Abstract
The neighborhood effect has become an important framework with which to study the mechanisms that maintain the coexistence of tree species. Phylogenetic relatedness among neighboring plants directly affects species coexistence and the maintenance of tree diversity. And some studies have reported that seedling performance is negatively correlated with phylogenetic relatedness, which termed phylogenetic negative density dependence. Soil-borne fungal pathogens affected seedling performance of phylogenetically related host species, i.e., phylogenetic Janzen–Connell effect. Seedlings may be particularly vulnerable to habitat and neighbor characteristics. Although previous studies have demonstrated the influence of neighborhood effects, phylogenetic relatedness, and habitat filtering on seedling survival, growth, and mortality, the effect of variation in these factors on seedling abundance remains unclear. To address this question, we used a 4-ha (200 m × 200 m) and monitored four-year (2020–2023) seedling dataset from a mid-montane humid evergreen broad-leaved subtropical forest in the Gaoligong Mountains, Yunnan, Southwestern China, and which consisted of 916 seedlings belonging to 56 species. The results of generalized linear mixed models showed no significant effect of conspecific adult neighbors on seedling abundance at any of the intervals evaluated. In contrast, we found evidence of phylogenetic distance density dependence in the forests of the Gaoligong Mountains. Specifically, there was a significant positive effect of the relative average phylogenetic distance between heterospecific adult neighbors and focal seedlings on focal seedling abundance in 2020; however, the relative average phylogenetic distance between heterospecific seedling neighbors and focal seedlings had a significant negative effect on seedling abundance over the four-year period (2020–2023). Among the habitat factors, only light (canopy opening) had a negative effect on seedling abundance in all four years. Light resources may be a limiting factor for seedlings, and determine seedling dynamics in subtropical forests. Overall, our results demonstrated that phylogenetic density dependence and habitat filtering affected subtropical seedling abundance. Our findings provide new evidence of the impact of phylogenetic density dependence on seedling abundance in a subtropical mid-montane humid evergreen broad-leaved forest and highlight the need to incorporate the neighborhood effect, phylogenetic relatedness, and habitat factors in models assessing seedling abundance.
Similar content being viewed by others
Introduction
A long-standing and fundamental question in ecology is how plant species coexist and how their diversity is maintained. Numerous mechanisms have been proposed to explain the coexistence of tree species at the local scale1,2. Conspecific negative density dependence (CNDD) was one of the first hypothesized mechanisms of species coexistence and has remained an important focus of research since its inception, i.e., Janzen-Connell hypothesis3,4 which proposes that tree species diversity is maintained by specific natural enemies (such as herbivores and fungal pathogens); and plant species diversity is maintained by higher seed and seedling mortality at higher densities of conspecific seedlings as well as conspecific adult tree neighbors3,4,5,6. CNDD is a widespread phenomenon that may influence species abundance patterns in various ways, thereby increasing forest diversity6,7,8.
To date, most studies examining the CNDD mechanism have mainly focused on seedling survival9,10,11, growth12, recruitment7,13, and mortality14,15. However, there are few studies in which the impact of neighborhood effects on species abundance has been investigated, thus the relationship between strength of CNDD and seedling abundance is yet to be explored8,16. As a result, the neighborhood effect on seedling abundance may be largely neglected in subtropical forests. The effects of conspecific and heterospecific neighbors on the probability of survival and mortality for individuals predict seedling dynamics for the next year, while species abundance is a direct measure of performance after seedling survival. Thus, studying the factors influencing seedling abundance in communities will further improve our understanding of the neighborhood effect that maintain tree species diversity in subtropical forest communities. The relationship between seedling abundance and the strength of the density dependence of neighbors is unclear and challenging to predict. Moreover, the distribution of seedling abundance has scarcely been studied directly and has mainly been extrapolated by analyzing the spatial pattern distribution of species and intra- and interspecific association17, such as X2 test, point pattern analysis and null model18.
Species abundance has been suggested to contribute substantially to the variation in CNDD among species in a community5,8,15,19, suggesting that neighbors of the same species play a role in determining whether a given species is common or rare in the community5,20. Seiwa et al.21 concluded that the close relationship between species abundance and the strength of the neighborhood effect is useful for determining forest management goals. Rare species suffered more strengths of conspecific neighbors than common species5. Conversely, the effects of heterospecific neighbors were not found to be related to species abundance5. There were three main types of relationships between density dependence and species abundance: positive, negative, and uncorrelated5. Further studies on density dependence are required to reconcile these inconsistent results. Variation in plant species abundance is a consequence of CNDD5,22. Moreover, species abundance is also strongly affected by biotic interactions, such as competition, mutualism, parasitism, and herbivory21. Schroeder et al.23 used spatially explicit model to track the community dynamics of microbial mutualists, pathogens, and their plant hosts, who found that a positive correlation between host abundance and plant-soil feedback will develop largely due to the effects of microbial community dynamics. Species interactions influence species abundance5.
A simple division of neighbors into conspecific and heterospecific may hide the potentially large variation in which heterospecifics are similar to focal seedlings6,14,24,25,26. An increasing number of studies have demonstrated that closely related neighbors have stronger negative effects on the survival or growth of focal species. These effects are referred to as phylogenetic negative density dependence (PNDD)13,27,28,29,30. The Janzen-Connell hypothesis is extended with respect to considering pathogen exclusion experiments, that negative density dependence of phylogenetic relatedness among neighbors on seedling performance are mediated by associated host-specific pathogens28. Meanwhile, phylogenetic positive density dependence (PPDD) has also been reported in many forest types13,31,32,33. The strength of the phylogenetic density dependence may play a role in shaping the abundance and temporal distribution of species within communities.
The seedling stage has long been recognized as the key bottleneck of forest regeneration34. Apart from neighbor interactions, another crucial driver of seedling abundance is abiotic environmental conditions. Among abiotic factors, the vulnerable seedling stage is largely influenced by the canopy, which modifies the surrounding habitat. Consequently, light is likely to be the most limiting factor, with lower light intensities in the understory. Soil moisture also becomes a relatively more important limiting factor as light availability increases35. Soil fertility36 and seasonal drought37 have been identified as important limiting factors on seedlings. Moreover, many components of the niche that are correlated with topography may vary through time, leading plant species to respond differently to the same habitat in different years.
The extent to which neighborhood effects, phylogenetic distance among neighbors, and habitat heterogeneity jointly affect seedling abundance is unclear. Therefore, identifying the factors that influence tree seedling abundance is essential for understanding, predicting, conserving, and managing forests more effectively38. To fill this gap in the literature, we analyzed the influence of neighbor densities, phylogenetic distance between neighbors, and habitat factors on seedling abundance using generalized linear mixed models with seedling abundance in the Gaoligong Mountains, Yunnan, China, from 2020 to 2023. We aimed to provide a mechanistic understanding of the effects of neighborhood interactions and habitat on seedling abundance. We investigated the following research three major questions with the corresponding hypotheses: (1) How do conspecific neighbor densities affect seedling abundance? We hypothesize (H1) that seedling abundance is influenced by the effect of CNDD. (2) Is there evidence of phylogenetic density dependence on seedling abundance? We hypothesize (H2) phylogenetic distance among heterospecific neighbors negatively affect seedling abundance and that seedling abundance would decrease with closer phylogenetic distance between neighbors (PNDD). (3) What is the consequence of habitat filtering on seedling abundance? We hypothesize (H3) that seedling abundance may also vary according to spatiotemporal patterns and habitat heterogeneity (light, topography and soil properties), with a high survival rate and low mortality of seedlings in favorable habitats (e.g., high light) favoring species abundance.
Materials and methods
Study area and sampling site
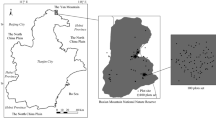
This study was conducted within a 4-ha (200 m × 200 m) Gaoligong Mountains forest dynamics plot (24°50′9.8″–24°50′17.3″ N, 98°45′53.1″–98°46′1.3″ E) in the Gaoligong Mountains National Nature Reserve, Yunnan, China (Fig. 1a, b). The forest of this area is a typical mid-montane humid evergreen broad-leaved forest with an average ambient air temperature of 15.5 °C and average annual precipitation of 1,650 mm, approximately 87%39 of which falls in the rainy season from May to October. The soil in the forest is yellow–brown loam39. The plot was established in 2009–2010, according to the standards of the Center of Tropical Forest Science (http://www.ctfs.si.edu/). All living and freestanding woody plant stems with a diameter at breast height (DBH) ≥ 1 cm were tagged, mapped, measured, and identified to species. The topography varied across the plot, with higher elevations in the east and lower terrain in the west, ranging from 2135 to 2329.65 m above sea level. The distinctive vegetation type was dominated by species in the families Fagaceae (e.g., Lithocarpus hancei and Quercus lamellosa), Lauraceae (Neolitsea lunglingensis and Litsea chinpingensis), and Theaceae (Polyspora longicarpa, Camellia tsaii, and Camellia taliensis).
Seedling census
To study seedling regeneration, the 4-ha forest plot was divided into 100 subplots (20 m × 20 m), and 100 seedling quadrats (2 m × 2 m) were established in November 2020, spaced evenly within the top-right corners of each subplot (Fig. 1c). In the initial seedling quadrats, all free-standing woody seedlings (lianas, shrubs, and trees) ≥ 20 cm tall with a DBH < 1 cm within each quadrat were tagged, identified to species, and their heights were measured (Fig. 1d). A total of 914 seedlings of 55 woody plant species were recorded. Subsequently, a census of all seedling quadrats was performed at the end of the rainy season (November) annually from 2021 to 2023. In each re-census, we determined the status (alive or dead) of any previously recorded woody seedlings and remeasured the height of any live seedlings. All newly recruited seedlings within the 20-cm height threshold were identified, tagged, and measured at each census. The data obtained from the focal seedling recordings from 2020 to 2023 were subjected to further statistical analysis.
Habitat variables
To evaluate the influence of habitat on focal seedling abundance, we measured the following habitat variables: light, topography, and soil properties.
Understory light availability data were collected by taking hemispherical photographs using a Nikon Z5N1933 camera with an F2.8 12-mm Senyo round fisheye lens 1.3 m above the ground in each seedling quadrat in the winter of 2021. The digital hemispherical photographs were analyzed using the software program Gap Light Analyzer40 (GLA Version 2.0), which is used to extract the canopy structure and gap light. Photographs with the best contrast and least overexposure were selected for analysis to ensure a consistent level of contrast41.
Three topographic factors (elevation, convexity, and slope) were measured in each seedling quadrat following the methods described by Harms et al.42. The elevation was measured as the mean elevation of the four corners of each seedling quadrat. Convexity was calculated according to the elevation of the grid of interest minus the mean elevation of the eight neighboring quadrats. As for the edge focal quadrats, convexity was calculated as the elevation of the center point minus the mean of the four corners43. The slope was defined as the mean angular deviation from the horizontal plane of each of the four triangular planes formed by connecting three of the four vertices of each quadrat.
To characterize the soil conditions and chemical composition, we randomly collected three soil core samples (approximately 500 g) at a depth of 0–5 cm (this soil horizon has the greatest effect on seedlings and the root distribution of the seedling is relatively shallow44) in each seedling quadrat in May 2022. Superficial leaf litter and humus layers were removed prior to soil sampling. We used a rapid nitrogen, phosphorus, and potassium meter (TRREC N01, Shandong Sean) to measure the soil pH, electrical conductivity (EC), available phosphorus (AP), and available potassium (AK). The soil samples were taken to laboratory, and then which were air-dried, smashed, sieved using 1-mm and 0.15-mm mesh and stored in plastic bags for later additional analysis. Soil organic matter content (C) was measured in soil oxidized with H2SO4–K2Cr2O7.Total nitrogen (TN) was evaluated using the micro-Kjeldahl method and a mixture of H2SO4 and K2SO4–CuSO4–Se catalyst. The soil was digested in NaOH solution, total phosphorus (TP) and total potassium (TK) were determined using the Mo-Sb anti spectrophotometric method and HF–HClO4, flame photometer method, respectively. Soil temperature and moisture were measured at three randomly selected points in each seedling quadrat using a soil hygrothermograph (YDSC–A01) at a depth of approximately 5 cm, and the mean values were used for the analysis.
To reduce the collinearity of environmental variables, a principal component analysis (PCA) was conducted on the aforementioned habitat variables using the prcomp function in the “stats” package. However, rather than including canopy openness in the PCA, we inserted it directly into the models. The first three components (PC1, PC2, and PC3) accounted for 66.15% of the total variance of the three environmental variables, and were used in subsequent analyses. PC1, PC2 and PC3 represented 32.59%, 20.56% and 13.00% of the variation (Table S1), respectively. PC1 was mainly associated with high elevation, EC, C, AP, AK, TN, TP, and soil moisture, and low TK. PC2 was characterized by high C and TN but low EC, AP, AK, TK, and soil moisture. PC3 was associated with low elevation, convexity, slope, and soil pH.
Neighborhood variables
We quantified the local adult neighbor densities of focal seedlings. However, we did not consider seedling neighbors, which are defined as the number of seedlings within each seedling quadrat32 and would therefore interfere with the main results of the analysis. Focal seedling abundance was extremely significantly correlated with conspecific seedling neighbor density in models, thereby the effects of phylogenetic density dependence and habitat filtering on seedling abundance were covered up (i.e., not significant) (Table S2). We defined seedling abundance as the number of seedling individual in each seedling quadrat.
We calculated the densities of conspecific adult neighbor (CN) within a 20-m radius as the sum of basal area (BA) of the conspecific adult neighbor divided by the distance between each tree and the center of the focal seedling quadrat. Similarly, the densities of heterospecific adult neighbor (HN) was calculated as follows:
where N is the number of conspecific or heterospecific adult neighbors of a focal seedling, BAi is the basal area of neighboring adult i, and Distancei is the distance between the center of the focal seedling and its adult neighbor i. To correct for the edge effect, seedlings located within 20 m from the 4-ha plot boundary were not included.
Phylogenetic distance
To evaluate the effects of heterospecific neighbors on focal seedling abundance, the “V.PhyloMaker 2” package was used to create a phylogenetic tree basing on the mega-tree GBOTB.extended.LCVP.tre for the Gaoligong Mountains45. We used the LCVP database to standardize spellings and nomenclature for the woody plant names of this plot in the phylogeny, and removed duplicate plant names. For those families that were present in GBOTB.extended.TPL.tre, we added them to the phylogeny based on their locations in GBOTB.extended.TPL.tre. We calculated the relative average phylogenetic distance (APd’) for focal seedlings and adult neighbors24 as follows:
where \(S\_APd^{\prime}\) represents the relative average phylogenetic distance between heterospecific seedling neighbors and focal seedlings, and \(A\_APd^{\prime}\) represents the relative average phylogenetic distance between heterospecific adult neighbors and focal seedlings. MPdobs represents the observed phylogenetic distance, calculated as the mean phylogenetic distance of all heterospecific neighbors to the focal seedling. The expected phylogenetic distance (MPdrand) is a null model that generates 999 random neighborhoods at a given species richness using the “picante” package46. A positive \(APd^{\prime}\) value indicates that the neighbors are less closely related to the focal seedlings than expected under the model, whereas a negative \(APd^{\prime}\) value suggests that the neighbors are more closely related than expected.
Statistical analysis
To identify the key factors influencing seedling abundance, we modeled individuals using a log-link function with a generalized linear mixed model in the following full model form:
In this model, \({s}_{ij}\) is the individual number of surviving seedlings i in quadrat j, which is considered as the response variable; \({p}_{ij}\) is the predicted abundance for each seedling; \({\beta }_{0}\) is overall mean intercept; the coefficients \({\beta }_{1-3}\) represent the habitat, density of conspecific and heterospecific adults, and phylogeny, respectively, as fixed effects. The random effects include the quadrat \({\mu }_{\begin{array}{c}j\end{array}}\) and species \({\mu }_{\text{species}}\) to control for spatial autocorrelation, given that the neighborhoods of seedlings present in the same quadrats are inherently more similar than that of seedlings present in other quadrats. \({\varepsilon }_{ij}\) is the error term of the model for seedling i in quadrat j.
We analyzed each census interval separately to assess the temporal variability in the importance of the predictor variables. All variables were standardized by subtracting the mean value of the variable and dividing it by one standard deviation before the analysis. The mean values and ranges of the variables were included in the seedling abundance models (Table 1).
To test our hypotheses, we compared six candidate models evaluated with different factors as fixed effects in four intervals: (1) a null model with only random effects, (2) a habitat-only model, (3) a density-dependent model, (4) a density + habitat model, (5) a phylogenetic density model, and (6) a phylogenetic + habitat model. We used the Akaike information criterion (AIC) to identify the best-fit model, where models with delta AIC values of < 2 were considered to have equal predictive validity. To compare the strength of evidence for the best-fit model relative to other models, the likelihood values were normalized according to Akaike weights (AICw), i.e., divided by the sum of the likelihoods of all models47.
The coefficient estimate (CE) was calculated to represent the strength of the effects of the variables, where CE > 0 indicates a positive effect on seedling abundance, and CE < 0 indicates negative effects. Specifically, a positive CE of phylogenetic distance indicates that the phylogenetic similarity of heterospecific neighbors is negatively correlated with seedling abundance (i.e., phylogenetic negative density dependence) and vice versa. The results of coefficient estimate showed 95% confidence intervals, which meant 95 of every 100 such intervals will contain the true value.
All statistical analyses were performed using the R v4.4.148. The “ggplot2” package was used to present the results graphically.
Results
In the annual censuses in 2020, 2021, 2022, and 2023, a total of 914, 916, 834, and 854 living seedlings of 55, 54, 53, and 52 focal woody seedling species, respectively, were recorded in the 100 focal seedling quadrats.
The explanatory powers of the six models of seedling abundance showed clear differences across the four census years (Table 2). In the 2020, 2021, and 2023 intervals, the phylogenetic density models and phylogenetic + habitat models were equivalent, and the AICw values of phylogenetic + habitat models were higher. However, the AIC values of the null models were the lowest and best fit for the 2022 interval (AICw = 0.3962). Based on the comparison of AIC values, the phylogenetic + habitat model was the best-fit model for all four census years.
With respect to the model containing all factors in the four intervals, seedling abundance was best explained by the phylogenetic + habitat model, which showed large variations in the effects of neighbor density and environmental variables on seedling abundance at different intervals (Fig. 2). In all years, there were no significant effects of CN, PC1, PC2, and PC3 on seedling abundance, whereas a significant negative effect of light on seedling abundance was observed in all four years (CE = − 0.343, P < 0.05; CE = − 0.359, P < 0.01; CE = − 0.271, P < 0.05; CE = − 0.307, P < 0.05) (Fig. 2; Table S3). We found seedling abundance was also negatively correlated with species-specific coefficients of light (Fig. S1). Thus, the number of seedlings was lower in habitats with higher canopy openness.
Estimated coefficients (mean ± 2SE) of the association of neighborhood effect and habitat variables with seedling abundance in the Gaoligong Mountains forest. Error bars represent 95% confidence intervals. Filled circles indicate significant effects (P < 0.05). CN, the densities of conspecific adult neighbor; S_APd′, relative average phylogenetic distance between heterospecific seedling neighbors and focal seedlings; A_APd′, relative average phylogenetic distance between heterospecific adult neighbors and focal seedlings; Light, understory light availability; PC1–PC3, first three principal components.
In addition, the phylogenetic density of heterospecific seedlings (S_APd′) was found to have a significant negative effect on seedling abundance in the four census years (CE = − 0.475, P < 0.001; CE = − 0.592, P < 0.001; CE = − 0.300, P < 0.05; CE = − 0.460, P < 0.01). In contrast, the phylogenetic density of adult tree neighbors (A_APd′) was significant positively correlated with seedling abundance only in 2020 (CE = 0.215, P < 0.05) (Fig. 2a; Fig. S2; Table S3). Thus, both neighbor and habitat filtering affected seedling abundance, and the strength of these effects varied according to interannual dynamics.
Discussion
In the present study, we explored the effects of neighborhood and habitat factors on seedling abundance in the Gaoligong Mountains of Southwestern China. Our results revealed that the phylogenetic + habitat models of seedling abundance were the best predictors, with interannual variations in the coefficients of variables. We found that the PPDD of seedling neighbors and the PNDD of adult neighbors affected seedling abundance. Our results also confirmed that seedling abundance responses are strongly linked to the light environment. In the following section, we discuss these results in detail.
Phylogenetic density dependence has a greater influence on seedling abundance than conspecific density dependence
We first hypothesis (H1) expected that the effect of CNDD on seedling abundance, however, this expectation was not met at this site. Our results are consistent with the findings of Metz et al.14, who showed that seedling abundance was unrelated to conspecific density dependence in study plots on a highly diverse Amazonian rain forest. However, this is inconsistent with the results from a subtropical evergreen broad-leaved forest, where the strength of conspecific density dependence was stronger for species with higher abundance16. This is likely because the number of conspecific neighbors was lower at our site. Another reason may be related to the variety of definitions of focal seedlings49. Seedlings that were more than 20 cm tall in this study may have passed the stage at which they were vulnerable to habitat variables. In contrast, Zhu et al.13 found that smaller seedlings are more sensitive to conspecific density dependence, and this effect weakens with increasing seedling growth. And Seedlings were negatively impacted by conspecific seedling neighbors in three periods, but were most strongly impacted by first stage conspecific seedling neighbors. Furthermore, species with a relatively large seed mass, slow growth rate, and strong defense ability may suffer from fewer negative effects from conspecific neighbors, thus improving species abundance50,51. Species with strong habitat preferences would survive well in their optimal habitat, where conspecific neighbors tend to be high17,19, thereby masking the negative effect of conspecific adult neighbors on seedling abundance in the forest.
We found evidence of PPDD in the study area which this result supported our hypothesis H2; the phylogenetic distance between focal seedlings and their heterospecific seedling neighbors was negatively associated with seedling abundance. These results are consistent with those of Lebrija-Trejos et al.31 and Wu et al.32. This outcome is likely the result of closely related species sharing similar habitat resources13,26,32. In addition, seedling abundance may be further promoted by PPDD as a result of plant-microorganism associations52. Closer phylogenetic distance of focal seedling and neighbors tend to share symbiotic partners in the soil, for instance, mycorrhizas colonized in the root tissues and facilitated the transport of nutrients and water26. Furthermore, the extensive underground mycorrhizal networks created by ectomycorrhizal fungi may benefit to seedling survival26, it was further conducive to the increase of seedling abundance.
It is worth noting that we detected a pattern of PNDD in adult neighbors in 2020, which indicates that the presence of heterospecific adult neighbors with a closer phylogenetic distance will be more detrimental to the survival of focal seedlings. Consequently, seedling abundance increased with increasing phylogenetic distance between heterospecific adult neighbors and focal seedlings. The PNDD of adult neighbors was not significant in the other three intervals (2021–2023), suggesting that the strength of the PNDD of adult neighbors varied over time. Our results are further supported by previous studies showing that seedling survival increases when the surrounding adults are distantly related to the focal seedlings14,33. Compared with seedlings at later stages, seedlings at earlier stages are too fragile to defend against natural enemy attacks; therefore, the strength of PNDD should decrease as the life stage progresses30. The sizes of focal species and neighbors were unequal; it has been shown that there is a stronger negative effect of heterospecific neighbors on seedlings growing in lower abundance53. Another possible explanation for the effect of PNDD is that closely related species with similarly important functional traits are more likely to associate host-specific fungal pathogens28. Closely related species may increase the possibility of being attacked by natural enemies27,53,54,55.
Considering previously reported results as well as our own findings, it appears that—in subtropical forests—phylogenetic density dependence contributes more strongly to the maintenance of seedling abundance than does conspecific density dependence.
Effects of habitat filtering on seedling abundance
As hypothesized H3, seedling abundance was affected habitat heterogeneity. However, we only found that light of habitat factors was negatively correlated with seedling abundance at all intervals, and the strength of the negative effects varied over time. Thus, the light variable (i.e., canopy openness) is a good indicator of how seedling abundance responds to changing conditions. In nearly all cases, seedling abundance was less sensitive to changes in the surrounding topography and soil properties. These results may be due to the low spatial variation in topography and soil properties in our subtropical forest plot. The maximum difference in elevation is only 194.65 m, and soil properties (e.g., soil moisture) may not be a critical limiting factor given that the annual precipitation is over 1650 mm (Fig. S3). Thus, light plays a more important role than do topography or soil properties.
Our results contrast with those of previous studies showing a positive effect of light availability on seedling survival and mortality in deciduous broad-leaved forests56,57. Generally, higher light levels enhance seedling survival56,57,58,59. However, we found that high light levels decreased the seedling abundance. Seedlings, in particular, may be negatively affected under high light conditions because of either strong competition from light-demanding species or photoinhibition60. The lower seedling abundance of each species in our study plot under such high light conditions could be due to desiccation or sun scalding of seedlings growing under more open canopies61. To some degree, different effects may represent life-history variation in response to light: seedlings of shade-tolerant species tend to have higher survival in shade, whereas seedlings of light-demanding species experience high mortality under low light62,63. We did not detect any associations of topographic factors with seedling abundance. However, Metz64 illustrated that the composition of individuals filling canopy gaps is strongly influenced by topographic niche. It is also possible that the effect of a topographic environment with low variation on seedling responses is reversed or overwhelmed by other processes. Yin et al.65 found that under the similar limited light conditions, the increased soil nutrients and water content may still be insufficient to meet the developmental requirement of seedlings, that light still limited seedling abundance. Additionally, the negative effect of light on seedling abundance could be due to directly sunscald and photoinhibition, or indirectly by reduced humidity and soil moisture as a result of the loss of forest canopy66.
Hence, our results indicate that using long-term data will be essential to further our understanding of habitat filtering of seedling abundance over time. Therefore, continuous monitoring of seedling abundance and its associated factors is necessary. Interannual variation of habitat variables and neighbor densities have strong impacts on seedling abundance and help to predict the responses of communities to the changing environment59.
Conclusion
Through a long-term (four-year) seedling census in the Gaoligong Mountains, we examined the influences of neighborhood effect, phylogenetic relatedness, and habitat on seedling abundance. The results demonstrate that the effects of conspecific adult neighbors appear to be less relevant during the seedling stage. The finding that CNDD was not relevant for seedlings in this study stands in contrast with other studies, particularly in BCI. However, phylogenetic relatedness among heterospecific seedling neighbors tended to have strongly negative effects on seedling abundance. Furthermore, we found a significant negative relationship between light exposure and seedling abundance. Our results highlight that it is crucial to consider the effects of both phylogenetic relatedness and habitat factors on seedling abundance, and that these effects may vary over time. Overall, this work contributes to a better understanding of the roles of neighborhood effects, phylogenetic density dependence, and habitat filtering in shaping seedling abundance. Accordingly, continuous long-term community-wide monitoring is essential to understand the importance of neighbor density and habitat filtering on seedling performance. For forest management, the knowledge of the mechanisms of neighbor density and habitat filtering in impacting seedling abundance which can provide a theoretical reference for maintaining species diversity in forest communities, and thus setting management goals for forest.
Data availability
All data generated during this study are included in the main body of this article. More information about dataset is available at https://datadryad.org/stash/share/h2bdltgfvsyZhH6BbrhREA0oBhIJdGB2u0F2QqjMxA.
References
Wright, S. J. Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130, 1–14 (2002).
Volkov, I., Banavar, J. R., He, F. L., Hubbell, S. P. & Maritan, A. Density dependence explains tree species abundance and diversity in tropical forests. Nature 438, 658–661 (2005).
Janzen, D. H. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 (1970).
Connell, J. H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In Dynamics of Populations (eds Den Boer, P. J. & Gradwell, G. R.) 298–312 (Centre for Agricultural Publishing and Documentation, 1971).
Comita, L. S., Muller-Landau, H. C., Aguilar, S. & Hubbell, S. P. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329, 330–332 (2010).
Comita, L. S. et al. Testing predictions of the Janzen-Connell hypothesis, a meta-analysis of experimental evidence for distance and density-dependent seed and seedling survival. J. Ecol. 102, 845–856 (2014).
Harms, K. E., Wright, S. J., Calderon, O., Hernandez, A. & Herre, E. A. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404, 493–495 (2000).
LaManna, J. A. et al. Plant diversity increases with the strength of negative density dependence at the global scale. Science 356, 1389–1392 (2017).
Hubbell, S. P., Ahumada, J. A., Condit, R. & Foster, R. B. Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol. Res. 16, 859–875 (2001).
Lin, L. X., Comita, L. S., Zheng, Z. & Cao, M. Seasonal differentiation in density-dependent seedling survival in a tropical rain forest. J. Ecol. 100, 905–914 (2012).
Zhu, Y. et al. Density-dependent survival varies with species life-history strategy in a tropical forest. Ecol. Lett. 21, 506–515 (2018).
Mensah, S., Lokossou, C. J. M., Assogbadjo, A. E. & Kakaï, R. G. Seasonal variation of environment and conspecific density-dependence effects on early seedling growth of a tropical tree in semi-arid savannahs. Glob. Ecol. Conserv. 43, e02455 (2023).
Zhu, Y., Comita, L. S., Hubbell, S. P. & Ma, K. P. Conspecific and phylogenetic density-dependent survival differs across life stages in a tropical forest. J. Ecol. 103, 957–966 (2015).
Metz, M. R., Sousa, W. P. & Valencia, R. Widespread density-dependent seedling mortality promotes species coexistence in a highly diverse Amazonian rain forest. Ecology 91, 3675–3685 (2010).
Bachelot, B., Kobe, R. K. & Vriesendorp, C. Negative density dependent mortality varies over time in a wet tropical forest, advantaging rare species, common species, or no species. Oecologia 179, 853–861 (2015).
Wang, W. T. et al. Species abundance is jointly determined by functional traits and negative density dependence in a subtropical forest in southern China. J. Plant Ecol. 14, 491–503 (2021).
Getzin, S., Wiegand, T., Wiegand, K. & He, F. L. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 96, 807–820 (2008).
Liu, P. C. et al. Competition and facilitation co-regulate the spatial patterns of boreal tree species in Kanas of Xinjiang, northwest China. For. Ecol. Manag. 467, 118167 (2020).
Zhu, Y., Mi, X. C., Ren, H. B. & Ma, K. P. Density dependence is prevalent in a heterogeneous subtropical forest. Oikos 119, 109–119 (2010).
Johnson, D. J., Beaulieu, W. T., Bever, J. D. & Clay, K. Conspecific negative density dependence and forest diversity. Science 336, 904–907 (2012).
Seiwa, K., Masaka, K., Konno, M. & Iwamoto, S. Role of seed size and relative abundance in conspecific negative distance dependent seedling mortality for eight tree species in a temperate forest. For. Ecol. Manag. 453, 117537 (2019).
Mangan, S. A. et al. Negative plant-soil feedback predicts tree species relative abundance in a tropical forest. Nature 466, 752–755 (2010).
Schroeder, J. W., Dobson, A., Mangan, S. A., Petticord, D. F. & Herre, E. A. Mutualist and pathogen traits interact to affect plant community structure in a spatially explicit model. Nat. Commun. 11, 2204 (2020).
Webb, C. O., Gilbert, G. S. & Donoghue, M. J. Phylogenetic-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 87, 123–131 (2006).
Johnson, D. J. et al. Conspecific negative density-dependent mortality and the structure of temperate forests. Ecology 95, 2493–2503 (2014).
Huang, T. H., Huang, C. L., Lin, Y. C. & Sun, I. F. Seedling survival simultaneously determined by conspecific, heterospecific, and phylogenetically related neighbors and habitat heterogeneity in a subtropical forest in Taiwan. Ecol. Evol. 12, e8525 (2022).
Paine, C. E. T. et al. Phylogenetic density dependence and environmental filtering predict seedling mortality in a tropical forest. Ecol. Lett. 15, 34–41 (2012).
Liu, X. B. et al. Experimental evidence for a phylogenetic Janzen-Connell effect in a subtropical forest. Ecol. Lett. 15, 111–118 (2012).
Comita, L. S. et al. Changes in phylogenetic community structure of the seedling layer following hurricane disturbance in a human-impacted tropical forest. Forests 9, 556–572 (2018).
Pu, X. C. & Jin, G. Z. Conspecific and phylogenetic density-dependent survival differs across life stages in two temperate old-growth forests in Northeast China. For. Ecol. Manag. 424, 95–104 (2018).
Lebrija-Trejos, E., Wright, S. J., Hernández, A. & Reich, P. B. Does relatedness matter? Phylogenetic density-dependent survival of seedlings in a tropical forest. Ecology 95, 940–951 (2014).
Wu, J. J. et al. How does habitat filtering affect the detection of conspecific and phylogenetic density dependence?. Ecology 97, 1182–1193 (2016).
Cao, J. et al. Seedling density dependence regulated by population density and habitat filtering: Evidence from a mixed primary broad-leaved Korean pine forest in Northeastern China. Ann. For. Sci. 75, 25. https://doi.org/10.1007/s13595-018-0706-x (2018).
Walck, J. L., Hidayati, S. N., Dixon, K. W., Thompson, K. & Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 17, 2145–2161 (2011).
Coomes, D. A. & Grubb, P. J. Impacts of root competition in forests and woodlands: A theoretical framework and review of experiments. Ecol. Monogr. 70, 171–207 (2000).
Furey, G. N. & Tilman, D. Plant biodiversity and the regeneration of soil fertility. PNAS 118, e2111321118 (2021).
Krishnadas, M. et al. Seasonal drought regulates species distributions and assembly of tree communities across a tropical wet forest region. Glob. Ecol. Biogeogr. 30, 1849–1962 (2021).
Kuang, X. et al. Conspecific density dependence and community structure: insights from 11 years of monitoring in an old-growth temperate forest in Northeast China. Ecol. Evol. 7, 5191–5200 (2017).
Chai, Y. et al. Tree species richness and abundance in a four-ha plot in a mid-montane humid evergreen broad-leaved forest in Gaoligong Mountains of Yunnan province, China. J. Food Agric. Environ. 12, 1128–1134 (2014).
Frazer, G. W., Canham, C. D. & Lertzman, K. P. Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, user manual and program documentation. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York (1999).
Norden, N. et al. Interspecific variation in seedling responses to seed limitation and habitat conditions for 14 Neotropical woody species. J. Ecol. 97, 186–197 (2009).
Harms, K. E., Condit, R., Hubbell, S. P. & Foster, R. B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 89, 947–959 (2001).
Legendre, P. et al. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 90, 663–674 (2009).
John, R. et al. Soil nutrients influence spatial distributions of tropical tree species. PNAS 104, 864–869 (2007).
Jin, Y. & Qian, H. V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 44, 335–339 (2022).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach 2nd edn. (Springer, 2002).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024).
Martini, F. On the definition of tree seedlings. Plant Ecol. 225, 75–79 (2024).
Lebrija-Trejos, E., Reich, P. B., Hernández, A. & Wright, S. J. Species with greater seed mass are more tolerant of conspecific neighbours: A key driver of early survival and future abundances in a tropical forest. Ecol. Lett. 19, 1071–1080 (2016).
Kunstler, G. et al. Plant functional traits have globally consistent effects on competition. Nature 529, 204–207 (2016).
Jiang, Y., Wang, Z. H., Chu, C. J., Kembel, S. W. & He, F. L. Phylogenetic dependence of plant-soil feedback promotes rare species in a subtropical forest. J. Ecol. 110, 1237–1246 (2022).
Chen, L. et al. Forest tree neighborhoods are structured more by negative conspecific density dependence than by interactions among closely related species. Ecography 41, 1114–1123 (2018).
Queenborough, S. A., Burslem, D. F., Garwood, N. C. & Valencia, R. Taxonomic scale-dependence of habitat niche partitioning and biotic neighbourhood on survival of tropical tree seedlings. Proc. R. Soc. B 276, 4197–4205 (2009).
Shuai, F. M., Wang, Y. F. & Yu, S. X. Density dependence in forests is stronger in tropical and subtropical climates among closely related species. Ecography 37, 659–669 (2014).
Liu, H. K. et al. Disentangling the effects of biotic neighbors and habitat heterogeneity on seedling survival in a deciduous broad-leaved forest. For. Ecol. Manag. 519, 120339 (2022).
Liu, H. K. et al. Habitat heterogeneity and biotic interactions mediate climate influences on seedling survival in a temperate forest. For. Ecosyst. 10, 100138 (2023).
Lin, F. et al. The contribution of understory light availability and biotic neighborhood to seedling survival in secondary versus old-growth temperate forest. Plant Ecol. 215, 795–807 (2014).
Holík, J., Janík, D. & Adam, D. Light can modify density-dependent seedling mortality in a temperate forest. J. Veg. Sci. 32, e12992 (2021).
Krause, G. H., Koroleva, O. Y., Dalling, J. W. & Winter, K. Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ. 24, 1345–1352 (2001).
Uriarte, M., Muscarella, R. & Zimmerman, J. K. Environmental heterogeneity and biotic interactions mediate climate impacts on tropical forest regeneration. Glob. Change Biol. 24, 692–704 (2018).
Wright, S. J. et al. Functional traits and the growth mortality trade-off in tropical trees. Ecology 91, 3664–3674 (2010).
Jurinitz, C. F., de Oliveira, A. A. & Bruna, E. M. Abiotic and biotic influences on early-stage survival in two shade-tolerant tree species in Brazil’s Atlantic forest. Biotropica 45, 728–736 (2013).
Metz, M. R. Does habitat specialization by seedlings contribute to the high diversity of a lowland rain forest?. J. Ecol. 100, 969–979 (2012).
Yin, J. et al. Seasonal variations in the mechanisms of understory herb diversity in a temperate forest in Northeast China. Ecol. Indic. 160, 111827 (2024).
Comita, L. S. et al. Abiotic and biotic drivers of seedling survival in a hurricane-impacted tropical forest. J. Ecol. 97, 1346–1359 (2009).
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 31901102).
Author information
Authors and Affiliations
Contributions
LW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing—original draft, Writing—review & editing. YC: Investigation. JW: Funding acquisition, Project administration, Resources, Supervision, Writing—review & editing. XY: Investigation. JS: Investigation. ZF: Investigation. FC: Investigation. LH: Investigation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Chai, Y., Wu, J. et al. Phylogenetic relatedness and habitat affect seedling abundance of a mid-montane humid evergreen broad-leaved forest in the Gaoligong Mountains, Southwestern China. Sci Rep 14, 21019 (2024). https://doi.org/10.1038/s41598-024-72374-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72374-3