Abstract
Undifferentiated pleomorphic sarcoma (UPS) is the largest subgroup of soft tissue sarcomas. This study determined the value of perfusion-weighted imaging with dynamic-contrast-enhancement (PWI/DCE) morphologic, qualitative, and semiquantitative features for predicting UPS pathology-assessed treatment effect (PATE). This retrospective study included 33 surgically excised extremity UPS patients with pre-surgical MRI. Volumetric tumor segmentation from PWI/DCE was obtained at Baseline (BL), Post-Chemotherapy (PC), and Post-Radiation Therapy (PRT). The surgical specimens’ PATE separated cases into Responders (R) (≥ 90%, 16 patients), Partial-Responders (PR) (89 − 31%, 10 patients), and Non-Responders (NR) (≤ 30%, seven patients). Seven semiquantitative kinetic parameters and maps were extracted from time-intensity curves (TICs), and 107 radiomic features were derived. Statistical analyses compared R vs. PR/NR. At PRT, 79% of R displayed a “Capsular” morphology (P = 1.49 × 10-7), and 100% demonstrated a TIC-type II (P = 8.32 × 10-7). 80% of PR showed “Unipolar” morphology (P = 1.03 × 10-5), and 60% expressed a TIC-type V (P = 0.06). Semiquantitative wash-in rate (WiR) was able to separate R vs. PR/NR (P = 0.0078). The WiR radiomics displayed significant differences in the first_order_10 percentile (P = 0.0178) comparing R vs. PR/NR at PRT. The PWI/DCE TIC-type II curve, low WiR, and “Capsular” enhancement represent PRT patterns typically observed in successfully treated UPS and demonstrate potential for UPS treatment response assessment.
Similar content being viewed by others
Background
Soft tissue sarcomas (STS) are a rare and diverse group of malignant tumors with several subgroups, including adipocytic (lipomatous), fibroblastic/myofibroblastic, fibrohistiocytic, vascular, pericytic (perivascular), skeletal muscle, smooth muscle, gastrointestinal stromal, chondro-osseous, peripheral nerve sheath, and undifferentiated small round cell sarcomas1. Undifferentiated Pleomorphic Sarcoma (UPS) is the largest subgroup of STS and accounts for approximately 20% of all cases2. It is considered a diagnosis of exclusion when all identifiable lines of histopathologic differentiation have been excluded. These tumors can occur in any part of the body at any age but are usually more common in older patients3. It represents an archetypical cellular STS imaging tumor model that is significantly different from myxoid, chondroid, lipomatous, and fibrous-rich STS sub-types3.
Treatment-induced changes in STS
After undergoing treatment, STS may exhibit a decreased cell count with degenerative changes, necrosis (treatment-induced necrosis (TIN)), and hyalinization (treatment-induced fibrosis, (TIF)). Additionally, treatment can lead to intratumoral hemorrhage ((treatment-induced hemorrhage or (TIH)), deposition of hemosiderin, and recruitment of foamy macrophages. Evaluating the response of STS to systemic or radiation therapy can be difficult because viable tumor cells within the lesion may be replaced with benign tissue such as TIF and granulation instead of undergoing liquefaction or hemorrhagic necrosis. This makes it difficult to determine the degree of treatment response, as the tumor may have decreased, increased, or remained the same overall size. Established response criteria, such as the Response Evaluation Criteria in Solid Tumors (RECIST)4, require a significant reduction in tumor size to be considered a positive response. Some STS patients receive systemic therapy with anthracyclines and alkylating agents, and radiation therapy is commonly administered before or after surgery. Early indicators of tumor susceptibility to treatment can help identify effective agents and discontinue ineffective ones. Pathology-Assessed Treatment Effect (PATE) is a robust prognostic indicator in some sarcomas5,6. Values ranging from 75 to 95% PATE have been published to define responders. These studies have consistently shown higher 5- and 10-year survival rates and lower rates of tumor recurrences among responders with a high PATE5. Increased TIN after neoadjuvant therapies may lead to higher rates of successful radical resection, lower relapse rates, and facilitate limb salvage in tumors that were deemed unresectable before treatment because of neurovascular encasement or truncal involvement6,7.
The role of conventional and functional magnetic resonance imaging (MRI)
Limitations of size-based response criteria have encouraged the pursuit of additional metrics capable of reliably assessing tumor response and providing early prognostic markers. Conventional MRI focuses on the size and anatomic features of the lesions and has demonstrated high diagnostic accuracy, sensitivity, and specificity in detecting recurrent STS8. Functional MRI adds a multiparametric assessment of tumor biology, including cellularity and vascularity, by utilizing sequences such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced imaging (DCE)9.
Perfusion-weighted imaging with dynamic contrast enhancement (PWI/DCE)
Dynamic contrast-enhanced MRI is a diagnostic imaging technique that involves performing a series of rapid tumor scans following the administration of contrast material and assessing tissue perfusion kinetics over time2. The analysis of DCE-MR imaging can be done in a qualitative, semiquantitative, or quantitative manner2,9. This technique can identify viable malignant tumor tissue by demonstrating distinct perfusion patterns. Malignant tissues usually display rapid early enhancement compared to benign tumors10. Although some evidence suggests that DCE MRI can differentiate between malignant and benign tumors11,12, there is contradictory information due to overlapping PWI/DCE patterns between some benign and malignant lesions. PWI/DCE is also helpful in navigating biopsies and identifying histologic changes after therapy. DCE combined with DWI has increased diagnostic accuracy for identifying therapeutic responses compared to conventional, anatomic post-contrast imaging alone. Advanced imaging can identify post-therapeutic histologic changes, such as the development of granulation tissue and fibrosis, that could otherwise be indistinguishable from the viable tumor on conventional imaging12,13.
Purpose and novelty of the study
We aim to demonstrate that several clinically available vascularity-dependent PWI/DCE morphologic, qualitative, and semiquantitative features that independently and combined in a model, can correlate with and predict the degree of PATE in surgical specimens, thereby reliably assessing overall therapeutic efficacy and outperforming traditional size-based treatment assessment metrics, such as RECIST, World Health Organization (WHO)1, and volumetric measurements. To our knowledge, this study represents the first demonstration of a PWI/DCE-derived features-based and clinically usable model for predicting treatment response in extremity UPS patients arising from a highly specialized national reference sarcoma center.
Materials and methods
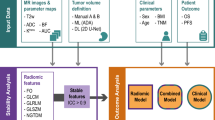
Figure 1 presents a general overview of the methodology performed in this study.
Summary of the methodology of this study. First, PWI/DCE images were acquired from UPS patients at baseline (BL), post-chemotherapy (PC), and post-radiation therapy (PRT). Second, tumors were manually contoured in three dimensions by an MD-trained research assistant, generating tumor volume of interest (VOI) segmentations. Third, features were extracted from the tumor VOIs, including morphologic patterns, time-intensity curves, semiquantitative parameters, and radiomics. Finally, statistical analyses were performed on the extracted features comparing responders vs. partial/non-responders.
General disclosures and statements
-
Methodology: All included data collection, management, and processing methods were performed in accordance with all the institutional and generally accepted relevant clinical and research guidelines and regulations.
-
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
-
IRB and waiver of consent: Due to the study’s retrospective nature, The UT MD Anderson Cancer Center Institutional Review Board waived the need to obtain informed consent (IRB identifier PA16-0857 Protocol Name: “Utility of imaging of bone and soft tissue tumors and disease and treatment-related changes for diagnosis, prognosis, treatment response, and outcome”).
Study design
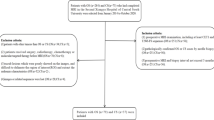
The study included the analysis of 5,135 MRI scans performed for extremity STS in our institutional 29-magnet fleet (consisting of 1.5T and 3.0T scanners from two manufacturers) between February 2021 and May 2023. Within this time range, 643 UPS studies were completed, including pre-operative primary tumor assessment and post-operative surveillance studies. We excluded all post-operative surveillance and primary myxoid-UPS cases, yielding 33 extremity cases. All 33 cases underwent surgical resection preceded by at least one pre-operative MRI study within the defined time range mentioned above. As our study aims to evaluate the effectiveness of PWI/DCE metrics in characterizing treatment response, we established advanced/functional imaging MRI protocols to evaluate STS of the extremities. PWI/DCE metrics were compared at different time points against the standard references, including: (1) Pathology-assessed treatment effect (PATE) on surgical specimens, and (2) conventional tumor treatment size-based metrics such as RECIST, WHO, and volume.
UPS patient population
This retrospective study included 33 patients ranging in age from 36 to 85 years. Twenty were male (61%) and 13 were female (39%). The average age of the patients was 64 years (Table 1). Out of the 33 patients, 10 underwent BL MRI studies outside our institution without PWI/DCE, while their subsequent PC and PRT MRIs were obtained at our institution. These 10 patients were grouped under the “Conventional Imaging” category. The remaining 23 patients had a complete set of advanced MRI studies, including BL, PC, and PRT, performed at our institution. This latter group was classified as the “Functional Imaging” group. Eighteen patients did not receive neoadjuvant chemotherapy, and 3 did not receive neoadjuvant radiotherapy before surgical excision. Therefore, they were excluded from the PC and PRT analyses, respectively. Overall, 23 patients were included in the BL group, 15 in the PC group, and 30 in the PRT group (Table 1).
Reference standard PATE
The 33 patients were categorized into three groups based on the surgical specimen’s PATE percentage. Tumors demonstrating equal to or greater than 90% PATE were classified as responders (R), tumors with a PATE in the 31–89% range were labeled as partial responders (PR), and tumors with a PATE of 30% or less were considered non-responders (NR). The three groups were compared, including 16 subjects in the R group, 10 in the PR group, and 7 in the NR group (Table 1). The combined PR/NR group had 17 subjects.
Reference standard RECIST, WHO, and volume
All 33 patients were analyzed for RECIST, WHO, and volumetric measurements. Maximum diameter, area, and volume were measured at PC and PRT with respect to BL, comparing responders and partial/non-responders. RECIST, WHO, and volume criteria for partial response (PR) threshold were set at 30%, 50%, and 50% decrease, respectively. Progressive disease (PD) threshold was set at 20%, 25%, and 25% increase, respectively14. When compared with BL, all R, and PR/NR patients at PRT displayed size changes that would fall within the range of stability, namely between + 20% and − 30% for RECIST and + 25% and − 50% for WHO and volume criteria. All R and PR/NR cases demonstrated pseudo-progression at PC, crossing the threshold of + 20% for RECIST and + 25% for WHO and volume assessments. (Fig. 2).
MR image acquisition
Standardized PWI/DCE sequence2 parameters were tailored according to MRI vendor and field strength. Following a power-injector delivered intravenous dose of a gadolinium contrast agent at a 3 cc/sec rate, a fast volumetric T1-shortening sensitive three-dimensional (3D) gradient echo acquisition lasting 4 min was performed, with a temporal resolution of 7–12 s. Sequence parameters of the dynamic sequence were: FOV = 38–42 cm, matrix = 256 × 160 (in-plane resolution 1.5–1.65 mm), TR = 3.4-3.8ms, TE = 1.3-1.6ms, flip angle = 12, slice thickness = 4 mm. During the pre-operative treatment, multiple MR studies were acquired for each patient and compiled into three classes: Baseline (BL, pre-therapy), post-systemic chemotherapy (PC), and pre-operative/post-radiation (PRT) time points. For STS, we conducted one pre-therapy baseline study, one to three studies during systemic therapy, and at least one post-radiation study one to two months after radiation therapy and immediately before surgical resection. While the BL is a single time point, multiple time points were classified as PC or PRT for each patient.
Imaging storage and post-processing
The acquired MRI data sets were transferred to the institutional Picture Archiving and Communication System (PACS) (IntelliSpace PACS, Philips, Amsterdam, Netherlands). MR images were retrieved from PACS, and each tumor was manually contoured in three dimensions by an MD-trained research assistant to create tumor volume of interest (VOI) segmentation. Conventional tumor size metrics for all three x, y, and z planes were registered for all time points and used to estimate RECIST, WHO, and volumetric assessment metrics. MIM version 7.1.4 (MIM Software Inc., Cleveland, USA) was used for post-processing to outline, process, and generate VOIs from PWI/DCE images. The segmented tumor volume files were subsequently exported to an institutional network storage drive as DICOM RT-Struct files for further analysis. An in-house developed Cancer Radiomic and Perfusion Imaging (CARPI) automated framework15, capable of intensity histogram-based first-order and high-order radiomic feature extraction from advanced MRI sequences, processed all the collected RT-Struct files containing segmented VOI data. CARPI also extracted 7 semiquantitative kinetic parameters and maps: Wash-in rate (WiR), wash-out-rate (WoR), peak enhancement (PE), the wash-in area under the curve (WiAUC), time-to-peak (TTP), wash-out area under the curve (WoAUC), and total area under the curve (AUC) from TICs15,16. One-hundred and seven radiomic features were then derived, including shape (14 features), first-order statistics (18 features), and texture (75 features), which were automatically recorded in a database management system (DBMS) based on PostgreSQL (The PostgreSQL Global Development Group, Berkeley, USA). Statistical analyses compared responders vs. partial/non-responders.
Qualitative PWI/DCE analysis
Qualitative analysis refers to the subjective analysis of the time-signal intensity curve (TIC), which visually represents the net contrast resulting from inflow and clearance during the passage of the gadolinium bolus over time. In PWI/DCE, the TIC usually displays three stages of perfusion: (1) upslope, which reflects contrast wash-in; (2) plateau, which represents the steady state of contrast within the interstitial fluid but may not be visible in all lesions; and (3) downslope, which reflects contrast wash-out as gadolinium passes out of the tissues under examination. The TIC shape was subjectively assigned to one of five curve types based on their morphology by an experienced radiologist: Type I, II, III, IV, and V. Lesions were categorized into TIC III, IV, and V when displaying a rapid wash-in/early upstroke curve9,13.
Semiquantitative PWI/DCE analysis
The semiquantitative analysis measures empirical perfusion parameters derived from the TIC morphology. It includes maximum/peak enhancement (Emax, PE - curve peak), rate of enhancement/wash-in rate (Eslope/WiR -upslope gradient), area under the curve (AUC), and time to peak enhancement (TTP -time between base of upslope and Emax). Additional semiquantitative metrics include wash-out rate (WoR), wash-out area under the curve (WoAUC), and wash-in area under the curve (WiAUC)17,18,19,20.
Quantitative PWI/DCE analysis
In the field of musculoskeletal (MSK) imaging, quantitative analysis involves pharmacokinetic modeling, which requires arterial input function to measure gadolinium concentration changes over time. The most common pharmacokinetic model used in MSK imaging is the extended Tofts model, which generates four perfusion parameters. These parameters include the transfer constant (Ktrans), exchange rate constant (Kep), extravascular extracellular space fractional volume (Ve), and plasma fractional volume (VP)21,22,23. Given that most quantitative analyses occur primarily in the research setting, the relatively high number of available overlapping publications, and the limited availability of FDA-approved clinically applicable vendor-neutral solutions for MSK analysis, we decided to focus mainly on assessing the value of morphologic, qualitative TIC-related and semiquantitative mapping-derived features that can be easily assessable in the daily clinical setup.
Statistical analysis
The PWI/DCE morphology patterns and TIC curve types in R, PR, and NR were compared using one-way chi-squared tests. The PWI/DCE semiquantitative parameters and radiomic features in R vs. PR/NR were compared using two-tailed non-parametric Wilcoxon rank-sum tests. Finally, receiver operating characteristic (ROC) analysis of the most relevant PWI/DCE features discriminating R from PR/NR was performed. All statistical analyses were implemented in Python 3.10.13 using the SciPy library version 1.12.0 and the Scikit-Learn library version 1.4.1. Statistical significance was assessed at 5% (P < 0.05).
Results
Table 2 summarizes the results from the statistical analyses on the PWI/DCE-derived features performed in this study.
PWI/DCE morphologic patterns observed after radiation therapy (PRT)
PWI/DCE morphologic patterns observed at PRT were categorized into six groups (Fig. 3): “Capsular,” “Unipolar,” “Bipolar,” “Non-Nodular,” “Semi-Lunar,” and “Solid Enhancement.”
Representative PWI/DCE morphologic patterns from UPS patients at PRT classified by PATE are presented in Fig. 4.
PWI/DCE morphology patterns are classified by pathology-assessed treatment effect (PATE). Representative PWI/DCE morphology patterns from UPS patients with > 90% PATE (top panels), 31–89% PATE (middle panels) showing unipolar enhancement (red arrows), and 5–30% PATE (bottom panels) showing bipolar enhancement (red arrows).
In the responder’s group (n = 14) (R), 79% (n = 11) displayed a “Capsular” pattern (P = 1.49 × 10−7), 14% (n = 2) had a “Unipolar,” and 7% (n = 1) had a “Non-Nodular” pattern (Fig. 5). In the partial responder’s group (n = 10) (PR), 80% (n = 8) displayed a “Unipolar” morphology (P = 1.03 × 10−5), and 20% (n = 2) had a “Bipolar” pattern. In the non-responder’s group (n = 6) (NR), 50% (n = 3) displayed a “Bipolar” pattern (P = 0.1562), 33% (n = 2) a “Semi-Lunar” pattern, and 17% (n = 1) a “Solid-Enhancement” pattern. A significant statistical association was observed between the PRT “Capsular” enhancement pattern and the responder’s group (R) (P = 1.5 × 10−7) and between partial-responders (PR) and the “Unipolar” pattern (P = 1.0 × 10−5) (Fig. 5).
Qualitative analysis and time-intensity curves (TICs)
Time-intensity curves were classified as type I, II, III, IV, and V. Figure 6 summarizes the statistical association between the observed TIC types and UPS patient response.
PWI/DCE time-intensity curve (TIC) types at baseline, post-chemotherapy, and post-radiation are organized by response. At post-radiation/pre-surgical, 100% of good responders demonstrated a TIC-type 2, 60% of partial responders expressed a TIC-type 5, and 83.3% of non-responders expressed a TIC-type 5. All p-values resulted from one-way chi-squared tests.
At BL, 50% (n = 6) of responders (R) expressed a TIC-type V (P = 0.26), 86% (n = 6) of partial responders (PR) expressed a TIC-type V (P = 0.0027), and 50% (n = 2) of non-responders (NR) demonstrated a TIC-type IV (P = 0.57). At PC, 87% (n = 7) of responders expressed a TIC-type II (P = 0.0046), 60% (n = 3) of partial responders expressed a TIC-type V (P = 0.25), and 50% (n = 1) of non-responders demonstrated a TIC-type V (P = 0.60). Finally, at PRT, 100% (n = 14) of responders (R) showed a TIC-type II (P = 8.3 × 10-7), 60% (n = 6) of partial responders expressed a TIC-type V (P = 0.06), and 83% (n = 5) of non-responders expressed a TIC-type V (P = 0.03).
Combined qualitative and morphologic patterns
11/14 (79%) Responders at PRT presented a capsular pattern and a TIC Type 2, while only 2/8 (25%) presented both features at PC.
Semiquantitative perfusion kinetic parameters and the importance of WiR
Statistical significance for wash-in rate (WiR; P = 0.0078) and wash-out rate (WoR; P = 0.023) was observed, separating responders vs. partial/non-responders (Figs. 7 and 8).
Boxplots of seven semiquantitative perfusion kinetic parameters comparing responders vs. partial/non-responders at post-radiation (PRT). P-values resulted from two-tailed non-parametric Wilcoxon rank-sum tests. The wash-in rate (WiR) decreased 34% in responders relative to partial/non-responders, showing the highest statistical association with response. a.u.: Arbitrary Units.
Comparison of seven PWI/DCE kinetic parameter maps from a responder (A) vs. a non-responder (B) at post-radiation (PRT). Table (C) summarizes the p-values resulting from two-tailed non-parametric Wilcoxon rank-sum tests comparing these parameters from the entire population of responders (n = 14) vs. partial/non-responders (n = 16) at PRT. TTP: Time to Peak; PE: Peak Enhancement; WiR: Wash-In Rate; WoR: Wash-Out Rate; WiAUC: Wash-In Area Under the Curve; WoAUC: Wash-Out Area Under the Curve; AUC: Area Under the Curve.
On average, WiR decreased by 34% in responders relative to partial/non-responders. In responders (R), the WiR significantly reduced by 47% at PC (P = 0.0031) and by 40% at PRT (P = 0.0055) with respect to baseline (Fig. 8). No significant differences were observed in partial/non-responders at PC or PRT with respect to baseline. The PR/NR only showed a 5% WiR reduction at PRT compared to BL (Fig. 9).
First-order radiomics
The radiomic analysis of the WiR maps displayed significant differences in first order_10th-percentile (P = 0.018) between responders and partial/non-responders (Fig. 10).
WiR map radiomics scatter plot (A) showing means with 95% confidence intervals and boxplots (B) of top 10 radiomic features comparing responders (R) vs. partial/non-responders (PR/NR) at post-radiation (PRT). All P-values resulted from two-tailed non-parametric Wilcoxon rank-sum tests. s.v.: Standardized Value.
Response model
ROC curves were generated for capsular pattern, TIC type 2, and WiR to quantify the classification performance in responders (n = 14) vs. partial/non-responders (n = 16) at PRT (Figs. 10 and 11). The model combining the capsular pattern and TIC type 2 yielded the highest area under the curve (AUC = 0.97) compared to the other models based solely on the capsular pattern (AUC = 0.89), TIC type 2 (AUC = 0.88), and WiR (AUC = 0.79).
Discussion
RECIST, WHO, and volumetric treatment assessment. The problem of pseudoprogression and stability
STS of low- and high-grades exhibit varying pathological responses to treatment, such as hyaline fibrosis, necrosis, and granulation tissue24. Pseudoprogression is a phenomenon where the tumor enlarges, followed by a subsequent decrease in size without any changes in therapy or evidence of progression on later imaging or pathological assessments13,25. It was previously thought to occur in up to 10% of STS responders and 30% of patients with an intermediate response. Tumor volume may increase up to 40% after radiation, and certain cases may show an increase in volume despite showing 95% tumor necrosis19,26. Therefore, an increase or lack in a reduction in tumor size during pre-operative radiotherapy for STS does not directly indicate inferior local tumor control, compromised survival, or overall lack of response to pre-operative radiotherapy27. Our study results show a high prevalence of pseudoprogression at PC and universal stability at PRT in both responders and partial/non-responders (Fig. 2), indicating that RECIST, WHO, and volumetric measurements are unreliable for predicting histopathological effects, differentiating between responders and partial/non-responders, and assessing overall therapeutic effectiveness.
Vascularity as a biomarker and the role of PWI/DCE in assessing STS
The single most useful oncological application of perfusion imaging is to determine tumor response to neoadjuvant chemotherapy and radiotherapy because sarcomas (particularly bone sarcomas) may not decrease and may even increase in size after effective treatment due to intra-tumoral hemorrhage, edema, or necrosis, rendering tumor size a poor indicator of tumor response28. Successful treatment response can be predicted with high specificity if no greater than 5% of the STS volume displays early arterial enhancement6. In summary, the PWI/DCE provides additional and valuable information and typically adds no more than 4–5 min in scan time to the MRI exam. As a result, it has become a standard, integral component of routine extremity tumor MRI protocols at our institution since 20212,13.
PWI/DCE features in UPS treatment assessment
A combination of morphologic, qualitative, and semiquantitative PWI/DCE features has helped differentiate responders from partial/non-responders (Figs. 5, 6 and 7). Our results have shown that a “Capsular” pattern is a typical PWI/DCE morphologic feature in the responder group (P = 1.49 × 10−7). A clinical radiologist can readily recognize this pattern without the need for post-processing software as is required for first- and high-order radiomic feature extraction. However, suboptimal responders tend to display a “Unipolar” or “Bipolar” pattern at PRT. The finding of the described patterns appears to follow a set pattern where “capsular” is the latest pattern of response, following “unipolar” and “bipolar,” respectively. This progression may be partly linked to the presence of an underlying “sarcoma organ model” with a dominant vascular supply at the superior pole, a secondary vascular supply at the inferior pole, and a likely arterial perfusion gradient from superior to inferior and from peripheral to central/equatorial, as illustrated in Fig. 12.
Sarcoma organ model of undifferentiated pleomorphic sarcoma. On the left, a 3D CT-angiogram (CTA) reconstruction demonstrates a superior pole vascular supply dominance. A secondary, inferior vascular pole is also seen. On the right, a post-radiation PWI/DCE wash-in map in a UPS with < 90% TIN demonstrates arterial perfusion gradient from hypervascular residual solid components in proximity to arterial supply (superior pole/unipolar enhancement) to a central/equatorial necrosis in correlation with hypoxic tumor. The figure was modified with permission from the authors of RF Valenzuela et al.30.
PWI/DCE qualitative, semiquantitative, and quantitative analysis: The PWI/DCE qualitative analysis enables the classification of the measured TIC from the lesion into one of 5 categories (types I-V) based on the observed TIC shape characteristics. TICs III, IV, and V display a rapid wash-in/early upstroke, representing a typical malignant tissue hallmark2. A limitation of this method is that it requires an experienced radiologist to manually and subjectively perform the TIC classification.
Our results demonstrated that the TIC-type II displayed the strongest statistical association with response at both PC (P = 0.0046) and PRT (P = 8.32 × 10−7). At the same time, TIC-types III, IV, and V were associated with ineffective treatment of UPS. The TIC universally transitions to type II following successful therapy with greater than 90% PATE (Figs. 6 and 13). Therefore, after effective treatment, there is significant resolution of the arterial hypervascularity previously represented as the rapid early uptake seen in TICs III, IV, and V, converting into a slow-ascending curve without an early rapid uptake characterized by a TIC II. This transition is highly associated with successful therapy at PRT in responders.
Pre-therapeutic and post-therapeutic time-intensity curves of cellular STS/Non-Myxoid UPS. The three left panels show type III, IV, or V kinetic curves associated with untreated UPS. Following successful therapy with greater than 90% necrosis, the kinetic curve transitions to type II (right panel). Note: All P-values resulted from one-way chi-squared tests. The figure was modified with permission from the authors of RF Valenzuela et al.30.
The semiquantitative analysis measures empirical perfusion parameters derived from the TIC morphology, including maximum/peak enhancement (Emax/PE-curve peak), rate of enhancement/wash-in rate (Eslope/WiR-upslope gradient), the area under the curve (AUC), and time to peak enhancement (TTP-time between base of upslope and Emax). Successful treatment response ( > = 90% intra-tumoral necrosis) is likely to be present when there is a 60% reduction in the TIC upslope (Eslope/WiR)26,27,29,30. Conversely, poor responders show a minimal reduction or even an increase in Eslope/WiR27. A limitation of this method is that it requires specific post-processing software to accurately compute the semi-quantitative parameters from the TICs. In our study, 100% of responders displayed a TIC type II, inherently implying the loss of the rapid arterial upstroke observed in TIC types III, IV, and V, seen at BL. WiR reduction was 40% in R vs. 5% in PR/NR at PRT. Our findings also demonstrated that the WiR semiquantitative parameter, which represents a biomarker of arterial flow in active tumors, shows the potential to distinguish between responders and partial/non-responders (Fig. 7) since it significantly decreased by 40% in the responder group (R) at PRT. Moreover, the WiR analysis across treatment (Fig. 9) displayed a significant decrease in responders at both PC and PRT with respect to BL, indicating decreased arterial flow in successfully treated UPS. Representative PWI/DCE semiquantitative maps from two UPS patients at PRT (Fig. 8) demonstrated lower WiR map values for the responder with 90% PATE compared to the non-responder with 15% PATE. The radiomic analysis of the WiR maps (Figs. 9 and 10) highlighted the value of the first-order 10th-percentile intensity histogram-derived feature in discriminating responders from partial/non-responders.
Quantitative analysis involves pharmacokinetic modeling, which requires an arterial input function to measure gadolinium concentration changes over time. Most PWI/DCE quantitative analyses performed in the MSK field occur primarily in research settings, and there is limited availability of FDA-approved clinically applicable vendor-neutral solutions for MSK analysis. Therefore, our study mainly focused on investigating the value of morphologic, qualitative TIC-related, and semiquantitative mapping-derived features that can be easily assessable in the daily clinical setup.
Selection of the best PWI/DCE-based response model
The model combining the capsular pattern and TIC type 2 yielded the highest area under the curve (AUC = 0.97), appearing as a superior UPS response predictor. The use of the capsular pattern (AUC = 0.89) alone is a helpful approach for routine clinical practice as it does not require post-processing software and outperforms the use of TIC type 2 (AUC = 0.88) and WiR (AUC = 0.79) when not used in combination. Recent studies have reported that several MRI-derived features, such as tumor size, necrosis, peritumoral edema, peritumoral contrast enhancement, intratumoral contrast enhancement, and polycyclic/multilobulated tumor configuration, demonstrate the potential to become predictors of STS malignancy grade31,32 and possibly providing helpful information for the development of STS response assessment models.
Study limitations
The present study was limited by the sample size included in the analyses (n = 33). Therefore, future studies with a significantly larger number of UPS patients will be needed to validate these promising results further. In addition, subsequent studies will investigate using PWI/DCE morphological patterns, TICs, semi-quantitative parameters, and radiomics as features in machine learning or deep learning models to automatically assess UPS patient response to treatment. Nevertheless, the proposed PWI/DCE morphologic, semi-quantitative, and radiomic features have the potential to outperform conventional size-based metrics such as RECIST, WHO, and volumetric measurements in predicting treatment-induced histopathologic changes and overall treatment effectiveness for UPS.
Conclusion
Advanced MRI protocols, including perfusion-weighted imaging with dynamic contrast-enhancement (PWI/DCE) imaging, demonstrated the potential to perform significantly better than traditional methods like RECIST, WHO, and volumetric measurements in predicting treatment-induced histopathologic changes and overall treatment effectiveness for undifferentiated pleomorphic sarcoma (UPS). Conventional size-based metrics have comparably low reliability in assessing the effectiveness of treatment, as demonstrated by the high rate of primary tumor post-chemotherapy (PC) pseudoprogression and universal stability at post-radiotherapy (PRT). PWI/DCE can identify viable, enhancing vascularized components of a soft tissue sarcoma, making it an excellent tool for evaluating treatment response. It can provide valuable information for assessing UPS treatment, strongly correlating with the percentage of treatment response (PATE) through vascularity-dependent metrics. PWI/DCE can successfully predict treated UPS with a 90% or higher PATE, potentially outperforming traditional size-based treatment assessment metrics. This is demonstrated by PWI/DCE’s ability to show a TIC-type II, low WiR, and “Capsular”-like enhancement at PRT. The combined use of TIC-type II and “Capsular”-like enhancement at PRT displays the best response-predicting model performance. Unsuccessfully treated UPS may demonstrate “unipolar” or “bipolar” enhancement with TIC types III, IV, or V during pre-surgical evaluation. This information can be used to optimize effective therapy while pausing ineffective treatments earlier in the course of therapy. As the present study conclusions may be limited by the relatively small sample size, larger sample studies may be needed to validate these promising results further.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sbaraglia, M., Bellan, E. & Dei Tos, A. P. The 2020 WHO classification of soft tissue tumours: News and perspectives. Pathologica113(2), 70 (2021).
Valenzuela, R. F. et al. Multiparametric MRI for the assessment of treatment effect and tumor recurrence in soft-tissue sarcoma of the extremities. J. Radiol. Oncol.7(3), 058–065 (2023).
Jo, V. Y. & Fletcher, C. D. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathol.-J. RCPA46(2), 95–104 (2014).
Eisenhauer, E. A., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45(2), 228–247 (2009).
Wardelmann, E. et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC–STBSG) recommendations for pathological examination and reporting. Eur. J. Cancer53, 84–95 (2016).
Soldatos, T. et al. Multiparametric assessment of treatment response in high-grade soft-tissue sarcomas with anatomic and functional MR imaging sequences. Radiology278(3), 831–840 (2016).
Tsagozis, P., Brosjö, O. & Skorpil, M. Preoperative radiotherapy of soft-tissue sarcomas: Surgical and radiologic parameters associated with local control and survival. Clin. Sarcoma Res.8, 1–7 (2018).
Sedaghat, S. et al. Diagnostic value of MRI for detecting recurrent soft-tissue sarcoma in a long-term analysis at a multidisciplinary sarcoma center. BMC Cancer21, 1–8 (2021).
Subhawong, T. K. & Wilky, B. A. Value added: functional MR imaging in management of bone and soft tissue sarcomas. Curr. Opinion Oncol.27(4), 323–331 (2015).
Noebauer-Huhmann, I.-M. et al. Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: Preliminary results. Eur. Radiol.25, 2041–2048 (2015).
Lee, S. K. et al. Multiparametric quantitative analysis of tumor perfusion and diffusion with 3T MRI: Differentiation between benign and malignant soft tissue tumors. Br. J. Radiol.93(1115), 20191035 (2020).
Leplat, C., et al. Contrast-Enhanced 3-T perfusion MRI with quantitative analysis for the characterization of musculoskeletal tumors: Is it worth the trouble? Am. J. Roentgenol. 2018, 1092–1098.
Valenzuela, R. F. et al. Advanced imaging in musculoskeletal oncology: Moving away from RECIST and embracing advanced bone and soft tissue tumor imaging (ABASTI)-part I-tumor response criteria and established functional imaging techniques. In Seminars in Ultrasound, CT and MRI. (Elsevier, 2021).
Subhawong, T.K. et al. MRI volumetrics and image texture analysis in assessing systemic treatment response in extra-abdominal desmoid fibromatosis. Radiology: Imaging Cancer3(4), e210016 (2021).
Sierra, E. D. et al. Cancer radiomic and perfusion imaging automated framework: Validation on musculoskeletal tumors. JCO Clin. Cancer Inform.8, e2300118 (2024).
Liu, D. J. et al. Weight-gain induced changes in renal perfusion assessed by contrast-enhanced ultrasound precede increases in urinary protein excretion suggestive of glomerular and tubular injury and normalize after weight-loss in dogs. PloS one15(4), e0231662 (2020).
Lavini, C. et al. Pixel-by-pixel analysis of DCE MRI curve patterns and an illustration of its application to the imaging of the musculoskeletal system. Magn. Resonance Imaging25(5), 604–612 (2007).
Toms, A. P. et al. Limitations of single slice dynamic contrast enhanced MR in pharmacokinetic modeling of bone sarcomas. Acta Radiol.50(5), 512–520 (2009).
van Rijswijk, C. S. et al. Dynamic contrast-enhanced MR imaging in monitoring response to isolated limb perfusion in high-grade soft tissue sarcoma: Initial results. Eur. Radiol.13, 1849–1858 (2003).
Hawighorst, H. et al. Evaluation of angiogenesis and perfusion of bone marrow lesions: Role of semiquantitative and quantitative dynamic MRI. J. Magn. Resonance Imaging: Off. J. Int. Soc. Magn. Resonance Med.10(3), 286–294 (1999).
Yankeelov, T. E. & Gore, J. C. Dynamic contrast enhanced magnetic resonance imaging in oncology: Theory, data acquisition, analysis, and examples. Curr. Med. Imaging3(2), 91–107 (2007).
Calamante, F. Arterial input function in perfusion MRI: A comprehensive review. Prog. Nucl. Magn. Resonance Spectrosc.74, 1–32 (2013).
Tofts, P. S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Resonance Imaging7(1), 91–101 (1997).
Fields, B. K. et al. Quantitative magnetic resonance imaging (q-MRI) for the assessment of soft-tissue sarcoma treatment response: A narrative case review of technique development. Clin. Imaging63, 83–93 (2020).
Miki, Y. et al. The significance of size change of soft tissue sarcoma during preoperative radiotherapy. Eur. J. Surg. Oncol.36(7), 678–683 (2010).
Guo, J. et al. Assessing vascular effects of adding bevacizumab to neoadjuvant chemotherapy in osteosarcoma using DCE-MRI. Br. J. Cancer113(9), 1282–1288 (2015).
Verstraete, K. & Lang, P. Bone and soft tissue tumors: The role of contrast agents for MR imaging. Eur. J. Radiol.34(3), 229–246 (2000).
Tirkes, T. et al. Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics33(5), 1323–1341 (2013).
Amit, P. et al. Role of dynamic MRI and clinical assessment in predicting histologic response to neoadjuvant chemotherapy in bone sarcomas. Am. J. Clin. Oncol.37(4), 384–390 (2014).
Valenzuela, R. F. et al. Perfusion weighted imaging with dynamic contrast enhancement (PWI/DCE) for the treatment assessment of extremity soft-tissue non-myxoid undifferentiated pleomorphic sarcoma. European Congress of Radiology 2024 (2024).
Sedaghat, S. et al. Configuration of soft-tissue sarcoma on MRI correlates with grade of malignancy. Radiol. Oncol.55(2), 158–163 (2021).
Schmitz, F., Sedaghat, S. Inferring malignancy grade of soft tissue sarcomas from magnetic resonance imaging features: A systematic review. Eur. J. Radiol. 111548 (2024).
Acknowledgements
The John S. Dunn, Sr. Distinguished Chair in Diagnostic Imaging. M.R Evelyn Hudson Foundation Endowed Professorship.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.RV and ED wrote the main manuscript textMK prepared the tumor segmentationED performed all data analysisRV and BA performed all data curation.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Valenzuela, R.F., Duran-Sierra, E., Canjirathinkal, M. et al. Perfusion-weighted imaging with dynamic contrast enhancement (PWI/DCE) morphologic, qualitative, semiquantitative, and radiomics features predicting undifferentiated pleomorphic sarcoma (UPS) treatment response. Sci Rep 14, 21681 (2024). https://doi.org/10.1038/s41598-024-72780-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72780-7
Keywords
This article is cited by
-
Radiologists and treatment response assessment: conventional and functional radiological criteria for predicting therapeutic response in virus-related and non-virus-related cancers
Infectious Agents and Cancer (2025)
-
A follow-up study on the novel use of contrast-enhanced susceptibility-weighted imaging for extremity desmoid fibromatosis response assessment
Scientific Reports (2025)
-
Prognostic factors and the impact of neoadjuvant chemotherapy on the development of metastasis in patients with undifferentiated pleomorphic sarcoma of soft tissue
International Journal of Clinical Oncology (2025)
-
Building a pre-surgical multiparametric-MRI-based morphologic, qualitative, semiquantitative, first and high-order radiomic predictive treatment response model for undifferentiated pleomorphic sarcoma to replace RECIST
Cancer Imaging (2025)