). This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Nippon Medical School (approval number: M-2021–020). Informed consent was obtained on the Nippon Medical School website through an opt-out process.
Quantification of pathological findings
Five 500 µm × 500 µm square areas were randomly selected in Elastica Masson–Goldner (EMG)-stained images of the lungs of each mouse, and the degree of fibrosis in the area was assessed by assigning an Ashcroft score21 to the EMG-stained images by two pathologists. The Ashcroft scores were averaged for each individual. The number of infiltrating cells, immunohistochemistry-positive cells, and immunohistochemistry-positive area ratio for each mouse lung were assessed using the QuPath software (Ver. 0.4.3)22. Whole slide image data were obtained using a Leica SCN400F slide scanner (Leica Biosystems, Wetzlar, Germany). Five or ten 500 µm × 500 µm square areas were randomly selected in each mouse lung and analysed by positive cell detection or positive pixel count. Values obtained were averaged for each individual.
Statistical analysis
Data were analysed using GraphPad Prism 8 and are presented as mean ± standard deviation. One-way analysis of variance and Tukey’s test or the unpaired t-test were used for between-group comparisons. Normal distribution was tested using the Shapiro–Wilk test. Body weight changes were analysed using a two-way repeated measures analysis of variance and Sidak’s multiple comparison test. Statistical criteria for significant differences were set at P < 0.05. All the experiments were repeated twice.
Results
Effect of DSF on BLM-induced lung fibrosis in mice
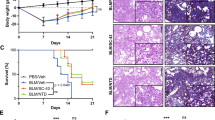
BLM administration induced body weight loss, which was mitigated by DSF treatment (Fig. 1A). Blood surfactant protein-D (SP-D) level, which is a clinically useful marker of disease activity in lung fibrosis23, slightly increased on day 14 and considerably increased on day 28 after the start of BLM administration (Fig. 1B). In the DSF-treated group, SP-D levels remained low on day 14 and were significantly lower on day 28 than those in the DSF non-treated group (Fig. 1B).
Effect of DSF on BLM-induced lung fibrosis in mice. (A) Time course of percentage change in body weight following BLM administration. (B) SP-D concentration in the blood of mice on days 0, 14, and 28. (C–H) Representative cross-sectional micro-CT images of mouse lungs on days 14 (C, D, E) and 28 (F, G, H) in the untreated (C, F), DSF non-treated (D, G), and DSF-treated (E, H) groups. (I) Percentage of the abnormally hyperabsorbed area (between − 400 and − 200 Hounsfield units) in the whole lung field on days 0, 14, and 28. n = 5 per group. *P < 0.05, **P < 0.01. Sidak’s multiple comparison test for (A) and Tukey’s test for (B) and (I) were used to compare DSF non-treated and DSF-treated groups.
We used micro-CT lung images from all the mice in each group to assess the proportion of fibrotic lesions in the whole lung (Fig. 1C–H). Abnormal lung areas indicated by high-absorption areas were obscured on day 14 (Fig. 1D,E) but were clearly observed on day 28 (Fig. 1G). Conversely, DSF treatment significantly reduced BLM-induced lung fibrotic lesions (Fig. 1H,I).
Histological analysis revealed that the DSF non-treated group had large fibrotic lesions with inflammatory cell infiltration in the subpleural alveolar region on day 28 (Fig. 2A,B,D,E). These are typical lesions in this continuous subcutaneous BLM infusion model. In contrast, the fibrotic lesions in the DSF-treated group were milder and contained fewer inflammatory cells (Fig. 2C,F,G,H). In addition, the content of hydroxyproline, a surrogate for collagen content, in the lung tissue of DSF-treated mice was significantly reduced compared with that in DSF non-treated mice (Fig. 2I).
Histological analysis and hydroxyproline content of BLM-induced lung fibrosis in mice. (A–F) Haematoxylin and eosin staining (A–C) and Elastica Masson–Goldner (EMG) staining (D–F) of lung tissues on day 28 in untreated (A, D), DSF non-treated (B, E), and DSF-treated (C, F) mice. Higher magnification images in the lower left correspond to the areas indicated by blue squares. Scale bars = 500 μm. (G) Number of infiltrating cells in the lung tissue on day 28 counted in HE images (A–C). (H) Ashcroft score of lung tissues on day 28 evaluated in EMG images (D–F). (I) Hydroxyproline content in the lung tissue on day 28. n = 5 per group. *P < 0.05, **P < 0.01 (Tukey’s test). ns, not significant.
Effect of DSF on type I collagen deposition, myofibroblast and macrophage infiltration in the lung tissue of the BLM-induced lung fibrosis mouse model
On day 28, we evaluated the expression of type I collagen, a major component of ECM, using immunohistochemical staining. Deposits of type I collagen were abundant in the fibrotic lesions of the lungs of DSF non-treated mice but were few in those of DSF-treated mice (Fig. 3A–C,J). Additionally, α-SMA-positive myofibroblasts, the main source of ECM, were increased in the fibrotic lung lesions of DSF non-treated mice but were only slightly increased in those of DSF-treated mice (Fig. 3D–F,K). Numerous F4/80+ cells (macrophages) were also found in the fibrotic lung lesions of DSF non-treated mice, whereas only a few were found in the DSF-treated mice (Fig. 3G–I,L). Therefore, the oral administration of DSF potently attenuated lung fibrosis in mice and inhibited macrophage infiltration into the lung tissue.
Immunohistochemical analysis of BLM-induced lung fibrosis in mice. (A–F) Representative immunostaining images for type I collagen (A–C), α-SMA (D–F), and F4/80 (G–I) in the lung tissue of untreated (A, D, G), DSF non-treated (B, E, H) and DSF-treated (C, F, I) mice on day 28. Higher-magnification images in the lower left correspond to the areas indicated by blue squares. Scale bars = 500 μm. (J, K) Percentage of type I collagen positive and α-SMA positive area in lung tissue on day 28. (L) Number of F4/80+ cells in the lung tissue on day 28 counted in immunostaining images (G–I). n = 5 per group. *P < 0.05, **P < 0.01 (Tukey’s test). ns, not significant.
Effect of DSF on inflammatory cells in the lung tissue and peripheral blood in the BLM-induced lung fibrosis mouse model
To further investigate the effect of DSF on BLM-induced lung fibrosis, we conducted flow cytometric analysis of inflammatory cells in the lungs and peripheral blood, focusing mainly on macrophages on day 28, according to the gating procedure shown in Supplementary Fig. 1. The total number of cells in the lung tissue was not significantly different between the DSF non-treated and DSF-treated mice (Fig. 4A). However, the macrophages found in the lung tissue of DSF-treated mice were significantly reduced compared with those DSF non-treated mice (Fig. 4B). Furthermore, DSF treatment reduced both CD86+ M1 and CD206+ M2 macrophages (Fig. 4C,D), which are associated with lung fibrosis progression24,25,26. Furthermore, the proportion of monocytes (Ly-6Chi monocytes) in the lungs or peripheral blood was not significantly different between DSF non-treated and DSF-treated mice (Fig. 4E,G). Likewise, the proportion of neutrophils in the lungs or peripheral blood was not significantly different between the two groups (Fig. 4F,H). Therefore, DSF significantly reduced macrophage infiltration but not neutrophil infiltration in BLM-induced lung fibrosis.
Flow cytometric analysis of lung tissues and peripheral blood of BLM-induced lung fibrosis in mice. (A) Total number of viable cells in the lung tissue of untreated, DSF non-treated, and DSF-treated mice on day 28. (B–F) Percentages of total monocytes/macrophages, CD86+ M1 macrophages, CD206+ M2 macrophages, Ly-6Chi monocytes, and neutrophils among total viable cells in the lung tissue of untreated, DSF non-treated, and DSF-treated mice on day 28. (G, H) Percentages of monocytes and neutrophils in total viable cells in the peripheral blood of untreated, DSF non-treated, and DSF-treated mice on day 28. n = 5 per group. *P < 0.05, **P < 0.01 (Tukey’s test). ns, not significant.
Effect of DSF on monocyte/macrophage migration toward lung cell-derived chemoattractants
We examined whether DSF directly inhibits monocyte migration in vitro using the Boyden Chamber method. To mimic monocyte migration from the bone marrow to the lung tissue in response to chemoattractant derived from the lung microenvironment, as our mouse model in vivo, we used mouse primary bone marrow cells containing monocytes and the lung cell culture supernatant (LCCS) obtained from lung tissue-derived primary murine cells cultured for 72 h as a chemoattractant (Fig. 5A). In this experiment, monocytes migrated efficiently toward the culture supernatant (Fig. 5B). However, the CCR2 inhibitor BMS CCR2 22 inhibited this migration, indicating that this migratory system is CCR2-dependent (Fig. 5B). Indeed, the culture supernatants of lung tissue-derived cells mainly contained CCL2, a ligand for CCR2, and, to a lesser extent, CCL3 and CCL4, which are both ligands for CCR5 (Fig. 5C). Thus, this in vitro model mimicked the migration of bone marrow-derived monocytes into lung tissue during BLM-induced inflammation in our mouse model.
Effect of DSF on monocyte migration in vitro. (A) Schematic diagram of the Boyden chamber-based monocyte chemotaxis assay toward lung cell culture supernatant (LCCS). Monocytes in input cells and migrated cells were quantified by flow cytometry, and the migration efficiency (number of monocytes in migrated cells/number of monocytes in input cells [%]) was calculated. (B) Migration efficiency of monocytes after CCR2 inhibitor (BMS CCR2 22) pretreatment. n = 3 or 4 per group. (C) Concentrations of 13 chemokines (CXCL1, CXCL5, CXCL9, CXCL10, CXCL13, CCL2, CCL3, CCL4, CCL5, CCL11, CCL17, CCL20, and CCL22) as migration factors in the supernatant of lung tissue-derived cells of C57BL/6J mice after 72 h of culture. (D) Migration efficiency of monocytes pretreated with DSF, DDC, Cu(DDC)2, and cyanamide (ALDH inhibitor). n = 3 per group. (E) Percentage of viable bone marrow cells pretreated with DSF, DDC, Cu(DDC)2, and cyanamide (ALDH inhibitor). The percentage of viable cells not treated with inhibitors was used as the reference (100%). n = 3 per group. *P < 0.05, **P < 0.01 (Tukey’s test).
We analysed whether DSF or several other DSF-related inhibitors could inhibit monocyte migration using this in vitro model. DSF successfully inhibited the migration of these monocytes in a concentration-dependent manner at 5 and 50 μM concentrations (Fig. 5D) without significant cytotoxicity (Fig. 5E). DSF itself was previously reported to be more potent in inhibiting FROUNT than its metabolites DDC and Cu(DDC)28. In this study, DSF inhibited monocyte migration more strongly than DDC and Cu(DDC)2 at low concentrations (5 µM). However, those metabolites inhibited monocyte migration as much as DSF at high concentrations (50 µM) (Fig. 5D). In contrast, cyanamide, an ALDH inhibitor, did not inhibit monocyte migration (Fig. 5D). These results suggested that DSF directly and efficiently inhibited the migration of bone marrow-derived monocytes and were consistent with the previous report that DSF itself is more potent in inhibiting FROUNT than its metabolites DDC and Cu(DDC)28.
Effect of DSF on the expression of Ccl2, Spp1, Col1a1, and Timp-1 in the lung tissue of the BLM-induced lung fibrosis mouse model
To investigate the molecules contributing to the antifibrotic effect of DSF in BLM-induced lung fibrosis, we examined the changes in the mRNA expression of lung fibrosis-related genes in the lung tissue. On day 14, the mRNA expression levels of Ccl2, Spp1, and Timp-1 increased in DSF non-treated mice but were significantly suppressed in DSF-treated mice (Fig. 6A). On day 28, the mRNA expression levels of Ccl2, Col1a1, and Timp-1 increased in DSF non-treated mice but significantly decreased in DSF-treated mice (Fig. 6B). Thus, in addition to suppressing the mRNA expression of Col1a1, which contributes to collagen deposition, on day 28, DSF treatment significantly reduced the expression of profibrotic factor genes in the lung tissue as early as day 14, when dense fibrosis had not yet been observed.
Effect of DSF on profibrotic gene expression in the lung tissues of BLM-induced lung fibrosis in mice. (A, B) Relative mRNA expression of Ccl2, Spp1, Col1a1, and Timp-1 in the lung tissue of untreated, DSF non-treated and DSF-treated mice on day 14 (A) and day 28 (B). n = 4 or 5 per group. *P < 0.05, **P < 0.01 (Tukey’s test). ns, not significant.
Analysis of S100A4+ macrophages in the BLM-induced lung fibrosis mouse model and IPF
According to recent studies, S100A4+ macrophages are involved in the pathogenesis of lung fibrosis18,27. We investigated the expression of S100A4 on day 28 in a mouse model of BLM-induced lung fibrosis and found that S100A4+ cells were found mainly in the fibrotic lesions (Fig. 7A–C). Double-fluorescence staining analysis indicated that macrophages were the predominant S100A4+ cells in this model (Fig. 7D), and their numbers were significantly attenuated in DSF-treated mice compared to those in DSF non-treated mice (Fig. 7E). Furthermore, we assessed S100A4 expression in human lung tissue from patients with idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) and control subjects without fibrotic lung disease using multiplex immunofluorescence imaging to examine these macrophages specifically. Our data demonstrated that S100A4 expression in CD68+ macrophages was substantially elevated in the fibrotic regions of the lungs from patients with IPF/UIP compared to that in CD68+ macrophages from control normal lung subjects (Fig. 8A–C). Additionally, we observed that FROUNT was more prominently expressed in S100A4+CD68+ macrophages (Fig. 8B white allowheads) than in S100A4-CD68+ macrophages (Fig. 8B yellow allowheads) within the fibrotic regions of patients with IPF/UIP (Fig. 8B,D).
Effect of DSF on S100A4+ macrophages in the lung tissues of BLM-induced lung fibrosis in mice. (A–C) Representative immunostaining images for S100A4 in the lung tissue of untreated (A), DSF non-treated (B) and DSF-treated (C) mice on day 28. Higher-magnification images in the lower left correspond to the areas indicated by dashed squares. Scale bars = 200 μm. (D) Representative bright-field and double-fluorescence staining images in the lung tissues of BLM-treated mice on day 28. DAPI (4′,6-diamidino-2-phenylindole) is shown in blue, CD68 in green, and S100A4 in red. The bright-field image was generated from fluorescent images using inForm software. Higher-magnification images correspond to the area indicated with dashed squares. Arrowheads indicate S100A4+CD68+ macrophages. Scale bars = 25 μm. (E) Number of S100A4+ cells in the lung tissue on day 28 counted in immunostaining images (A–C). n = 5 per group. *P < 0.05, **P < 0.01 (Tukey’s test). ns, not significant.
Analysis of multi-fluorescent staining of lung tissues of IPF. (A, B) Representative immunofluorescent staining images in normal lung (A) and in fibrotic areas of the lungs of IPF patients (B). DAPI is shown in pink, α-SMA in brown, CD34 in light blue, CD68 in green, S100A4 in red, and FROUNT in blue. Higher-magnification images on the right correspond to the area indicated with dashed squares. White arrows indicate CD68+ macrophages in normal lung. White arrowheads indicate S100A4+CD68+ macrophages and yellow arrowheads indicate S100A4-CD68+ macrophages in fibrotic areas of the lungs of IPF patients. Scale bars = 25 μm. (C) S100A4 signals of each CD68+ macrophage in normal lung tissues and in fibrotic areas of the lungs of IPF patients. (D) FROUNT signals in S100A4-CD68+ macrophages and S100A4+CD68+ macrophages in fibrotic areas of the lungs of IPF patients. n = 5 or 6 per group. **P < 0.01 (unpaired t-test).
Discussion
Here, we demonstrated that DSF reduced macrophage infiltration into the lung tissues, suppressed the expression of profibrotic cytokines and the inhibitor of ECM-degrading enzymes, and attenuated BLM-induced lung fibrosis in mice. DSF reduced macrophage infiltration in vivo and directly inhibited the migration of bone marrow-derived monocytes in vitro. Thus, DSF is a potential therapeutic agent targeting macrophages in fibrotic lung diseases.
We chose BLM continuous subcutaneous administration with an osmotic pump to establish the BLM-induced lung fibrosis mouse model28. In contrast to intratracheal administration, which tends to produce a variable airway-centric distribution of fibrotic lesions, this subcutaneous infusion model can produce relatively large subpleural fibrotic lesions that are more uniform and highly reproducible, partially resembling human IPF. These features of fibrotic lesions were confirmed by histological analysis and micro-CT imaging. Using this lung fibrosis model, we demonstrated that DSF significantly reduced BLM-induced lung fibrosis. This reduction was evidenced by decreased blood SP-D levels, improvements in radiological and histological evaluations, and a reduction in the hydroxyproline content of the lung tissues.
Flow cytometry and immunohistochemistry revealed that the DSF-treated group had fewer macrophages in the lung tissue than the DSF non-treated group. This result is consistent with previous studies reporting that DSF treatment reduced macrophage infiltration around metastases in a mouse model of lung adenocarcinoma metastasis8. Furthermore, both M1 and M2 macrophage subtypes were reduced in the DSF-treated group. M1 macrophages secrete proinflammatory cytokines such as TNF-α and IL-1β, thereby promoting fibrosis24. M2 macrophages activate fibroblasts via secretion of cytokines such as TGF-β and PDGF and produce TIMPs that inhibit ECM degradation25,26. Thus, considering that both M1 and M2 macrophages are involved in lung fibrosis, reducing both subtypes may reduce lung fibrosis in this study.
Although the number of monocytes in the peripheral blood and their accumulation in the lung tissue was not significantly different between the DSF-treated and DSF non-treated groups, the DSF-treated group showed less macrophage infiltration in the lung tissue, suggesting that DSF may inhibit monocyte extravasation from capillaries into the lung tissue. Deficiency of CCR2, a critical chemokine receptor for monocyte recruitment, has been reported to reduce monocytes in the peripheral blood29 and enhance susceptibility to infection30 because monocyte migration from the bone marrow into the bloodstream is inhibited29. However, we found that DSF-treated mice showed no decrease in monocytes in the peripheral blood. This result indicates the advantage of DSF treatment over CCR2 inhibition in reducing the risk of bacterial infection associated with reduced monocytes in the peripheral blood. Moreover, the percentage of neutrophils in the lung tissue did not differ between the DSF-treated and DSF non-treated groups in this study. The chemokine receptor CCR2 is mainly expressed in monocytes, whereas CCR5 is expressed in monocytes and NK cells31. Neutrophils do not express CCR2/CCR5 under steady-state conditions in humans and mice32,33. DSF inhibits cell migration signals mediated by blocking the interaction of CCR2/CCR5 with FROUNT8. Thus, the effect of the inhibition of monocyte extravasation from capillaries and the difference in the infiltration of macrophages and neutrophils into the lung tissue in this study might be attributed to the inhibitory effect of DSF on FROUNT, an intracellular regulator of CCR2/CCR5 signalling.
We previously demonstrated that DSF inhibits the migration of human monocytic THP-1 cells when CCL2 is used as a chemotactic factor8. In contrast, the current study revealed that DSF inhibits the migration of primary mouse bone marrow monocytes using soluble factors secreted from primary mouse lung cells as chemotactic factors. This indicates that DSF directly inhibits monocyte migration in a more in vivo-like environment.
In this study, the DSF metabolites DDC and Cu(DDC)2 also inhibited monocyte migration; however, their inhibitory activity was lower than that of DSF. In contrast, cyanamide, which is not a DSF metabolite but an ALDH inhibitor, did not inhibit migration. These results are consistent with previous studies reporting that DSF and its metabolites have the potential to inhibit the FROUNT/CCR2 interaction, with DSF having the highest activity over its metabolites8. Considering that both DSF and its metabolites inhibit the interaction of CCR2/CCR5 and FROUNT with different intensities8, our in vitro data imply that the inhibition of cell migration by DSF may have been influenced by its action via FROUNT.
These in vitro and in vivo data suggest that the inhibition of monocyte migration and macrophage infiltration into the lungs of BLM-treated mice by DSF might be attributed to DSF’s inhibition of FROUNT, a molecule that regulates the intracellular signalling pathways of CCR2 and CCR5 in monocytes.
Quantitative PCR analysis showed that DSF suppressed the expression of Ccl2, Spp1, Col1a1, and Timp-1, which was upregulated by BLM administration. These four genes encode profibrotic factors. Generally, CCL2 not only stimulates monocyte/macrophage migration but also enhances fibrocyte recruitment into the alveolar zone and promotes its differentiation into fibroblasts, causing excessive collagen deposition34. Osteopontin is encoded by Spp1 and is upregulated in BLM-induced lung fibrosis35 and IPF lung tissue20,36,37. Osteopontin promotes fibroblast migration38 and proliferation39. TIMP-1 inhibits matrix metalloproteinases involved in ECM degradation and has a fibrogenic effect on BLM-induced lung fibrosis40. Given that Ccl21,41, Spp139, and Timp-142 are mainly expressed in macrophages, these profibrotic factors could be suppressed by DSF’s macrophage regulation.
DSF has various functions, including anti-inflammatory effects15,16,17,43,44. It has been reported that DSF, when administered intraperitoneally to mice that had been intratracheally injected with BLM, has a fibrosis-inhibiting effect, with a focus on the inhibition of TGF-β signalling in fibroblasts45. However, we administered DSF orally, which is the same route of administration as in clinical practice with established safety, and focused our analysis on its effects on monocytes/macrophages, particularly its effects on migration. Indeed, DSF inhibited the migration of monocytes/macrophages both in an in vivo mouse model of lung fibrosis and in an in vitro model and reduced BLM-induced lung fibrosis. To the best of our knowledge, this is the first study to reveal the inhibitory effect of DSF on monocyte/macrophage migration in lung fibrosis.
In our study, using a BLM-induced lung fibrosis mouse model, we observed a notable decrease in the number of S100A4+ cells in the lungs of DSF-treated mice compared to that in DSF non-treated mice. Double-staining analysis revealed a predominance of CD68+ macrophages among the S100A4+ cell population in BLM-treated mice, consistent with the findings reported in the literature18,27. Given the established role of S100A4 secreted by macrophages in the enhancement of fibrosis through the proliferation and activation of lung fibroblasts18,27, DSF may exert its antifibrotic effect by reducing the number of S100A4+ macrophages in lung tissue.
Furthermore, our multi-fluorescence staining study showed that S100A4+ macrophages in the fibrotic regions of IPF express FROUNT. Notably, the FROUNT signals were significantly more intense in S100A4+ macrophages than in S100A4- macrophages within the fibrotic areas of IPF patients. Although direct verification of the involvement of FROUNT in macrophage migration in IPF has not been done in this study, these findings could imply a potential role for FROUNT in the migration of macrophages, particularly those expressing S100A4, within the fibrotic landscape of IPF. IPF is a progressive and fatal disease often treated with pirfenidone and nintedanib.
Pirfenidone has diverse effects, including the suppression of inflammatory cytokines46, growth factors47,48, and oxidative stress49. Nintedanib is a tyrosine kinase inhibitor of the receptors for PDGF, FGF and VEGF50. These antifibrotic drugs can slow IPF progression but cannot entirely cure IPF. Moreover, side effects, such as diarrhoea, anorexia, and photosensitivity, often hinder patients from continuing treatment. Although monocyte-derived macrophages are considered potential therapeutic targets for IPF18,19,20, there are no clinically available drugs for IPF that target macrophages. Clinical trials of CCL2-neutralizing antibodies in IPF have not shown their efficacy51, so the need to regulate other CCR2-binding chemokines such as CCL7 has been considered. In contrast, a hypothetical approach, such as targeting FROUNT, a molecule that binds to the intracellular region of chemokine receptors and promotes chemokine signalling, could potentially regulate signalling through multiple chemokines such as CCL2, CCL3, CCL4 and CCL7, or through both CCR2 and CCR5, and could be highly effective in the treatment of fibrosis.
In this study, DSF showed an inhibitory effect on macrophage responses and was therapeutically beneficial in BLM-induced lung fibrosis. FROUNT and the FROUNT-binding region of CCR2/CCR5 are highly conserved across species and previous studies also demonstrated that DSF inhibited human monocyte migration8. Therefore, antifibrotic efficacy of DSF demonstrated in this study may be translated into human diseases. Since oral DSF administration has been confirmed to be safe for humans, the DSF drug repositioning strategy could be a feasible and promising option for the development of therapeutic agent for fibrotic lung diseases, including IPF.
In conclusion, this study demonstrated that oral DSF administration could suppresses macrophage infiltration into lung tissue and attenuates BLM-induced lung fibrosis, providing experimental evidence of macrophage-targeted treatment for lung fibrosis. Our findings warrant future research to investigate the clinical efficacy of DSF as an antifibrotic agent.
Data availability
Data are available to interested researchers upon reasonable request to the corresponding author.
References
Kolahian, S., Fernandez, I. E., Eickelberg, O. & Hartl, D. Immune mechanisms in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 55, 309–322. https://doi.org/10.1165/rcmb.2016-0121TR (2016).
Ishida, Y. et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am. J. Pathol. 170, 843–854. https://doi.org/10.2353/ajpath.2007.051213 (2007).
Okuma, T. et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J. Pathol. 204, 594–604. https://doi.org/10.1002/path.1667 (2004).
Baran, C. P. et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 78–89. https://doi.org/10.1164/rccm.200609-1279OC (2007).
McCubbrey, A. L. et al. Deletion of c-FLIP from CD11b hi macrophages prevents development of bleomycin-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 58, 66–78. https://doi.org/10.1165/rcmb.2017-0154OC (2018).
Toda, E. et al. FROUNT is a common regulator of CCR2 and CCR5 signaling to control directional migration. J. Immunol. 183, 6387–6394. https://doi.org/10.4049/jimmunol.0803469 (2009).
Terashima, Y. et al. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat. Immunol. 6, 827–835. https://doi.org/10.1038/ni1222 (2005).
Terashima, Y. et al. Targeting FROUNT with disulfiram suppresses macrophage accumulation and its tumor-promoting properties. Nat. Commun. 11, 609. https://doi.org/10.1038/s41467-020-14338-5 (2020).
Jacobsen, E. & Martensen-Larsen, O. Treatment of alcoholism with tetraethylthiuram disulfide (antabuse). J. Am. Med. Assoc. 139, 918–922. https://doi.org/10.1001/jama.1949.02900310022006 (1949).
Lipsky, J. J., Shen, M. L. & Naylor, S. Overview–in vitro inhibition of aldehyde dehydrogenase by disulfiram and metabolites. Chem. Biol. Interact. 130–132, 81–91. https://doi.org/10.1016/s0009-2797(00)00224-6 (2001).
Skrott, Z. et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 552, 194–199. https://doi.org/10.1038/nature25016 (2017).
Duan, L. et al. Inhibitory effect of disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 446, 1010–1016. https://doi.org/10.1016/j.bbrc.2014.03.047 (2014).
Cen, D. et al. Disulfiram induces apoptosis in human melanoma cells: A redox-related process. Mol. Cancer Ther. 1, 197–204 (2002).
Thakare, R., Shukla, M., Kaul, G., Dasgupta, A. & Chopra, S. Repurposing disulfiram for treatment of Staphylococcus aureus infections. Int. J. Antimicrob. Agents 53, 709–715. https://doi.org/10.1016/j.ijantimicag.2019.03.024 (2019).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745. https://doi.org/10.1038/s41590-020-0669-6 (2020).
Marikovsky, M., Ziv, V., Nevo, N., Harris-Cerruti, C. & Mahler, O. Cu/Zn superoxide dismutase plays important role in immune response. J. Immunol. 170, 2993–3001. https://doi.org/10.4049/jimmunol.170.6.2993 (2003).
Zhao, M. et al. Disulfiram and diphenhydramine hydrochloride upregulate miR-30a to suppress IL-17-associated autoimmune inflammation. J. Neurosci. 36, 9253–9266. https://doi.org/10.1523/JNEUROSCI.4587-15.2016 (2016).
Li, Y. et al. S100A4(+) Macrophages are necessary for pulmonary fibrosis by activating lung fibroblasts. Front. Immunol. 9, 1776. https://doi.org/10.3389/fimmu.2018.01776 (2018).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404. https://doi.org/10.1084/jem.20162152 (2017).
Morse, C. et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. https://doi.org/10.1183/13993003.02441-2018 (2019).
Ashcroft, T., Simpson, J. M. & Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41, 467–470. https://doi.org/10.1136/jcp.41.4.467 (1988).
Bankhead, P. et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 7, 1–7. https://doi.org/10.1038/s41598-017-17204-5 (2017).
Kuroki, Y., Takahashi, H., Chiba, H. & Akino, T. Surfactant proteins A and D: Disease markers. Biochim. Biophys. Acta 1408, 334–345. https://doi.org/10.1016/s0925-4439(98)00079-9 (1998).
Zhang, L. et al. Macrophages: Friend or foe in idiopathic pulmonary fibrosis?. Respir. Res. 19, 170. https://doi.org/10.1186/s12931-018-0864-2 (2018).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. https://doi.org/10.1038/nri3073 (2011).
van Geffen, C. et al. Regulatory immune cells in idiopathic pulmonary fibrosis: Friends or foes?. Front. Immunol. 12, 663203. https://doi.org/10.3389/fimmu.2021.663203 (2021).
Zhang, W. et al. S100a4 is secreted by alternatively activated alveolar macrophages and promotes activation of lung fibroblasts in pulmonary fibrosis. Front. Immunol. 9, 1216. https://doi.org/10.3389/fimmu.2018.01216 (2018).
Harrison, J. H. & Lazo, J. S. High dose continuous infusion of bleomycin in mice: A new model for drug-induced pulmonary fibrosis. J. Pharmacol. Exp. Ther. 243, 1185–1194 (1987).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317. https://doi.org/10.1038/ni1309 (2006).
Kurihara, T., Warr, G., Loy, J. & Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186, 1757–1762. https://doi.org/10.1084/jem.186.10.1757 (1997).
Mack, M. et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166, 4697–4704. https://doi.org/10.4049/jimmunol.166.7.4697 (2001).
Bonecchi, R. et al. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J. Immunol. 162, 474–479 (1999).
Speyer, C. L. et al. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am. J. Pathol. 165, 2187–2196. https://doi.org/10.1016/S0002-9440(10)63268-3 (2004).
Moore, B. B. et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am. J. Pathol. 166, 675–684. https://doi.org/10.1016/S0002-9440(10)62289-4 (2005).
Kaminski, N. et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 97, 1778–1783. https://doi.org/10.1073/pnas.97.4.1778 (2000).
Hou, J. et al. Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J. 288, 3530–3546. https://doi.org/10.1111/febs.15669 (2021).
Pardo, A. et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2, e251. https://doi.org/10.1371/journal.pmed.0020251 (2005).
Fujisawa, Y., Matsuda, K. & Uehara, T. Osteopontin enhances the migration of lung fibroblasts via upregulation of interleukin-6 through the extracellular signal-regulated kinase (ERK) pathway. Biol. Chem. 401, 1071–1080. https://doi.org/10.1515/hsz-2020-0125 (2020).
Takahashi, F. et al. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 24, 264–271. https://doi.org/10.1165/ajrcmb.24.3.4293 (2001).
Manoury, B., Caulet-Maugendre, S., Guénon, I., Lagente, V. & Boichot, E. TIMP-1 is a key factor of fibrogenic response to bleomycin in mouse lung. Int. J. Immunopathol. Pharmacol. 19, 471–487. https://doi.org/10.1177/039463200601900303 (2006).
Brieland, J. K. et al. Effect of acute inflammatory lung injury on the expression of monocyte chemoattractant protein-1 (MCP-1) in rat pulmonary alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 7, 134–139. https://doi.org/10.1165/ajrcmb/7.2.134 (1992).
Selman, M. et al. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment?. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L562-574. https://doi.org/10.1152/ajplung.2000.279.3.L562 (2000).
Jia, Y. & Huang, T. Overview of antabuse® (disulfiram) in radiation and cancer biology. Cancer Manag. Res. 13, 4095–4101. https://doi.org/10.2147/CMAR.S308168 (2021).
Gandhi, N. M., Gopalaswamy, U. V. & Nair, C. Radiation protection by disulfiram: Protection of membrane and DNA in vitro and in vivo against gamma-radiation. J. Radiat. Res. 44, 255–259. https://doi.org/10.1269/jrr.44.255 (2003).
Jiang, H. et al. Tetraethylthiuram disulphide alleviates pulmonary fibrosis through modulating transforming growth factor-beta signalling. Pharmacol. Res. 174, 105923. https://doi.org/10.1016/j.phrs.2021.105923 (2021).
Oku, H., Nakazato, H., Horikawa, T., Tsuruta, Y. & Suzuki, R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 446, 167–176. https://doi.org/10.1016/s0014-2999(02)01757-0 (2002).
Gurujeyalakshmi, G., Hollinger, M. A. & Giri, S. N. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am. J. Physiol. 276, L311-318. https://doi.org/10.1152/ajplung.1999.276.2.L311 (1999).
Iyer, S. N., Gurujeyalakshmi, G. & Giri, S. N. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Ther. 291, 367–373 (1999).
Mitani, Y. et al. Superoxide scavenging activity of pirfenidone-iron complex. Biochem. Biophys. Res. Commun. 372, 19–23. https://doi.org/10.1016/j.bbrc.2008.04.093 (2008).
Hilberg, F. et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 68, 4774–4782. https://doi.org/10.1158/0008-5472.CAN-07-6307 (2008).
Raghu, G. et al. CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur. Respir. J. 46, 1740–1750. https://doi.org/10.1183/13993003.01558-2014 (2015).
Acknowledgements
We are grateful to Dr. H. Shimizu, Ms. A. Ishikawa, Ms. N. Kuwahara, Ms. K. Wakamatsu, Ms. M. Kataoka, Mr. T. Arai, and Ms. Y. Sawa for their expert technical assistance. This study was supported by JSPS KAKENHI Grant Numbers 18K08140, 20K08553, 21H02755, and 20K07553 and by AMED under Grant Number JP23ek0109667.
Author information
Authors and Affiliations
Contributions
YO, ET, HU, YTerashima, and ASaito: Conception and design, data collection, data analysis and interpretation, and manuscript writing. SK, YK, and MT: Data collection and analysis. KM, YY, TN, and AShimizu: conception, design, and supervision. YTerasaki: Conception and design, data collection, data analysis and interpretation, manuscript writing, and supervision. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Okabe, Y., Toda, E., Urushiyama, H. et al. Antifibrotic effect of disulfiram on bleomycin-induced lung fibrosis in mice and its impact on macrophage infiltration. Sci Rep 14, 23653 (2024). https://doi.org/10.1038/s41598-024-71770-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71770-z