Abstract
In this work, we demonstrate that palladium-immobilized triazine dendrimer on magnetic nanoparticles in proper solvents, provides an impressive, atom-economical and compelling approach for the selective synthesis of 2,3-diphenylindole or pentaphenylpyrrole derivatives via annulation of diphenylacetylene with diverse anilines. Both the annulation methods were taken place under copper- and phosphine-free conditions with high yields at air atmosphere. Likewise, bis-indoles were obtained with excellent yields under optimized reaction conditions. Besides, the catalyst was isolated and reused for seven cycles without decrease potential of catalytic activity. Two mechanistic pathways were proposed and geometry optimizations, electronic properties as well as vibrational characterizations of all structures were performed with density functional theory (DFT). Also, the investigation of atomic basin properties of these molecular systems was carried out utilizing the quantum atoms-in-molecules theory (QTAIM). The results showed that 2,3-diphenylindole and pentaphenyl pyrrole molecular systems can be used as intramolecular acceptor/donor (n-like/p-like) sections.
Similar content being viewed by others
Introduction
The discovery of metal-catalyzed strategies is widespread interest in diverse organic transformations such as oxidation1,2, reduction3,4,5,6, coupling7,8,9,10 as well as cyclization reactions11,12,13. On this point, dendrimers have played a significant role due to their special physical properties which make them as suitable hosts for metal complexes and nanoparticles. Furthermore, they show excellent recyclability and reusability with maintaining their activities14,15,16,17.
Annulation of an alkyne with a nucleophile via oxidative coupling reaction in the presence of transition metals such as Pd18,19,20, Pt21,22, Ni23, Au24,25,26 and In27,28 is an attractive hot-topic because this method afforded an atom-economical manner for the straightforward one-step synthesis of heterocyclic molecules.
In the chemical synthetic opportunities, nitrogen-based heterocycles have attracted particular attention in the scope of drug discovery for treatment of many diseases and biological studies. Among numerous heterocycles, the pyrrole and indole moieties are core structures of important synthetic pharmaceuticals, natural products and electrically conducting materials29,30,31,32. Thanks to many considerable advances in pyrrole and indole synthesis catalyzed by diverse metals, most of these methods suffer from drawbacks and limitations including sensitivity to air, long reaction times or low yields, low turnover frequency (TOF) and harsh reaction conditions33,34,35,36,37,38. Moreover, this organic transformation is performed in the presence of hazardous axially and co-catalyst such as phosphines and copper ions37. Therefore, there is a strong demand for their fine, efficient and site-selective synthesis which is a useful task in organic chemistry.
By combination of transition-metal catalysis aspects, potential of dendrimers, and our interest in palladium-catalyzed C–C coupling reactions39,40,41, we are pleased to report a unique palladium containing 1,3,5-triazine dendrimer stabilized nanomagnetic particles (Pd-TDSN) catalyzed as a microreactor structure for efficient synthesis of 2,3-diphenylindoles and pentaarylpyrroles under solvent-controlled conditions (Scheme 1).
Results and discussion
In this contribution, we reported previously immobilization and stabilization of transition-metal ions and nanoparticles by diverse triazine dendrimers as excellent hosts and unique spherical three-dimensional organic structures42,43,44,45,46. In continue, a novel triazine dendrimer (TD) was synthesized through multistep convergent approach and characterized (Scheme 2). The magnetite (Fe3O4) nanoparticles were synthesized through the co-precipitation technique47. Subsequently, the nanoparticles were coated and stabilized with triazine dendrimer through self-assembling process to produce the TDSN. During the self-assembling procedure, the dendrimer was attached to magnetic nanoparticles via carboxylic acid groups. This three-dimensional network is ready for immobilization of ions and molecules as well as nanoparticles. As shown in Fig. 1, the Pd-TDSN catalyst was prepared via reaction between Na2PdCl4 and TDSN.
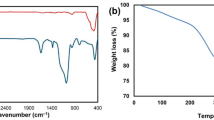
The FT-IR spectra of magnetic nanoparticels (a) and the TDSN (b) were shown in Fig. S1 (see supporting information). As can be seen, TDSN spectrum shows the stretching vibrations of C–H bonds at about 2890–2923 cm−1, triazine ring at 1580 cm−1 (C=N) and carbonyl of carboxyl group at 1695 cm−1. In addition, the stretching characteristic bands at 1230 cm−1 and 1120 cm−1 can be attributed to C–O and C–S bonds, respectively. These observations are good evidences for the attachment of dendritic structure on the surface of magnetic nanoparticles.
Moreover, the chemical composition and bonding environments of the Pd-TDSN evaluated by X-ray photoelectron spectroscopy48 (XPS) and showed that the nanoparticles were composed mainly of C, N, Fe and Pd elements (Fig. 2a, survey scan). Figure 2b showed C 1s peaks of C–C bonds (symmetric skeletal backbone) at 284.5 eV, C–O/S at 286.2 eV, carbon of the triazine ring 288.0 eV, and C=O at 295.5 eV. Besides, N 1s spectrum exhibited the nitrogen of triazine ring at 401.2 eV49,50 and tertiary amine groups at 403.7 and 405.7 eV51 (Fig. 2c). As can be seen Fig. 2d represented characteristic for multiplet splitting of Fe 2p peaks at 712.3, 714.6, 717.6, 725.4 and 728.5 eV which can attributed to Fe(II) 2p3/2, Fe(III) 2p3/2, 2p3/2 shoulder, Fe(II) 2p1/2, Fe(III) 2p1/2 signals, respectively. It worth to mentioned that Fe 2p peak shows small shift to higher binding energy due to stabilization of Fe3O4 nanoparticles by triazine dendrimer. In Fig. 2e, it can be observed that the Pd 3d5/2 and Pd 3d3/2 peaks have binding energies of 338.21 and 343.58 eV, respectively, which suggests that the catalyst contains Pd(II)52.
In Fig. 3a, the SEM image of TDSN demonstrated the retention of the initial nanosphere morphology, indicating successful structural inheritance following self-assembly and immobilization stages.
Furthermore, the energy dispersive X-ray analysis (EDX) (Fig. S2) clearly confirmed the presence of elements of the catalyst. The TEM image (Fig. 3b) exhibited dark nanomagnetic cores surrounded by grey dendrimer, confirming core-shell structure. Furthermore, an examination of the magnetic characteristics of both nanoparticles and TDSN was conducted through the use of a vibrating sample magnetometer (VSM). The Fig. 3c shows magnetization curves of magnetic nanoparticles (Fe3O4) and TDSN, swept from −1 to +1 T at room temperature. As clearly seen, both samples showed superparamagnetic behavior. It is worth mentioning that, the reduction of response to magnetic field indicated an increase of thickness of the triazine dendrimer as a shell layer on the magnetic nanoparticles. Hence, the amount of magnetization (Ms) for TDSN was found to be lower than magnetic nanoparticles.
We then commenced our studies on the potential catalytic activity of Pd-TDSN in the reaction of aniline (Anil) with diphenylacetylene (DPA) for the synthesis of 2,3-diphenylindole. To find the optimized reaction conditions, we explored the amount of the catalyst, base, additive, and the reaction temperature, solvent as well as molar ratios of substrates. Representative results are summarized in Table 1. To show the effect of base, the model reaction was performed using different bases in DMF solvent in the presence of Pd-TDSN catalyst (0.3 mol%) and LiCl as additive at 80 °C for 8 h (Table 1, entries 1–5). Among them, the Na2CO3 was found to be the most effective base to provide the desired product in 69% yield (entries 5). Moreover, the yield of the product was reduced to 60%, when NaCl was used instead of LiCl as additive (Table 1, entry 6). The role of additive will be discussed in the mechanism section. Then, different molar ratios of Anil to DPA were tested (entries 5, 7 and 8) and the highest yield was obtained with a molar ratio of 1.2:1 (entry 7). Then, solvent screening was carried out followed by process optimization. When DMSO or a 1:1 mixture of DMF/ THF, DMF/ 1,4-dioxane and DMF/ H2O, was used instead of DMF as reaction medium, the isolated yield was reduced to 34–73% (Table 1, entries 9–12). This can be attributed to the reducing of the polarity of reaction medium. Surprisingly, when cyclic ethers such as tetrahydrofuran (THF) or 1,4-dioxane were used as the solvent, the respected 2,3-diphenylindole was obtained in only 15% and 22% yields, respectively (Table 1, entries 13 and 14). Under these conditions, pentaphenylpyrrole was detected as the main product. Screening the catalyst amount (entries 7, 15 and 16) and reaction temperature °C (entries 7, 17 and 18) showed that 0.3 mol% catalyst and 80 °C (entry 7) are the best choices. Based on these findings, the most favorable conditions for synthesis of 2,3-diphenylindoles are utilizing Anil (1.2 mmol), DPA (1 mmol), Na2CO3 (1.5 mmol), LiCl (0.5 mmol), in the presence of Pd-TDSN (0.3 mol% Pd) and DMF as polar solvent at 80 °C (Table 1, entry 6). It should be noted that the synthesis reaction of indole did not occur in the presence of Fe3O4 nano particles.
After optimizing the reaction conditions, the scope of substrates tolerated in the cyclization reaction was investigated (Scheme 3). A diversity of anilines was used for this transformation. Based on the obtained results, anilines with electron-donating substituents such as methyl and methoxy were smoothly reacted with diphenylacetylene (DPA) and the desired products were isolated in high yields (84–90%). In addition, 4-acethylailine afforded ap3 in good yield in longer reaction time (12 h). In contrast, no indole was produced even after 48 h from anilines containing strong electron with-drawing groups (ortho, meta and para-nitroanilines). This can be attributed to dramatical reduction of possibility of the intramolecular electrophilic aromatic palladation. Also, the reaction of DPA with 2-tert-butyl aniline was tested as an investigation of the effect of the bulk group, but the reaction did not progress after 48 h.
It noteworthy that interesting results were obtained in the annulation reactions between 2- chloro-, 2-bromo- and 2-iodoaniline (ortho-substituted anilines) and diphenylacetylene in the presence of the catalyst (Scheme 4). When 2-chloroaniline was used as the substrate, the respected 7-chloro-2,3-diphenylindole was produced in high yield (76%) and with excellent regioselectivity without formation of 4-chloro-2,3-diphenylindole. Whereas, in the reaction of 2-bromoaniline and/or 2-iodoaniline with diphenylacetylene, the corresponding 2,3-diphenylindoles were obtained as the sole products in 70% and 85% yields, respectively, via Larock annulation.
The results disclose that the dendritic structure on nano particles provide micro environments or microreactors as an atom economy and efficient approach for accelerates the annulation reaction due to access to active sites and faster mass transfer.
To further expand the potential of the present protocol, the preparation of bis-indoles was also studied. As shown in Scheme 5, the “bis-annulation” between 1,3- or 1,4-phenylendiamine and diphenylacetylene was accomplished impressively in the presence of Pd-TDSN and obtained in good yields of the corresponding bis-indoles. As shown, two products are theoretically possible for both reactions. The 1H NMR spectrum of the product of the reaction of 1,3-phenylenediamine and phenyl acetylene (see SI) showed that the ratio among 3-BIN1 and 3-BIN2 isomers was about 70:30 (based on the peak area of N-H of indoles and two type of aromatic hydrogens). Whereas, under the same conditions, 1,4-phenylendiamine took part efficiently in this reaction to give only one regioisomeric bis-indole 4-BIN1.
Encouraged by the results obtained in annulation, we then investigated the efficiency of Pd-TDSN catalyst in the synthesis of pentaarlypyrrole derivatives. As outlined above, pentaphenylpyrroles could be efficiently obtained from the reaction of aniline and diphenylacetylene in cyclic ether (1,4-dioxane and THF) (Table 1, entries 13 and 14). Hence, we set out the optimization study for the preparation of these derivatives in the presence of Pd-TDSN (Table 2). Various reaction parameters, including the base and solvent type, temperature, catalyst quantity, and substrate molar ratios, were investigated. The representative results are provided in Table 2. As is shown, the optimized condition was using Anil (1 mmol), DPA (2 mmol), Na2CO3 (1 mmol) in the presence of Pd-TDSN (0.3 mol% Pd) and dioxane at 90 °C (Table 2, entry 5).
The scope and generality of this method for the synthesis of pentaarylpyrroles was investigated under optimized conditions. Scheme 6 demonstrated the efficient reaction between diphenylacetylene and anilines bearing various substituents, leading to high yields of the desired products, expect for 4-nitroaniline which did not show any progress even after 48 h.
According to the reaction conditions and based on the experimental observations, two plausible mechanisms for the formation of indole ap1 and pentphenylpyrrole p1 are proposed (Scheme 7) and computed (Figs. 4 and 5). In the case of pentaarylpyrrol synthesis, π complex A is initially formed between the triple bond of DPA and Pd(II) ion of Pd-TDSN in dioxane solvent. Next, an aminopalladation process occurs by the attack of aniline to A and provides B with cis configuration. Addition of the second molecule of diphenylacetylene to B gives the carbopalladium53,54 compound C, which upon intramolecular nucleophilic substitution of the chlorine in palladium center by nitrogen forms D as a cyclopalladated complex55. Finally, the desired pyrrole P1 is produced via an Reductive elimination reaction and the Pd0 is released which could be used for the next catalytic cycle.
In the case of indole synthesis, as mentioned above, the reaction is carried out in DMF solvent. As mentioned previously, DMF is able to coordinate to palladium ion56. Accordingly, the intermediate G may be formed via a σ–π rearrangement of π-complex F by the attack of aniline to this complex. Then, intramolecular electrophilic aromatic palladation in G leads to H, which upon deprotonation under mildly basic conditions affords the intermediate I. Finally, reductive elimination of palladium from I produces the desired indole ap1 and regenerates the catalyst for the next run. It is noteworthy that in the absence LiCl as additive, the reaction process is slow. It seems that the Cl- ions play an important role in the oxidative-addition/reductive-elimination process. Moreover, chloride ion can significantly influence the stability and activity of palladium (PdCl2) catalysts during recycling processes, often prevents the leaching of palladium into the reaction medium.
Although the main goal of this research is to experimentally investigate the mechanism of the study reaction pathway, TS (transition states) method was used in this section for the computational evaluation of intermediate structures, using DFT-B3LYP/6-311G -LANL2DZ level of theory. Sample of these results are shown in Fig. 6. As can be seen from this figure, the production of product ap1 requires less activation energy. Therefore, product ap1 is expected to be the main product of the reaction (analysis results obtained here can be a confirmation of the analysis of the results mentioned in the experimental part of the paper). The reason for this stability of product ap1 can be in its less spatial hindrance and the stability of its electronic-vibrational structure.
Computational studies of reaction mechanisms
To elucidate our expire mental results and further cast light on details of two proposed mechanisms, computational studies were performed with density functional theory (DFT)57 and quantum theory of atoms-in-molecules (QTAIM)58. Briefly, geometry optimizations, electronic properties and vibrational characterizations of all structures were carried out at UB3LYP/6-311G+ level of theory. For the palladium atoms of the molecules, LANL2DZ pseudopotential is used. Likewise, based on the QTAIM, the atomic/local electron density, \(\rho (r)\) (and its Laplacian, \(\nabla^{2} \rho (r)\)), electronic kinetic \(\left( {K_{elec} } \right)\), potential \(\left( {V_{elec} } \right)\) and thus total atomic electronic energies \(\left( {E_{elec} } \right)\) of each atomic basins can be obtained by summing the corresponding quantities of all \(N_{\Omega }\) atomic basins \(\left( \Omega \right)\) of the molecule as
Computational studies were conducted at the DFT level B97D3/6-311+g**/ to identify the obstacles associated with the proposed mechanisms' steps, specifically the conversion to P1 and ap1. Comparing the Gibbs free energy profiles of proposed mechanisms were presented in Fig. 5. As can be seen, the formation of A structure (Pd–acetyl π-complex) is the key intermediate for both catalytic cycles. Also, different conformations have different free energies of structures.
Fig. 5 displays the general free energy profile for two catalytic cycles. Kozuch and Shaik's energetic span model59,60 was utilized to calculate the activation energies for the synthesis of P1 and ap1, resulting in values of 31 and 22 kcal/mol, respectively. The rate constant (k) is determined by the driving force between the TOF-determining transition states (C in P1 and H in ap1 of catalytic cycles) and the TOF-determining intermediate (A). The results indicate that kP1 and kap1 are 1.19 × 10−10 s−1 and 4.18 × 10−4 s−1, respectively.
As mentioned above, the pyrrole and indole derivatives are of great significance due to their wide variety of applications as donor moieties in organic light-emitting devices (OLEDs), single molecular electronics, and non-linear optic materials. Therefore, electronic behavior of pathway of transformation of P1 and ap1 was studied by QTAIM. The local (contour maps) electron density \(\left(\rho (r)\right)\), Laplacian electron density \(\left({\nabla }^{2}\rho (r)\right)\) and electronic kinetic energy \(\left(K(r)\right)\) of the P1 and ap1 was represented in Fig. S3.
The QTAIM study of transformation of A to P1 and A to ap1 obviously showed changes of the electronic properties such as electron density and atomic kinetic energy of the atomic basins for structures.
It is noteworthy that each atomic basins of similar atoms (for example carbon atomic basins) do not show the same response, which can be attributed to the distribution of intramolecular charge and energy (atomic/local) changes in the molecular structures.
The study of atomic/local changes of molecular systems using QTAIM as a useful and acceptable theoretical method can open a new window for design of molecular structures with special capabilities such as intramolecular semiconductors, intramolecular junctions and intramolecular nanoparticles.
The results obtained showed the molecular systems intra-molecular charge/energy transfer (P1 and ap1) into sections that are either intramolecular acceptor/donor (n-like/p-like). This division facilitates the identification of the role played by each section (or atomic basin) in the mechanism of intra-molecular charge/energy transfer.
Catalyst recycling and reuse
Recovery and reusability of catalysts are important economic and environmental aspects, especially in organic synthesis. Due to the magnetization characteristic of the Pd-TDSN, the catalyst can be easily separated from the reaction mixture by an external magnet. Therefore, the recovery and reusability of the catalyst were examined in the model reaction of ap1 and P1 syntheses under the optimized conditions (Fig. 7). The catalyst could be reused for six runs without noticeable change in its activity. Also, ICP analysis showed no obvious leaching of palladium even after 7th run. All these observations reveal that the catalyst is stable during the reaction.
Conclusions
In summary, we have advanced an efficient method for synthesis of 2,3-diphenylindoles and pentaarylpyrroles via the annulation of diphenylacetylene with diverse aniline derivatives in the presence of palladium-immobilized triazine dendrimer on magnetic nanoparticles (Pd-TDSN) under mildly basic conditions. The products were obtained selectivity one’s choice of solvent. 2,3-Diphenylindoles were produced in DMF solvent in high yields. Besides, under same conditions, this catalytic system was effectively used for the synthesis of bis-indoles. On the other hand, 1,4-dioxane solvent, pentaarrylepyrroles were obtained in high yields. Ease of recovery and reuse of the catalyst make this method an economic and environmentally-benign process. Besides, the dendritic structure provides micro environment for easy access to active sites and faster mass transformation. The proposed mechanism of two transformation discussed and studied by using DFT/QTAIM theories. The Gibbs free energy of all intermediates and products were calculated and their diagram of transformation progresses were drawn. Furthermore, the results showed that these molecular systems can be used as suitable and elegant candidates for optical devices.
Experimental
General
All chemicals used in this work were purchased from Sigma-Aldrich, Fluka and Merck chemical companies at highest purity.
Preparation of Pd(II) immobilized on 1,3,5-triazin dendrimer stabilized nanomagnetic particles (Pd-TDSN)
In a round bottom equipped with a condenser, 2,4,6-trichloro-1,3,5-triazine (3 mmol), 1-Boc-piperazine (9 mmol) and diisopropylethylamine (DIPEA) (6 mmol) were dissolved in 25 mL of THF and stirred at room temperature for 4 h and then refluxed for 20 h. The solution was evaporated under vacuum. The residue was dissolved in CH2Cl2 (25 mL) and washed with brine (2 × 25 mL). The resulting light-yellow solution was dried over MgSO4, filtered, and evaporated under vacuum. Next, the obtained yellow powder was added to mixture of diluted with HCl (3 mL, 1%) and methanol (3 mL), stirred at room temperature for 1 h and evaporated under vacuum. Then, the residue was dissolved in 15 mL of CHCl3, washed with 0.1 M NaOH (aq) (2 × 5 mL), dried over MgSO4 and filtered. Finally, the solution was evaporated under vacuum to give TPT as a white product.
Preparation of DGT
In a round bottom, 2,4,6-trichloro-1,3,5-triazine (2.93 mmol) and diisopropylethylamine (DIPEA) (12 mmol)) was dissolved in 25 ml of THF and stirred at 0 °C for 2 min. Next, thioglycolic acid (6 mmol) was added dropwise for 10 min at 0 °C for 1 h. After completion of the reaction, the solution was evaporated under vacuum. The crude product was purified by silica-gel chromatography (CH2Cl2: MeOH; 10 :1) to give DGT as a white solid.
Preparation of TD
In a screw-cap glass tube with a magnetic stirrer, a mixture TPT (1 mmol), DGT (3 mmol) and diisopropylethylamine (DIPEA) were dissolved in 50 mL of DMF and stirred at 80 °C for 24 h. The reaction progress was checked by TLC (eluent: CH2Cl2/MeOH; 5:1). After the reaction is over, the residue was dissolved in 1,4-dioxane, washed with brine, dried over MgSO4, filtered and the solvent was evaporated to give a white powder with excellent purity.
Preparation of TDSN
150 mg of MNPs was dispersed in 10 mL of EtOH/DMF (1:1), and DIPEA was added dropwise to this solution for a period of 10 min and pH adjusted at 9. Then, TD (50 mg) was added to the mixture and sonicated for 24 h in a sonication bath. The resulting black precipitate (TDSN) was collected using a permanent magnet, washed several times with ethanol and acetone, and dried in a vacuum oven at 50 °C.
Preparation of Pd-TDSN
A mixture of TDSN (100 mg) and Na2PdCl4 (0.17 mmol) in 25 mL of MeOH was dispersed in 25 mL of MeOH by ultrasonic bath for 30 min. Then, the mixture was shacked overnight at room temperature. The magnetic catalyst was separated by an external magnetic field, washed with ether and dried under vacuum to afford Pd-TDSN as a black solid.
General procedure for synthesis of 2,3-diphenyl-1H-indole catalyzed by Pd-TDSN
A mixture of Anil (1 mmol), DPA (1 mmol), Na2CO3 (1 mmol), LiCl (0.5 mmol) and Pd-TDSN (0.3 mol%) in 2 mL DMF was stirred at 80 °C for the time indicated. The progress of reaction was monitored by TLC (ethyl acetate/n-hexane 1:5). After complication of reaction, the catalyst was easily separated by a permanent magnet and washed with EtOH (5 mL). The residue was dissolved in ethyl acetate (10 mL), washed with water (2 × 10 mL) and dried over MgSO4. The solution was filtered and evaporated under reduced pressure. The residue was purified by chromatography on silica gel (ethyl acetate/hexane, 1:15) to afford the pure product.
General procedure for synthesis of 1,2,3,4,5-pentaphenyl-1Hpyrrole by Pd-TDSN
A mixture of Anil (1 mmol), DPA (2 mmol), NaOAc (1 mmol) and Pd-TDSN (0.3 mol% Pd) in 2 mL of 1,4-dioxane was stirred at 90 °C. After whole consumption of the starting material monitored by TLC (ethyl acetate/n-hexane 1:5) the mixture was cooled to room temperature.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Xu, H. et al. Research progress of highly efficient noble metal catalysts for the oxidation of 5-hydroxymethylfurfural. Chemsuschem 15(13), e202200352 (2022).
Fu, X., Wan, C., Huang, Y. & Duan, X. Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media. Adv. Funct. Mater. 32(11), 2106401 (2022).
Fujita, E., Grills, D. C., Manbeck, G. F. & Polyansky, D. E. Understanding the Role of inter- and intramolecular promoters in electro- and photochemical CO2 reduction using Mn, Re, and Ru catalysts. Acc. Chem. Res. 55(5), 616–628 (2022).
Shao, P., Yi, L., Chen, S., Zhou, T. & Zhang, J. Metal-organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers. J. Energy Chem. 40, 156–170 (2020).
Iqbal, M. et al. Chemical design of palladium-based nanoarchitectures for catalytic applications. Small 15(6), 1804378 (2019).
Favier, I., Pla, D. & Gómez, M. Palladium nanoparticles in polyols: Synthesis, catalytic couplings, and hydrogenations. Chem. Rev. 120(2), 1146–1183 (2019).
Zhu, Y., Zeng, Y., Jiang, Z. T. & Xia, Y. Recent advances on transition-metal catalyzed cross-coupling reactions of gem-difluorinated cyclopropanes. Synlett (AAM) 34(01), 1–13 (2022).
Liu, S.-L., Ye, C. & Wang, X. Recent advances in transition-metal-catalyzed directed C–H alkenylation with maleimides. Org. Biomol. Chem. 20(24), 4837–4845 (2022).
Takale, B. S., Kong, F.-Y. & Thakore, R. R. Recent applications of Pd-catalyzed suzuki-miyaura and buchwald-hartwig couplings in pharmaceutical process chemistry. Organics 3(1), 1–21 (2022).
Devendar, P., Qu, R.-Y., Kang, W.-M., He, B. & Yang, G.-F. Palladium-catalyzed cross-coupling reactions: A powerful tool for the synthesis of agrochemicals. J. Agric. Food Chem. 66(34), 8914–8934 (2018).
Qiu, M., Fu, X., Fu, P. & Huang, J. Construction of aziridine, azetidine, indole and quinoline-like heterocycles via Pd-mediated C–H activation/annulation strategies. Org. Biomol. Chem. 20(7), 1339–1359 (2022).
Khan, S., Ahmad, T., Rasheed, T. & Ullah, N. Recent applications of vinylethylene carbonates in Pd-catalyzed allylic substitution and annulation reactions: Synthesis of multifunctional allylic and cyclic structural motifs. Coord. Chem. Rev. 462, 214526 (2022).
Ye, J. & Ma, S. Palladium-catalyzed cyclization reactions of allenes in the presence of unsaturated carbon–carbon bonds. Acc. Chem. Res. 47(4), 989–1000 (2014).
Crooks, R. M., Zhao, M., Sun, L., Chechik, V. & Yeung, L. K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 34(3), 181–190 (2001).
Ye, R., Zhukhovitskiy, A. V., Deraedt, C. V., Toste, F. D. & Somorjai, G. A. Supported dendrimer-encapsulated metal clusters: Toward heterogenizing homogeneous catalysts. Acc. Chem. Res. 50(8), 1894–1901 (2017).
Karakhanov, E., Maximov, A. & Zolotukhina, A. Heterogeneous dendrimer-based catalysts. Polymers 14(5), 981 (2022).
Ilunga, A. K. & Meijboom, R. A review of dendrimer-encapsulated metal nanocatalysts applied in the fine chemical transformations. Catal. Lett. 149(1), 84–99 (2019).
Cacchi, S., Fabrizi, G. & Moro, L. 2-Substituted-3-allenyl-benzo [b] furans through the palladium-catalysed cyclization of propargylic o-(alkynyl) phenyl ethers. Tetrahedron Lett. 39(28), 5101–5104 (1998).
Yue, D. & Larock, R. C. Synthesis of 2,3-disubstituted benzo[b]thiophenes via palladium-catalyzed coupling and electrophilic cyclization of terminal acetylenes. J. Org. Chem 67(6), 1905–1909 (2002).
Cacchi, S. Heterocycles via cyclization of alkynes promoted by organopalladium complexes. J. Organomet. Chem. 576(1–2), 42–64 (1999).
Patra, S. R., Sangma, S. W., Padhy, A. K. & Bhunia, S. Oxidative addition to the N–C bond Vs formation of the zwitterionic intermediate in platinum(II)–catalyzed intramolecular annulation of alkynes to form indoles: Mechanistic studies and reaction scope. J. Org. Chem 87(15), 9714–9722 (2022).
Shimada, T., Nakamura, I. & Yamamoto, Y. Intramolecular C−N bond addition of amides to alkynes using platinum catalyst. J. Am. Chem. Soc. 126(34), 10546–10547 (2004).
Nanda, S. K. & Mallik, R. 1, 2-Difunctionalizations of alkynes entailing concomitant C–C and C–N bond-forming carboamination reactions. RSC Adv. 12(10), 5847–5870 (2022).
Nakamura, I., Sato, T., Terada, M. & Yamamoto, Y. Gold-catalyzed cyclization of (ortho-Alkynylphenylthio)silanes: Intramolecular capture of the vinyl−au intermediate by the silicon electrophile. Org. Lett. 9(20), 4081–4083 (2007).
Kulandai Raj, A. S., Narode, A. S. & Liu, R.-S. Gold(I)-catalyzed reactions between N-(o-Alkynylphenyl)imines and vinyldiazo ketones to form 3-(Furan-2-ylmethyl)-1H-indoles via postulated azallyl gold and allylic cation intermediates. Org. Lett. 23(4), 1378–1382 (2021).
Skouta, R. & Li, C.-J. Gold-catalyzed reactions of CH bonds. Tetrahedron 64(22), 4917–4938 (2008).
Nakamura, I., Yamagishi, U., Song, D., Konta, S. & Yamamoto, Y. Gold-and indium-catalyzed synthesis of 3-and 6-sulfonylindoles from ortho-alkynyl-N-sulfonylanilines. Angew. Chem. Int. Ed. 46(13), 2284–2287 (2007).
Shen, Z.-L., Wang, S.-Y., Chok, Y.-K., Xu, Y.-H. & Loh, T.-P. Organoindium reagents: The preparation and application in organic synthesis. Chem. Rev. 113(1), 271–401 (2013).
Obaid, R. J. et al. Pharmacological significance of nitrogen-containing five and six-membered heterocyclic scaffolds as potent cholinesterase inhibitors for drug discovery. Process Biochem. 120, 250–259 (2022).
Srivastava, S. K. et al. Effects of substitution on the pyrrole N atom in derivatives of tetrahydronaltrindole, tetrahydrooxymorphindole, and a related 4, 5-epoxyphenylpyrrolomorphinan. J. Med. Chem. 47(26), 6645–6648 (2004).
Walsh, C. T., Garneau-Tsodikova, S. & Howard-Jones, A. R. Biological formation of pyrroles: Nature’s logic and enzymatic machinery. Nat. Prod. Rep. 23(4), 517–531 (2006).
Gale, P. A. Structural and molecular recognition studies with acyclic anion receptors. Acc. Chem. Res. 39(7), 465–475 (2006).
Chelucci, G. Metal-catalyzed dehydrogenative synthesis of pyrroles and indoles from alcohols. Coord. Chem. Rev. 331, 37–53 (2017).
Zhao, D., Shi, Z. & Glorius, F. Indole synthesis by rhodium (III)-catalyzed hydrazine-directed C–H activation: Redox-neutral and traceless by N–N bond cleavage. Angew. Chem. Int. Ed. 52(47), 12426–12429 (2013).
Romero, A. H. Fused heteroaromatic rings via metal-mediated/catalyzed intramolecular C–H activation: A comprehensive review. Top. Curr. Chem. 377(4), 1–70 (2019).
Neumann, J. J., Rakshit, S., Dröge, T., Würtz, S. & Glorius, F. Exploring the oxidative cyclization of substituted N-Aryl enamines: Pd-catalyzed formation of indoles from anilines. Chem. Eur. J. 17(26), 7298–7303 (2011).
Chen, X. et al. Palladium-catalyzed reaction of arylamine and diarylacetylene: Solvent-controlled construction of 2, 3-diarylindoles and pentaarylpyrroles. Eur. J. Org. Chem. 2012(23), 4380–4386 (2012).
Boyarskiy, V. P., Ryabukhin, D. S., Bokach, N. A. & Vasilyev, A. V. Alkenylation of arenes and heteroarenes with alkynes. Chem. Rev. 116(10), 5894–5986 (2016).
Rezaei, S. et al. Mono- and multifold C–C coupling reactions catalyzed by a palladium complex encapsulated in MIL–Cr as a three dimensional nano reactor. RSC Adv. 6(95), 92463–92472 (2016).
Landarani Isfahani, A. et al. Pd nanoparticles immobilized on nanosilica triazine dendritic polymer: A reusable catalyst for the synthesis of mono-, Di-, and trialkynylaromatics by sonogashira cross-coupling in water. Eur. J. Org. Chem. 2014(25), 5603–5609 (2014).
Isfahani, A. L. et al. Palladium nanoparticles immobilized on nano-silica triazine dendritic polymer (Pdnp-nSTDP): An efficient and reusable catalyst for suzuki-miyaura cross-coupling and heck reactions. Adv. Synth. Catal. 355(5), 957–972 (2013).
Nasr-Esfahani, M. et al. Copper immobilized on nanosilica triazine dendrimer (Cu(II)-TD@nSiO2)-catalyzed regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles and bis- and tris-triazoles via a one-pot multicomponent click reaction. J. Org. Chem 79(3), 1437–1443 (2014).
Ataee-Kachouei, T. et al. Ce(IV) immobilized on halloysite nanotube–functionalized dendrimer (Ce(IV)–G2): A novel and efficient dendritic catalyst for the synthesis of pyrido[3,2-c]coumarin derivatives. Appl. Organomet. Chem. 34(11), e5948 (2020).
Asadi, B., Mohammadpoor-Baltork, I., Mirkhani, V., Tangestaninejad, S. & Moghadam, M. Synthesis of Bi(III) immobilized on carboxyl-terminated triazine dendrimer stabilized magnetic nanoparticles: Improvement of catalytic activity for synthesis of indol-3-yl acrylates. ChemistrySelect 5(26), 7840–7848 (2020).
Nori, Z. Z. et al. Ultrafine Pt nanoparticles supported on a dendrimer containing thiol groups: An efficient catalyst for the synthesis of benzimidazoles and benzothiazoles from benzyl alcohol derivatives in water. RSC Adv. 10(55), 33137–33147 (2020).
Landarani-Isfahani, A. et al. Elegant pH-responsive nanovehicle for drug delivery based on triazine dendrimer modified magnetic nanoparticles. Langmuir 33(34), 8503–8515 (2017).
Cheng, W., Tang, K., Qi, Y., Sheng, J. & Liu, Z. One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 20(9), 1799–1805 (2010).
Martínez, J. M. L. et al. XPS studies on the Cu (I, II)–polyampholyte heterogeneous catalyst: An insight into its structure and mechanism. J. Mol. Catal. A Chem. 339(1–2), 43–51 (2011).
Cao, Y. et al. Vacuum heat-treatment of carbon nitride for enhancing photocatalytic hydrogen evolution. J. Mater. Chem. A 2(42), 17797–17807 (2014).
Sun, Y. et al. Chemically converted graphene as substrate for immobilizing and enhancing the activity of a polymeric catalyst. Chem. Commun. 46(26), 4740–4742 (2010).
Huang, C.-J. & Chang, Y.-C. In situ surface tailoring with zwitterionic carboxybetaine moieties on self-assembled thin film for antifouling biointerfaces. Materials 7(1), 130–142 (2013).
Tan, H.-Z. et al. Active Pd(ii) complexes: Enhancing catalytic activity by ligand effect for carbonylation of methyl nitrite to dimethyl carbonate. Catal. Sci. Technol. 7(17), 3785–3790 (2017).
Shi, Z., Zhang, B., Cui, Y. & Jiao, N. Palladium-catalyzed ring-expansion reaction of indoles with alkynes: From indoles to tetrahydroquinoline derivatives under mild reaction conditions. Angew. Chem. Int. Ed. 49(24), 4036–4041 (2010).
Wu, Y. T., Huang, K. H., Shin, C. C. & Wu, T. C. Palladium-catalyzed formation of highly substituted naphthalenes from arene and alkyne hydrocarbons. Chem. Eur. J. 14(22), 6697–6703 (2008).
Cui, X., Li, J., Fu, Y., Liu, L. & Guo, Q.-X. Regioselective Pd-catalyzed indolization of 2-bromoanilines with internal alkynes using phosphine-free ligands. Tetrahedron Lett. 49(21), 3458–3462 (2008).
Hosokawa, T., Nomura, T. & Murahashi, S.-I. Palladium–copper–DMF complexes involved in the oxidation of alkenes. J. Organomet. Chem. 551(1), 387–389 (1998).
Lyshevski, S. E. Nano and Molecular Electronics Handbook (CRC Press, Boca Raton, 2018).
Huang, L., Massa, L. & Karle, J. Reflections on quantum biochemistry: From context to contents. In Quantum Biochemistry: Electronic Structure and Biological Activity (ed. Matta, C. F.) (Wiley VCH, Weinheim, 2010).
Kozuch, S. & Shaik, S. How to conceptualize catalytic cycles? The energetic span model. Acc. Chem. Res. 44(2), 101–110 (2011).
Kozuch, S. A refinement of everyday thinking: The energetic span model for kinetic assessment of catalytic cycles. WIREs Comput. Mol. Sci. 2(5), 795–815 (2012).
Acknowledgements
We are grateful to the Research Council of the University of Isfahan for financial support of this work.
Author information
Authors and Affiliations
Contributions
A. L.-I; experimental work, writing—original main text. I.M.-B.; conceptualization, supervision, project administration, writing. M.M.; investigation, review and editing. V. M. and S.T.; data curation. R. S and H. H; theoretical study and data refinement.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Landarani-Isfahani, A., Mohammadpoor-Baltork, I., Moghadam, M. et al. Palladium-immobilized triazine dendrimer on magnetic nanoparticles: as reusable microreactor for solvent-dependent synthesis strategy of 2,3-diphenylindoles and pentaphenylpyrrole derivatives. Sci Rep 14, 22498 (2024). https://doi.org/10.1038/s41598-024-72224-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72224-2
This article is cited by
-
Electrochemical and colorimetric sensing of P-xylene using doped C60 fullerenes: a dual approach to medical and environmental applications
Scientific Reports (2025)
-
The catalyst-free green synthesis and QTAIM analysis of anilino-1,4-naphthoquinones as molecular wires
Scientific Reports (2025)
-
Doped C20 fullerenes as a new generation of efficient nanosorbents and nanosensors for rapid detection of dimethyltryptamine in drug detection
Scientific Reports (2025)