Abstract
In this study, we successfully established a novel gallbladder cancer cell line, designated as GBC-X1, derived from a primary tumor of a gallbladder cancer patient. By comprehensively analyzing the cell line’s phenotype, molecular characteristics, biomarkers, and histological characteristics, we confirmed that GBC-X1 serves as a valuable model for investigating the pathogenesis of gallbladder cancer and developing therapeutic agents. GBC-X1 has been continuously cultured for one year, with over 60 stable passages. Morphologically, GBC-X1 exhibits typical features of epithelial tumors. The population doubling time of GBC-X1 is 32 h. STR analysis validated a high consistency between GBC-X1 and the patient’s primary tumor. Karyotype analysis revealed an abnormal hypertetraploid karyotype for GBC-X1, characterized by representative karyotypes of 98, XXXX del (4) p (12) del (5) p (21) der (10). Under suspension culture conditions, GBC-X1 efficiently forms tumor balls, while subcutaneous inoculation of GBC-X1 cells into NXG mice leads to xenograft formation with a rate of 80%. Drug sensitivity testing demonstrated that GBC-X1 is resistant to oxaliplatin and sensitive to 5-FU, gemcitabine, and paclitaxel. Immunohistochemistry revealed positive expression of CK7, CK19, E-cadherin, MMP-2, CD44, SOX2, and TP53 in GBC-X1 cells, weak positive expression of Vimentin, and a Ki67 positive rate of 35%. Our research highlights GBC-X1 as a novel gallbladder cancer cell line and emphasizes its potential as an effective experimental model for investigating the pathogenesis of gallbladder cancer and drug development.
Similar content being viewed by others
Introduction
Gallbladder cancer commonly originates from the mucosal epithelium of the gallbladder and represents the most prevalent and highly prognostically unfavorable malignant tumor in the biliary system1,2. In 2020, there were approximately 19.3 million newly diagnosed cancer cases worldwide, resulting in nearly 10 million cancer-related deaths. Among these cases, gallbladder cancer accounted for 0.6% of newly diagnosed cancer patients and 0.9% of cancer-related deaths3. Gallbladder cancer has obvious geographical distribution characteristics, among which Bolivia in South America, Chile, northern India, Eastern Europe, and some Southeast Asian countries have higher incidence rate; The incidence rate is the lowest in European origin regions such as the United States, Australia, Canada, the United Kingdom and New Zealand4,5. Gallbladder cancer is one of the few tumors showing global gender differences. The incidence rate of women is three to six times higher than that of men6. The overall incidence rate in China is about 3.82/100,000, of which the incidence rate in Shanghai can reach 7.8/100,000. In recent years, the incidence rate in China has been rising, causing more and more attention7,8. Radical surgery remains the sole approach to achieving long-term survival for gallbladder cancer patients. However, this disease exhibits covert initial onset, marked malignancy, pronounced invasiveness, and a propensity for metastasis and dissemination. Early diagnosis is only possible for a meager 10–20% of patients9,10, with the majority being diagnosed at advanced stages, thus missing the opportunity for surgical intervention. The 5-year survival rate for patients with advanced gallbladder cancer ranges from a mere 5–15% 4,11.
In 1980, Koyama established the world’s first gallbladder cancer cell line, G-415. Since then, researchers from Japan and Korea have subsequently established multiple gallbladder cancer cell lines11,12. These cell lines closely mirror the characteristics of primary tumors, rendering the insights derived from their study more representative of the actual in vivo conditions13. Given the variation in tumor etiology, tumor heterogeneity, and genetic diversity influenced by race14,15,16,17, the establishment of a diverse range of gallbladder cancer cell lines assumes significant importance in facilitating basic research and drug development pertaining to this disease.
In this study, we successfully established a stable gallbladder cancer cell line, designated as GBC-X1, using surgically resected tumor tissue obtained from a gallbladder cancer patient. GBC-X1 represents a valuable experimental model for gallbladder cancer research.
Materials and methods
Source of human tissue
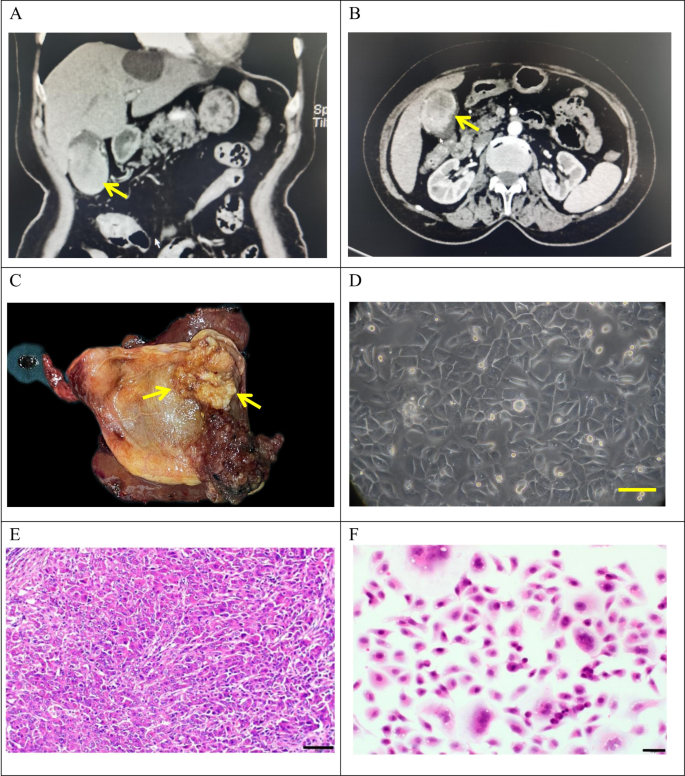
The tumor tissue was obtained from a 72-year-old female patient who underwent radical cholecystectomy at Lanzhou University Second Hospital on May 9, 2022. The patient presented with a gallbladder tumor, had no history of smoking and drinking, and had a past medical history of hepatitis B infection. Preoperative laboratory tests showed CEA levels of 9.9 ng/ml (reference range: 0-5.2 ng/ml), AFP levels of 1.2 U/ml (reference range: 0-5.8 U/ml), and CA19-9 levels of 49.6 U/ml (reference range: 0-35U/ml) (Table 1). Preoperative CT findings confirmed the presence of gallbladder cancer (Fig. 1A-B).
Clinical and pathological profle of GBC-X1.A and B: The patient’s preoperative CT scan results showed a huge mass at the bottom of the gallbladder (arrow). C: On the gross view of the postoperative specimen, a cauliflower-like mass can be seen in the gallbladder (arrow). D: Morphology of GBC-X1 cells under light microscopy ( scale bar = 50 μm). E: The patient’s primary tumor showed moderately to poorly differentiated. The tumor cells displayed irregular arrangements in glandular, tubular, cord-like, and nest-like patterns (scale bar = 50 μm). F: GBC-X1 cells were varying shapes, primarily short fusiform, characterized by enlarged nuclei, prominent nucleoli, limited cytoplasm, and the presence of multinucleated and megakaryocytic cells (scale bar = 50 μm).
A 4 × 4 cm neoplasm was observed in the gallbladder (Fig. 1C). Tissues were collected from the primary lesion for primary culture and subculture.
This study was approved by the Medical Ethics Committee of Lanzhou University Second Hospital (2023 A-381), and the patient provided an informed consent form.
Drugs
Gemcitabine was obtained from Jiangsu Haosen Pharmaceutical Group Co., Ltd., oxaliplatin from Jiangsu Hengrui Pharmaceutical Co., Ltd., 5-FU from Tianjin Jinyao Pharmaceutical Co., Ltd., and paclitaxel from Jiangsu Aosaikang Pharmaceutical Co., Ltd.
NXG mice
Two batches of NXG mice (2 and 3 mice), aged 5–6 weeks, weighing 11 g and 17 g, were used as experimental animals. The mice were purchased from Changzhou Cavens Experimental Animal Co., Ltd. and housed in the SPF level laboratory of the Animal Experimental Center at Lanzhou University. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23℃, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. The animals were fed an autoclaved rodent diet ad libitum. The mice bedding, feed and water were replaced every 2 days. All procedures followed the institutional and national guidelines for the care and use of laboratory animals. Animal health and behavior were monitored every day for 4 weeks. When the maximum diameter of the Xenograft tumor approaches 1.5 cm or when the Xenograft tumor grows for one month, the mice were killed. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection. Death was confirmed by loss of heartbeat, breathing and pupil response.
Animal experiments were reviewed and approved by the Medical Animal Experiment Ethics Committee of Lanzhou University Second Hospital (D2023-318). The all experiments were conducted in accordance with the ARRIVE guidelines and the Guidelines for the Care and Use of Laboratory Animals of China.
The following methods were similar or identical to those employed in previous studies18.
Primary culture, cell purification, and cell line establishment
The tumor tissue was rinsed with sterile PBS (catalog no.10010023, Gibco, USA) 3–5 times, minced as finely as possible, and then digested with type II collagenase (catalog no.17101015, Gibco, USA) and dispase (catalog no.17105041, Invitrogen, USA) in a shaking incubator at 37 ℃. Once the tissue blocks were partially digested, the supernatant was collected, filtered through a 100-mesh filter, and centrifuged at 300 g for 3 min. The supernatant was discarded, and the cell pellet was resuspended in PBS, followed by centrifugation at 300 g for 3 min. The entire culture medium (RPMI-1640(catalog no.c3010-0500, BI, Israel) + 10% FBS (catalog no.04-002-1 A, BI, Israel) + 1% penicillin-streptomycin (catalog no.03-031-1B, BI, Israel)) was added to the pellet and thoroughly mixed. The cell suspension was then evenly seeded into a six-well plate (catalog no.703001, NEST, China). The culture medium was changed after 48 h. Mechanical scraping was performed to remove any fibroblast contamination during the primary culture process. When the cells reached 80% confluency, they were trypsinized and passaged. The cell growth status was regularly monitored under an optical microscope. Starting from the third generation, cells were passaged at a 1:2 ratio and cryopreserved using a serum-free rapid cell cryopreservation solution (catalog no.MF699-01, Mei5 Biotechnology Co.,Ltd, China).
Cell growth curve
GBC-X1 cells (P25) in the logarithmic growth phase were trypsinized and adjusted to a cell density of 6 × 104/ml. Then 0.1 ml of the cell suspension was inoculated into each well of a 96-well plate. CCK-8 (catalog no.ck04, Dojindo, Japan) reagent was added simultaneously and incubated for 2 h for four consecutive days. The UV absorbance value at a wavelength of 450 nm was measured using an enzyme-linked immunosorbent assay reader, and a standard curve was plotted. Population doubling time were computed with several time points, using the “cell calculator++”tool [V. Roth MD, Doubling Time Calculator (2006), https://doubling-time.com/compute_more.php].
Short tandem repeat analysis
GBC-X1 cells (P15) in the logarithmic growth phase were collected after trypsin digestion (catalog no.03-050-1BCS, BI, Israel). STR analysis was performed by Suzhou Genetic Testing Biotechnology Company to establish the correlation between the cells and the primary tumor tissue.
Karyotype analysis
GBC-X1 cells (P42) in the logarithmic growth phase were treated with 0.25 µg/mL colchicine for 6 h at 37 ℃. Metaphase cells were collected, and fixed with methanol acetic acid (3:1). After trypsin digestion, the specimens were stained with Giemsa staining solution. The specimens were analyzed under a microscope. Select well dispersed and moderately stained mitotic phases for karyotype analysis.
Tumor sphere formation assay
Logarithmically growing cells were collected, and 1.5 × 105 cells were inoculated per well into a 6-well plate (Ultra-Low attachment Plate)(catalog no.3471, Corning, USA) after trypsin digestion. The cells were cultured in serum-free RPMI-1640 medium, and tumor sphere formation was assessed on days 7, 10, and 14 after seeding.
Scanning electron microscope
Cells from 30 passages in the logarithmic growth phase were used for the experiments. Cell slides were washed with physiological saline and quickly immersed in 4% glutaraldehyde (product of SPI-CHEM, USA) fixative solution. After rinsing three times with phosphate buffer, the cells were dehydrated using a stepwise gradient of tert-butanol (50%, 70%, 80%, 90%, 100%) for 5 min each. The dried samples were then placed in a JEOL JFD-320 cold dryer, and once the temperature reached room temperature, they were coated with a conductive paste using a JEOL JFCC-160 ion sputterer. The samples were then observed and photographed using a HITACHI Regulus 8100 scanning electron microscope.
Transmission electron microscope
Logarithmically growing GBC-X1 cells (P33) were collected by centrifugation. The cells were fixed in a fixative solution of 2% Paraformaldehyde − 2.5% Glutaraldehyde, followed by flushing with 0.1 mol/L dimethyl Sodium arsenate buffer (pH = 7.4). Subsequently, the cells were further fixed with 1% osmium tetroxide, washed with double-distilled water, dehydrated with a gradient of ethanol to propylene oxide, and embedded in SPI812 resin. Semi-thin sections of 1 µM using the Leica EMUC7 slicing mechanism. After observation and positioning under a light microscope with azure methylene blue staining, 60 nm ultra-thin sections were prepared using Leica EMUC7 slicing mechanism. These sections were stained with uranyl acetate and lead citrate and subjected to observation and image analysis using HITACHI H-7650 Transmission electron microscopy.
Drug sensitivity experiment
Logarithmically growing GBC-X1 cells (P45) were trypsinized and prepared as a single-cell suspension. A total of 10,000 cells per 100 µl were inoculated into each well of a 96-well plate, with six wells repeated in each group. After cells adhered to the wall, the experimental group was treated with different concentrations of anti-tumor drugs, while the control group was treated with corresponding drug solubilizers. After 72 h of drug action, the complete medium was replaced with 100 µl serum-free medium containing 10% (v/v) CCK8. After a 2-hour incubation, the optical density (OD) value at 450 nm was measured.
Transwell chamber migration assay
Cultivated cells in serum-free medium for 1 day in advance. GBC-X1 cells (P40) were collected after trypsin digestion, adjusted the cell concentration to 7.5 × 105/mL, and added 200ul of cell suspension to the upper chamber of Transwell. Added 700 ul of complete culture medium containing 15% FBS to the lower chamber of Transwell. Cultivated them for 48 h. Discarded the culture medium from the upper and lower chambers, gently washed the upper and lower chambers 1–2 times with 1 ml PBS, added 1 ml of 4% paraformaldehyde, fixed for 15 min, and then discarded. Added 1.5 ml of 0.1% crystal violet staining solution to the Transwell chamber, stained for 30 min, and discarded. Washed the Transwell upper chamber three times in ultrapure water, washed the bottom of the upper chamber membrane with a wet cotton ball, and then washed again once. Placed the Transwell upper chamber on a glass slide and observed and taked photos under a microscope.
Transwell chamber invasion assay
Cultivated cells (P40) in serum-free medium for 1 day. Diluted the Matrigel with serum-free medium at a ratio of 1:8 under 4℃ conditions. Took 50 ul of matrigel and added it to the Transwell upper chamber, evenly covering the polycarbonate film. Placed the Transwell upper chamber in a 37℃ cell culture incubator for 30 min, then discarded any excess liquid in the upper chamber. Collected the cells with trypsin, and adjusted the cell concentration to 7.5 × 105/mL, and added 200ul of cell suspension to the upper chamber of Transwell. Added 700 ul of complete culture medium containing 15% FBS to the lower chamber of Transwell. Cultivated them for 48 h. Discarded the culture medium from the upper and lower chambers, gently washed the upper and lower chambers 1–2 times with 1 ml PBS, added 1 ml of 4% paraformaldehyde, fixed for 15 min, and then discarded. Added 1.5 ml of 0.1% crystal violet staining solution to the Transwell chamber, stained for 30 min, and discarded. Washed the Transwell upper chamber three times in ultrapure water, washed the bottom of the upper chamber membrane with a wet cotton ball. Placed the Transwell upper chamber on a glass slide and observed and took photos under a microscope.
Xenograft formation experiment
Logarithmically growing cells (P40 and P45) were trypsinized and adjusted to a cell density of 1 × 107/ml. A total of 0.1 ml of cell suspension was inoculated on the right shoulder of five NXG mice. Tumor growth of the nude mice was observed and recorded the next day. After 4 weeks, the mice were euthanized, and the xenograft growth was observed upon dissection.
Immunohistochemical staining
Cells from the 42nd passage were digested and inoculated onto sterile slides for growth. After 48 h, the slides were washed with PBS, fixed with 4% paraformaldehyde for 15 min, air-dried, and treated with 0.5% Triton X-100 for 20 min. Paraffin sections of xenograft and primary tumors were prepared and baked overnight at 60 ℃. Dewaxing, gradient alcohol hydration, and antigen retrieval were performed using Dako’s Autostainer Link 48 instrument. The activity of peroxidase was blocked by incubating the slides with 3% hydrogen peroxide solution at 37 °C for 15 min. Then, 100 µL of normal goat serum was added dropwise and sealed at 37 ℃ for 15 min. The primary antibodies (CK7(catalog no. kit-0021, MXB, China), CK19(catalog no.kit-0030, MXB, China), Ki67(catalog no.RMA-0731, MXB, China), E-cadherin(catalog no.MAB-0738, MXB, China), Vimentin(catalog no. MAB-0735, MXB, China), MMP-2(catalog no. GB114959-50, Servicebio, China), CD44(catalog no. GB113500-50, Servicebio, China), SOX2(catalog no. GB11249-50, Servicebio, China), TP53(catalog no. GB111740-50, Servicebio, China) ) were incubated at 37 °C for 1 h. Color development was performed using the DAB staining solution kit (Dako), followed by rinsing with running water for 5 min. The slides were restrained with hematoxylin, dehydrated, and made transparent in xylene. Finally, the slides were sealed with neutral resin and observed under a microscope.
Statistical analysis
All statistical analyses were conducted using SPSS 26.0 software. The data were presented as mean ± SD. Student’s t-tests and ANOVA were used for group comparisons. A P-value of < 0.05 was considered statistically significant.
Results
Establishment of GBC-X1 Cell line
A novel gallbladder cancer cell line, designated as GBC-X1, was successfully established through primary culture and subsequent subculture of primary tumor tissue obtained from a gallbladder cancer patient. Under the light microscope, GBC-X1 cells exhibited typical adherence to culture surface and displayed characteristic epithelial cell morphology. The cells are primarily polygonal, with prominent nuclei and nucleoli. The presence of multinucleated cells and megakaryocytes, along with the loss of contact inhibition, was observed (Fig. 1D). The cell line maintained stable morphology and remained unchanged after repeated freezing and revival. The postoperative pathological diagnosis of the patient’s primary tumor confirmed moderately to poorly differentiated GBC. The tumor cells displayed irregular arrangements in glandular, tubular, cord-like, and nest-like patterns (Fig. 1E). H&E staining revealed varying shapes of GBC-X1 cells, primarily short fusiform, characterized by enlarged nuclei, prominent nucleoli, limited cytoplasm, and the presence of multinucleated and megakaryocytic cells, indicating typical features of malignant tumor cells (Fig. 1F).
Cell growth curve
GBC-X1 cells exhibited rapid proliferation with a short doubling time. The cells demonstrated consistent growth in RPMI-1640 culture medium supplemented with 10% fetal bovine serum. Using the CCK8 method, the population doubling time of GBC-X1 was determined to be 32 h (Fig. 2A).
Short tandem repeat analysis
The DNA typing results confirmed that both the submitted samples originated from the same individual, with a likelihood ratio (LR) of 5.2469 × 1026 (Table 2). This supports the notion that GBC-X1 shares the same origin as the primary tumor tissue and is free from contamination by existing cell lines. Thus, GBC-X1 represents a novel human gallbladder cancer cell line.
Chromosome analysis
Karyotype analysis revealed that GBC-X1 cells predominantly exhibited a hypertetraploid karyotype, characterized by significant variations in chromosome number and morphology. The representative karyotype was identified as 98, XXXX del (4) p (12) del (5) p (21) der (10) (Fig. 2B).
Tumor sphere culture
When GBC-X1 cells were cultured on an ultra-low attachment culture plate, they demonstrated robust proliferation in RPMI-1640 medium without serum and additional growth factors, forming tumorspheres. Over time, the size of spheres gradually increased (Fig. 2C-D).
Scanning electron microscope and transmission electron microscope
Scanning electron microscopy revealed that GBC-X1 cells appeared predominantly spherical and polygonal in shape, displaying abundant Microvilli on the cell surface, uniform morphology, and the presence of filopodia (Fig. 3A-D). Transmission electron microscopy exhibited enlarged nuclei situated close to the nuclear membrane, evident nuclear membrane shrinkage, increased nucleoli, decreased organelle count, significant mitochondrial swelling and vacuolization, decreased mitochondrial cristae, and the presence of desmosome structures between cells (Fig. 4A-D).
GBC-X1 ultrastructure under transmission electron microscopy. A: The nucleus of GBC-X1 is large and close to the nuclear membrane, with visible nuclear membrane shrinkage and an increase in the number of nucleoli (scale bar = 5 μm); B-D: The number of Organelle decreased, mitochondria swelled significantly (arrow), vacuolized, cristae decreased significantly, and desmosome structure was seen between cells (arrow) (scale bar = 1 μm).
Drug sensitivity experiment
Gemcitabine, paclitaxel, 5-FU, and oxaliplatin are commonly used chemotherapy drugs for gallbladder cancer. Our drug sensitivity experiment demonstrated that GBC-X1 cells exhibited resistance to oxaliplatin (IC50 = 10.65 µmol/L), while being sensitive to fluorouracil (IC50 = 0.50 µmol/L), gemcitabine (IC50 = 0.0035 µmol/L), and paclitaxel (IC50 = 0.55 µg/mL) (Fig. 5A-D).
Migration and invasion assay
In the migration assay, when GBC-X1 cells were seeded into the Transwell chamber for 24 h, the number of migrating cells was 9.33 ± 0.47(Fig. 6A), and after 48 h, the number of migrating cells was 195.67 ± 2.49(Fig. 6B); In the invasion assay, when cells were seeded into the Transwell chamber for 24 h, the number of transmembrane cells was 3.33 ± 0.47(Fig. 6C), while after 48 h, the number of transmembrane cells was 186 ± 4.90 (Fig. 6D).
Migration and invasion assay of GBC-X1 cell line. A and B: As time increases, the number of migrating cells of GBC-X1 significantly increases ( scale bar = 200 μm ). C and D: As time increases, the number of transmembrane cells significantly increases, but compared to migratory cells, the number of cells is less ( scale bar = 200 μm ).
Xenograft formation experiment
Assess the in vivo xenograft formation capability of GBC-X1, 1 × 106 cells were subcutaneously inoculated into five NXG mice(Fig. 7A-B). The results confirmed that GBC-X1 can form xenografts after subcutaneous inoculation in NXG mice, with a xenograft formation rate of 80%(Fig. 7C-D). No metastatic lesions were observed in the liver and lungs upon dissection of the mice (Fig. 7E-F). The xenograft sections stained with H&E exhibited irregular glandular-like structures formed by the cells, demonstrating moderate to low differentiation. The histological morphology of the xenograft closely resembled that of the primary tumor, with large, deeply stained nuclei and an increased number of mitotic figures (Fig. 7G). These findings suggest that GBC-X1 cells display vigorous proliferation, consistent with malignant tumor characteristics.
Tumorigenicity of the GBC-X1 cell line in vivo. A-D: GBC-X1 could form transplanted tumors in NXG mice, with a tumor formation rate of 80%. E and F: No metastatic lesions were found in the liver and lungs of the dissected mice after 4 weeks. G: The xenograft exhibited irregular glandular-like structures formed by the cells, demonstrating moderate to low differentiation (scale bar = 50 μm).
Immunohistochemical staining of GBC-X1 cells, xenograft, and primary tumor. GBC-X1 (A1), xenograft (B1), and primary tumor (C1) show positive expression of CK7. GBC-X1 (A2), xenograft (B2), and primary tumor (C2) show positive expression of CK19. GBC-X1 (A3), xenograft (B3), and primary tumor (C3) exhibit positive expression of E-cadherin. GBC-X1 (A4), xenograft (B4), and primary tumor (C4) weakly express Vimentin. The positive rate of Ki67 is 35% in GBC-X1 (A5), xenograft (B5), and primary tumor (C5). GBC-X1 (A6), xenograft (B6), and primary tumor (C6) exhibit positive expression of MMP-2. GBC-X1 (A7), xenograft (B7), and primary tumor (C7) exhibit positive expression of CD44. GBC-X1 (A8), xenograft (B8), and primary tumor (C8) exhibit positive expression of SOX2. GBC-X1 (A9), xenograft (B9), and primary tumor (C9) exhibit positive expression of TP53(scale bar = 50 μm). .
Immunohistochemical staining
Immunohistochemical staining demonstrated positive expression of GBC-X1 cells and xenograft showed elevated expression levels of CK7 (Fig. 8A1-B1), CK19 (Fig. 8A2-B2), E-cadherin (Fig. 8A3-B3),and weakly positive expression of Vimentin(Fig. 8A4-B4),. The primary tumor also exhibited positive expression of CK7(Fig. 8C1), CK19(Fig. 8C2), E-cadherin(Fig. 8C3), Vimentin(Fig. 8C4), confirming their shared origin. The Ki67 labeling index was determined to be 35%, indicating a high proliferation rate(Fig. 8A5-C5). Immunohistochemistry exhibited positive expression of MMP-2 (Fig. 8A6-C6), CD44 (Fig. 8A7-C7), SOX2(Fig. 8A8-C8) and TP53(Fig. 8A9-C9) in cell lines, xenograft and primary tumour.
Discussion
Gallbladder cancer patients are often diagnosed in the middle to late stages of the disease due to the lack of obvious early symptoms, resulting in a low proportion of surgeries and poor prognosis. Understanding the underlying mechanisms of GBC occurrence and development is crucial to explore effective treatment methods and improving patient survival rates4,9,10,11. However, progress in gallbladder cancer pathogenesis and drug development has been greatly hindered by the limited availability of gallbladder cancer cell lines.
In this study, we successfully established a new gallbladder cancer cell line, designated as GBC-X1, through primary culture and subculture of primary tumor tissue obtained from gallbladder cancer patients. GBC-X1 has been continuously cultivated for over a year and has undergone stable passaging for more than 60 generations. Morphologically, GBC-X1 cells display typical adherence to the culture surface, resembling epithelial cells with short spindle shapes, large nuclei, and prominent nucleoli. Multinucleated cells and megakaryocytes are also observed. STR analysis confirmed the high consistency of GBC-X1 with the patient’s primary tumor, ensuring that the cell line was free from contamination by other cell lines or microorganisms. The observed Ki67 expression level of 35% in GBC-X1 suggests active proliferation of GBC-X1, which is consistent with the population doubling time of 32 h. GBC-X1 was compared with different gallbladder cancer cell line developed previously in Table 3.
Tumor spheroids have been widely studied since their discovery by Sutherland and colleagues in the early 1970s19. They are formed by the spontaneous aggregation of cells, mediated by cell surface integrins binding to the extracellular matrix (ECM)20. Various factors, such as nutrients, oxygen, and growth factors, influence this process21. Notably, tumor-derived spheroids are enriched with tumor stem cells and are commonly used for chemotherapy drug screening22,23. In our study, GBC-X1 demonstrated the efficient formation of tumor spheroids in a serum-free medium without the addition of growth factors, highlighting its strong stem cell characteristics.
The expression of CK7 and CK19 serves as a marker for bile duct differentiation, as normal bile duct epithelial cells and the majority of bile duct cancers express these proteins. Therefore, positive expression of CK7 and CK19 confirms the biliary epithelium origin24,25,26. Epithelial-mesenchymal transition (EMT) is a cellular process associated with various tumor functions, including tumor initiation, malignant progression, tumor stem cell properties, tumor cell migration, blood invasion, metastasis, and chemotherapy resistance. EMT is characterized by the loss of the epithelial marker E-cadherin and the acquisition of the mesenchymal marker Vimentin expression27,28,29. In our study, GBC-X1 cells exhibited positive expression of both E-cadherin and Vimentin, indicating their EMT characteristics consistent with malignant tumors.
Aneuploidy are prominent features of human tumors: approximately 90% of solid tumors and 70% of hematopoietic malignancies display a certain degree of aneuploidy30,31,32. The presence of aneuploid karyotypes in tumor cells is closely associated with poor patient prognosis33,34. Triploid karyotypes may be linked to intrinsic drug resistance whereas karyotypes exceeding tetraploidy could be associated with acquired drug resistance35. The incidence of hypertetraploid in women with breast cancer was 5.9%, while in men it was 16%36,37 Bjelkenkrantz et al. found that hypertetraploid is associated with a high recurrence rate and disease progression of tumors38. GBC-X1 cells predominantly exhibit a hypertetraploid karyotype, represented by 98, XXXX del (4) p (12) del (5) p (21) der (10). These cells demonstrate resistance to oxaliplatin, but sensitivity to gemcitabine, fluorouracil, and paclitaxel. Thus, GBC-X1 serves as a valuable model for investigating the mechanisms underlying karyotype-related drug resistance.
The mouse xenograft model plays a crucial role in advancing our understanding of human tumor biology, enabling the study of carcinogenesis, tumor progression, and evaluation of therapeutic compound efficacy and toxicity39,40. However, not all cell lines are capable of forming xenografts when inoculated into nude mice41,42. In our study, GBC-X1 cells were successfully inoculated into NXG mice, resulting in the formation of subcutaneous transplant tumors at a rate of approximately 50%. This observation may be attributed to residual immunity in mice. Pathological examination confirmed that the morphology of GBC-X1 xenografts closely resembled that of the primary tumor, further validating GBC-X1 as a viable animal experimental model.
Oxaliplatin targets DNA as the target site, and platinum atoms form cross junctions with DNA to counteract its replication and transcription43. Gemcitabine is a pyrimidine based anti-tumor drug, and its main metabolite is incorporated into DNA in cells, mainly acting in the G1/S phase44. Fluorouracil is a pyrimidine analogue that belongs to the category of anti metabolic drugs45. Paclitaxel can cause the dynamic balance between microtubule proteins and the dimer of microtubule proteins, inducing and promoting microtubule protein aggregation, microtubule assembly, and preventing depolymerization, thereby stabilizing microtubules and inhibiting cancer cell mitosis and triggering cell apoptosis, effectively preventing cancer cell proliferation and playing an anticancer role46.
The doubling time of GBC-X1 cells in this study was 32 h, indicating that GBC-X1 is a highly proliferative cell line. Therefore, antiproliferative drugs such as gemcitabine, paclitaxel, and fluorouracil can inhibit cell proliferation; Cell proliferation is vigorous, and the intracellular DNA excision repair system may be overexpressed, leading to the occurrence of oxaliplatin resistance47. In the later stage, we will further clarify the mechanism of GBC-X1 resistance through molecular detection.
Of course, this study only identified and analyzed the establishment and biological characteristics of a new gallbladder cancer cell line GBC-X1, which may limit the universal application of this study in a wider population of GBC patients. The subsequent establishment of more new gallbladder cancer cell lines and their sequencing analysis may deepen our understanding of the occurrence, development and the drug resistance mechanism of gallbladder cancer.
In conclusion, we have presented a novel human gallbladder cancer cell line, GBC-X1, derived from a Chinese female patient. This new cell line serves as a valuable experimental model for advancing basic research and facilitating the development of new therapeutic approaches for gallbladder cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Siegel, R. L., Miller, K. D., Jemal, A. & Cancer statistics CA Cancer J Clin. 70, 7–30. (2020). https://doi.org/10.3322/caac.21590 (2020).
Menon, G. & Babiker, H. M. Gallbladder carcinoma. in StatPearls (StatPearls Publishing, Treasure Island, FL, 2024).
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Song, X. et al. Overview of current targeted therapy in gallbladder cancer. Signal Transduct Target Ther. 5, 230. (2020). https://doi.org/10.1038/s41392-020-00324-2 (2020).
Miranda-Filho, A. et al. Gallbladder and extrahepatic bile duct cancers in the Americas: incidence and mortality patterns and trends. Int. J. Cancer. 147, 978–989. https://doi.org/10.1002/ijc.32863 (2020).
Schmidt, M. A., Marcano-Bonilla, L. & Roberts, L. R. Gallbladder cancer: epidemiology and genetic risk associations. Chin. Clin. Oncol.8, 31. https://doi.org/10.21037/cco.2019.08.13 (2019).
Tuo, J. Y. et al. Report of incidence and mortality of gallbladder cancer in China, Zhonghua zhong liu za zhi [Chinese journal of oncology], 40, 894–899. (2014). https://doi.org/10.3760/cma.j.issn.0253-3766.2018.12.004 (2018).
Li, Y. S., Li, M. L. & Liu, Y. B. Current status and future prospects of basic research on gallbladder carcinoma. Chin. J. Practical Surg.41, 4 (2021).
Lamarca, A. et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat. Rev.84, 101936. https://doi.org/10.1016/j.ctrv.2019.101936 (2020).
Yuan, B. et al. Patient-derived organoids for personalized gallbladder cancer modelling and drug screening. Clin. Transl Med.12, e678. https://doi.org/10.1002/ctm2.678 (2022).
Xing, J. et al. Application and progress of cultured models of Gallbladder Carcinoma. J. Clin. Transl Hepatol.11, 695–704. https://doi.org/10.14218/JCTH.2022.00351 (2023).
Koyama, S. et al. Establishment of a cell line (G-415) from a human gallbladder carcinoma. Gan. 71, 574–575 (1980).
Mirabelli, P., Coppola, L. & Salvatore, M. Cancer Cell lines are useful Model systems for Medical Research. Cancers (Basel). 11, 1098. https://doi.org/10.3390/cancers11081098 (2019).
Guo, L. et al. GBCdb: RNA expression landscapes and ncRNA-mRNA interactions in gallbladder carcinoma. BMC Bioinform.24, 12. https://doi.org/10.1186/s12859-023-05133-2 (2023).
Lin, S. J. et al. Signatures of tumour immunity distinguish Asian and non-asian gastric adenocarcinomas. Gut. 64, 1721–1731. https://doi.org/10.1136/gutjnl-2014-308252 (2015).
Nassour, I. et al. Racial and ethnic disparities in a national cohort of ampullary cancer patients. J. Surg. Oncol.117, 220–227. https://doi.org/10.1002/jso.24835 (2018).
Hattori, E., Oyama, R. & Kondo, T. Systematic review of the current status of human sarcoma cell lines. Cells. 8, 157. https://doi.org/10.3390/cells8020157 (2019).
Xu, H. et al. Establishment and characterization of a new human ampullary carcinoma cell line, DPC-X1. World J. Gastroenterol.29, 2642–2656. https://doi.org/10.3748/wjg.v29.i17.2642 (2023).
Sutherland, R. M., McCredie, J. A. & Inch, W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst.46, 113–120 (1971).
Steinberg, M. S. Differential adhesion in morphogenesis: a modern view. Curr. Opin. Genet. Dev.17, 281–286. https://doi.org/10.1016/j.gde.2007.05.002 (2007).
Bates, R. C., Edwards, N. S. & Yates, J. D. Spheroids and cell survival. Crit. Rev. Oncol. Hematol.36, 61–74. https://doi.org/10.1016/s1040-8428(00)00077-9 (2000).
Gunti, S., Hoke, A. T. K., Vu, K. P. & London, N. R. Jr Organoid and Spheroid Tumor models: techniques and applications. Cancers. 13, 874. https://doi.org/10.3390/cancers13040874 (2021).
Nath, S. & Devi, G. R. Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol. Ther.163, 94–108. https://doi.org/10.1016/j.pharmthera.2016.03.013 (2016).
Takahashi, Y. et al. Application of immunohistochemistry in the pathological diagnosis of liver tumors. Int. J. Mol. Sci.22, 5780. https://doi.org/10.3390/ijms22115780 (2021).
Uhlén, M. et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom.4, 1920–1932. https://doi.org/10.1074/mcp.M500279-MCP200 (2005).
Malaguarnera, G. et al. Markers of bile duct tumors. World J. Gastrointest. Oncol.3, 49–59. https://doi.org/10.4251/wjgo.v3.i4.49 (2011).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. EMT: 2016 Cell.166, 21–45. https://doi.org/10.1016/j.cell.2016.06.028 (2016).
Pastushenko, I. & Blanpain, C. E. M. T. Transition States during Tumor Progression and Metastasis. Trends Cell Biol.29, 212–226. https://doi.org/10.1016/j.tcb.2018.12.001 (2019).
Bakir, B., Chiarella, A. M., Pitarresi, J. R., Rustgi, A. K. & EMT MET, plasticity, and Tumor Metastasis. Trends Cell. Biol.30, 764–776. https://doi.org/10.1016/j.tcb.2020.07.003 (2020).
Weaver, B. A. & Cleveland, D. W. Does aneuploidy cause cancer? Curr. Opin. Cell. Biol.18, 658–667. https://doi.org/10.1016/j.ceb.2006.10.002 (2006).
Xiao, R. et al. Aneuploid embryonic stem cells drive teratoma metastasis. Nat. Commun.15, 1087. https://doi.org/10.1038/s41467-024-45265-4 (2024).
Taylor, A. M. et al. Genomic and functional approaches to understanding Cancer Aneuploidy. Cancer Cell.33, 676–689e3. https://doi.org/10.1016/j.ccell.2018.03.007 (2018).
Chen, Y., Yang, Z., Wang, Y., Wang, J. & Wang, C. Karyotyping of circulating tumor cells for predicting chemotherapeutic sensitivity and efficacy in patients with esophageal cancer. BMC cancer. 19, 651. https://doi.org/10.1186/s12885-019-5850-7 (2019).
Wang, Y. et al. Vimentin expression in circulating tumor cells (CTCs) associated with liver metastases predicts poor progression-free survival in patients with advanced lung cancer. J. Cancer Res. Clin. Oncol.145, 2911–2920. https://doi.org/10.1007/s00432-019-03040-9 (2019).
Huang, M. et al. Aneuploid Circulating Tumor Cells as a predictor of response to Neoadjuvant Chemotherapy in Non-small Cell Lung Cancer. Int. J. Gen. Med.14, 6609–6620. https://doi.org/10.2147/IJGM.S330361 (2021).
Pinto, A. E., André, S., Nogueira, M., Mendonça, E. & Soares, J. Flow cytometric DNA hypertetraploidy is associated with unfavourable prognostic features in breast cancer. J. Clin. Pathol.50, 591–595. https://doi.org/10.1136/jcp.50.7.591 (1997).
Bezić, J. et al. Flow cytometric DNA hypertetraploidy tends to be more frequent in male than in female breast cancers. Virchows Arch.466, 185–189. https://doi.org/10.1007/s00428-014-1694-3 (2015).
Bjelkenkrantz, K., Lundgren, J. & Olofsson, J. Single-cell DNA measurement in hyperplastic, dysplastic and carcinomatous laryngeal epithelia, with special reference to the occurrence of hypertetraploid cell nuclei. Anal. Quant. Cytol.5, 184–188 (1983).
Chuprin, J. et al. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol.20, 192–206. https://doi.org/10.1038/s41571-022-00721-2 (2023).
Massa, A. et al. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 12, 2308. (2020). https://doi.org/10.3390/cancers12082308
Shultz, L. D. et al. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc.2014 (7), 694–708 (2014).
Shultz, L. D. et al. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 694–708. (2014). https://doi.org/10.1101/pdb.top073585 (2014).
Graham, J., Mushin, M., Kirkpatrick, P. & Oxaliplatin Nat. Rev. Drug Discov3, 11–12. https://doi.org/10.1038/nrd1287 (2004).
Pandit, B. & Royzen, M. Recent development of Prodrugs of Gemcitabine. Genes. 13, 466. https://doi.org/10.3390/genes13030466 (2022).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 3, 330–338. https://doi.org/10.1038/nrc1074 (2003).
Min, L. et al. Strategies and lessons learned from total synthesis of Taxol. Chem. Rev.123, 4934–4971. https://doi.org/10.1021/acs.chemrev.2c00763 (2023).
Cohen, R. et al. Statuts MMR et BRAF dans les cancers colorectaux: intérêts pour la prise en charge thérapeutique? [DNA mismatch repair and BRAF status in colorectal cancer: interest for the therapeutic management?]. Bull. Cancer. 102, S72–S81. https://doi.org/10.1016/S0007-4551(15)31220-0 (2015).
Acknowledgements
We would like to thank Bullet Edits (http://www.bulletedits.cn) for English language editing of the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China(Grants 82260555 and 82360510), Natural Science Foundation of Gansu Province (Grants 22JR11RA022, 22JR5RA901, 23JRRA0929 and 23JRRA1601), Lanzhou Science and Technology Plan Project (Grants 2022-3-45 and 2023-2-38), Intra-Hospital Fund of the First Hospital of Lanzhou University (Grant ldyyyn2022-12 ), Chengguan District Science and Technology Plan (Grant 2023SHFZ0018).
Author information
Authors and Affiliations
Contributions
C.P.C, H.T, X.M , T.T.C and Y.H.S analyzed and interpreted the results of the detection of this novel cell line, and wrote a part of the manuscript. H.X, L.L, L.M and B.Z performed identification of the cell line, and were major contributors in writing the manuscript. Z.F.W, W.L and H.Z were responsible for specimen collection. W.C.Z wrote part of the manuscript and modified the images and were involved in data processing. C.P.C, H.T, X.M, T.T.C and H.X performed the cell culture. C.P.C, H.T, X.M, Y.H.S, L.L produced the pathological sections. Z.F.W, W.L and H.Z organized the data for all the experiments. W.C.Z and H.X conceived and designed and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
We have obtained consents to publish this paper from all the participants of this study.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Lanzhou University Second Hospital (2023 A-381). Informed consent was obtained from the patient. The animal procedures were approved by the Medical Animal Experiment Ethics Committee of Lanzhou University Second Hospital (D2023-318).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chai, C., Tang, H., Miao, X. et al. Establishment and characterization of a novel human gallbladder cancer cell line, GBC-X1. Sci Rep 14, 21439 (2024). https://doi.org/10.1038/s41598-024-72830-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72830-0