Abstract
Evidence regarding the role of chronic low-grade inflammation in the progression of cardiometabolic diseases (CMDs) and cardiometabolic multimorbidity (CMM) is currently limited. This prospective cohort study, utilising data from the UK Biobank, included 273,804 adults aged 40–69 years initially free of CMD at baseline. CMM was defined as the coexistence of two or more CMDs, such as coronary artery disease, type 2 diabetes mellitus, hypertension and stroke. The aggregated inflammation score (INFLA-score), incorporating C-reactive protein, white blood cell count, platelet count and granulocyte-to-lymphocyte ratio, quantified chronic low-grade inflammation. Absolute risks (ARs), hazard ratios (HRs) and 95% confidence intervals (CIs) assessed the association of increased INFLA-score with the risk of CMMs and CMDs. The accelerated failure time model explored the effect of INFLA-score on the time to CMM onset, and a restricted cubic spline characterised the dose-dependent relationship between INFLA-score and CMM risk. After a median follow-up of 166.37 months, 13,755 cases of CMM were identified. In quartiles with increasing INFLA-score levels, CMM ARs were 4.41%, 4.49%, 5.04% and 6.01%, respectively; HR increased by 2%, 15% and 36%, respectively, compared to the lowest quartile. The INFLA-score and CMM risk relationship was nonlinear (P for nonlinear < 0.001), exhibiting a significant risk trend change at a score of 9. For INFLA-score < 9, CMM risk increased by 1.9% for each 1-point increase; for INFLA-score ≥ 9, the risk increased by 5.9% for each 1-point increase. Additionally, a higher INFLA-score was associated with an earlier onset of CMM (P < 0.001). Compared to the first INFLA-score quartile, the AFT model revealed adjusted median times to CMM occurrence were 2.92, 6.10 and 13.19 months earlier in the second, third and fourth quartile groups, respectively. Chronic low-grade inflammation is associated with a higher risk of cardiometabolic multimorbidity and earlier onset among middle-aged and older adults. Monitoring and screening the INFLA-score in adults without CMDs may improve early prevention of CMM.
Similar content being viewed by others
Introduction
Multimorbidity, characterised by the simultaneous presence of multiple chronic diseases in an individual, is associated with heightened disability and mortality risks1. According to the World Health Organization’s 2019 Global Health Estimates, ischaemic heart disease, stroke and diabetes are the first, second and ninth leading causes of death worldwide2. High systolic blood pressure stands as the leading contributor to the global burden of cardiovascular disease (CVD)3. When an individual presents with multiple concurrent chronic diseases, the risk of mortality is not merely additive, but rather, these diseases often interact synergistically, leading to an overall increased risk that surpasses the sum of individual disease risks. In such instances, patients face more complex health challenges, necessitating comprehensive medical attention and integrated treatment strategies. Cardiometabolic multimorbidity (CMM)—characterised by the co-occurrence of two or more cardiometabolic diseases (CMDs) such as coronary artery disease (CAD), hypertension, type 2 diabetes mellitus (T2DM) and stroke, represents a composite disease pattern of severity extending beyond individual disease, posing a significant global health challenge4.
In the United States, the prevalence of CMM among adults increased from 9.4% in 1999 to 14.4% in 20185. Similarly, a study involving over one million Chinese adults revealed a doubling of CMM prevalence from 2.4 to 5.9% between 2011 and 2016, with a surge to 11.6% among the elderly4. Moreover, individuals diagnosed with CMM face a 3.7–6.9 times higher risk of all-cause mortality, accompanied by a reduced life expectancy of 12–15 years at the age of 606. Despite these alarming statistics, research on CMM remains limited even though it involves shared cardiovascular risk factors and interconnected pathogenesis. Inflammation emerges as a unifying theme in the occurrence and maintenance of hypertension, the development of atherosclerosis, the rupture of arterial plaques and the onset of CVD7,8,9. C-reactive protein (CRP), a predictive biomarker for CVD and stroke10, is independently correlated with diabetes onset, with a risk increase of over threefold in individuals with the highest CRP levels11. Additionally, higher neutrophil and platelet counts, along with lower lymphocyte counts, are significantly associated with stroke risk12,13. Chronic inflammation thus serves as a shared marker for the occurrence of various CMDs, significantly influencing CMM progression and prognosis, and intricately linked to clinical factors, lifestyle and socioeconomic status14,15. Despite the broad biological process of the inflammation cascade, individual markers may not fully capture its complexity. The aggregated inflammation score (INFLA-score), a comprehensive measure of chronic low-grade inflammation that integrates white blood cell count, CRP, platelets and granulocyte/lymphocyte ratio, offers a holistic perspective. Each of these biomarkers reflects a different aspect of inflammation, and the INFLA-score offers a holistic view of an individual’s systemic inflammatory state. Andreis A et al. demonstrated that the INFLA-score identified ongoing inflammation in 78% of patients with pericarditis who had normal CRP levels16 and had superior predictive value for pericarditis compared to CRP alone. Similarly, Liu S et al. found that a higher INFLA-score was associated with an elevated incidence of CMD and that the INFLA-score captures more CMD risk than its components17. Despite this, evidence on the role of chronic low-grade inflammation in the progression of CMDs and CMM remains limited. To date, no studies have explored the prospective association of INFLA-score with the risk of CMM development. Therefore, our study aims to investigate the influence of INFLA-score on the developmental trajectory of CMM based on the UK Biobank. Furthermore, this study seeks to shed light on the association between INFLA-score and CMM risk, providing valuable insights into the prevention of CMM.
Methods
Study design and population
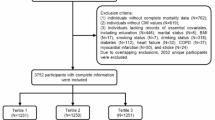
The UK Biobank, established between 2006 and 2010, is a comprehensive biomedical research database comprising over 502,000 adults aged 40–69 years from England, Scotland and Wales18. Health outcomes were systematically monitored through electronic health records, including Hospital Episode Statistics, cancer registry data and death registers. The study design and methods followed herein are detailed in a previous publication19. Initially, we enrolled 306,426 individuals without CMDs who completed inflammation markers tests. Participants with pre-existing conditions at baseline such as cancer (n = 27,392), pregnancy (n = 110) and those lacking complete follow-up data (n = 761) were excluded. To minimise reverse causality, an additional 4359 participants developing CMM within the initial two years of follow-up were also excluded. Consequently, 273,804 individuals were included in the main analysis (Fig. 1).
Ethical approval for the UK Biobank was obtained from the North West Multicentre Research Ethics Committee, Manchester, UK (REC reference: 11/NW/0382). The UK Biobank complied with the Declaration of Helsinki, and participants provided written informed consent. This study adhered to the STROBE guidelines for cohort studies.
Calculation of INFLA-score
The INFLA-score quantitatively assesses chronic low-grade inflammation by integrating four biomarkers—CRP, white blood cell count, platelet count and granulocyte-to-lymphocyte ratio (GrL)20. The granulocyte count is inclusive of neutrophil, eosinophil and basophil counts. Biomarkers were categorised into scoring levels based on their decile range: the highest range (7th to 10th deciles) received a positive score of + 1 to + 4, the fifth and sixth deciles were assigned a value of 0 and the lowest range (1st to 4th deciles) was assigned a negative score of − 4 to − 1. The INFLA-score is the sum of the scores obtained for these four indicators, ranging from − 16 to + 16, reflecting an individual’s chronic low-grade inflammation level, with a higher score suggesting heightened inflammatory status21,22.
Inflammation biomarker assay
At baseline recruitment, blood samples were collected and analysed within 24 h at the UK Biobanking Central Laboratory. Complete Blood Count was detected using a quantitative, automated LH750 haematology analyser (Coulter, Beckman Coulter, Brea, CA, USA). CRP was determined through immunoturbidimetric-high sensitivity analysis using a Beckman Coulter AU5800 system (Beckman Coulter (UK), Ltd) (Supplementary file 1).
Assessment of other variables
Various factors including age, sex, ethnicity, body mass index (BMI), socioeconomic status, alcohol consumption, blood pressure, biochemical parameters, smoking habits, physical activity, educational background, household income, medical history and medications were considered as potential covariates23,24. A directed acyclic graph (DAG) was generated using the DAGitty’s online platform (www.dagitty.net) to identify covariates requiring adjustment in the models. Data on age, sex, ethnicity, Townsend poverty index, education, annual household income, alcohol consumption and history of chronic diseases were collected using a touchscreen questionnaire. Ethnicity was categorised as White and non-White, based on self-reported ancestry, with 95% of participants reporting to be White. The Townsend poverty index is a composite measure that reflects poverty by considering factors such as unemployment, lack of car and home ownership and household overcrowding. A higher value indicated lower socioeconomic status25. Lipid and creatinine levels were also evaluated using enzymatic assays on the Beckman Coulter AU5800 instrument (Beckman Coulter (UK), Ltd). Glycated haemoglobin (HbA1c) levels were measured using high-performance liquid chromatography on a Bio-Rad Variant II Turbo analyser (Bio-Rad Laboratories, Inc.). BMI was calculated based on height and weight measurements obtained from the participants’ initial assessment. Blood pressure readings were obtained using an automated sphygmomanometer or a manual sphygmomanometer when necessary. Education status was categorised into six levels: (1) no qualification; (2) secondary education (or ordinary levels) or its equivalent; (3) advanced level education certification or equivalent; (4) other professional skills certification; (5) national vocational certification, Higher National Certificate or its equivalent; (6) College or university degree. Participants’ annual pre-tax household income was categorised into five income segments: (1) less than £18,000; (2) £18,000 to £29,999; (3) £30,000 to £51,999; (4) between £52,000 and £100,000; (5) exceeding £100,000. Lifestyle factors, medical history and medication use were extracted from the touchscreen questionnaire. Drinking and smoking status was classified as never, former and current. The physical activity of an individual was quantified as the summed metabolic equivalent task (minutes per week, MET), which included walking, moderate and vigorous activity. The DAG analysis (Fig. S1) was used to identify a minimally sufficient adjustment set in the main analysis. This set included age, sex, race, BMI, blood pressure, lipids, physical activity, hypercholesterolemia, lipid-lowering drugs, current aspirin use, alcohol consumption and smoking.
Study outcomes
The primary aim of this study was to investigate the association between the INFLA-score and CMM risk. The primary outcome was considered as the presence of CMM, which is defined as the coexistence of two or more of the following CMDs: CAD, T2DM, hypertension and stroke. The secondary aim was to examine the association between the INFLA-score and the risk of each CMD-CAD, T2DM, hypertension and stroke. Diagnostic algorithms for specific health conditions were developed using data from death registries, primary care records, hospitalisation records and self-reports26. CMD diagnoses relied on the International Classification of Diseases, Tenth Revision (ICD-10) codes. The ICD codes used for CAD were I20-25. T2DM was coded as E11, hypertension as I10 and stroke as I60-64. Follow-up for each participant was conducted from the date of enrolment until death, censoring or the end of the study period, whichever occurred first. Data were updated until May 1, 2023, for CAD, T2DM and hypertension, and April 1, 2023, for stroke, marking the end of the study’s follow-up period. Herein, CMM was followed up until May 1, 2023.
Statistical analysis
Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as means and standard deviations. The INFLA-score was categorised into quartiles, and group differences were assessed using the Chi-squared test for categorical variables and one-way analysis of variance for continuous variables. Quartile group incidence rates were expressed per 1000 person-years, and differences between groups were analysed using Poisson regression.
Absolute risks (ARs) and hazard ratios (HRs) were employed to assess the association between increased INFLA-score and risk of CMMs and CMDs. AR for specific conditions was calculated using a logistic regression model. This model assesses the impact of multiple variables on the onset of CMMs and CMDs, providing coefficients reflecting the effect of each variable on the event’s probability. These coefficients were expressed as log odds, with positive values indicating an association with increased event probability, while negative values indicated the opposite. The ‘plogis’ function in R was used to convert the log odds to ordinary probabilities, which estimates the probability of an event occurring in relation to a given variable. Finally, a new dataset containing representative values for all variables was constructed. Logistic regression models were then used to estimate the AR under different conditions.
HRs and 95% confidence intervals (CIs) for the association between the INFLA-score and CMM risk in the entire cohort were calculated using Cox proportional hazards models. The proportional hazard assumption was evaluated using the Schoenfeld Residuals test, with no observed violations. Two Cox regression models were developed: Model 1 was adjusted for age, sex and race, while Model 2 was further adjusted for variables from Model 1, as well as BMI, diastolic blood pressure, systolic blood pressure, HDL, LDL, TG, physical activity, hypercholesterolemia, current use of lipid-lowering drugs, current use of aspirin, alcohol consumption and smoking status. Multicollinearity between variables was assessed using the variance inflation factor (VIF), with covariates having a VIF ≥ 10 being excluded27. In the Cox regression model, the INFLA-score was evaluated as both a continuous and categorical variable in relation to CMM risk, and trend P values were calculated between categorical groups. Restricted cubic splines (RCS) with four knots (5%, 35%, 65% and 95%) were used to visually assess the dose–response relationship between the INLFA score and the risk of CMM and CMDs. The log-likelihood ratio test determined the P-value for non-linearity. If a nonlinear association was observed, a two-piecewise linear regression model identified the inflection point at which the relationship significantly changed28. Inflection point analysis is presented in detail in Appendix 1.
Subgroup analyses were conducted stratifying by clinical characteristics: age (< 55/≥ 55 years), sex (men/women), race (Whites/non-Whites), BMI (− 30/30 kg/m2), smoker and drinker (never/ever), and P values for interaction between groups were calculated through the likelihood ratio test.
Additionally, an accelerated failure time (AFT) model assessed the potential impact of different INFLA-score groups on the time to CMM. This model assumes that the covariates can be accelerated or delayed to the time trajectory of the event and does not rely on proportional risk assumptions, highlighting its potential application in diverse clinical studies29,30,31. In the multivariate AFT model, the effect of increasing INFLA-score quartile levels on time to CMM onset was assessed using the first INFLA-score quartile (Q1) as the reference group. The difference in median time to onset of CMM between the two groups was expressed in months and calculated by subtracting the comparison group from Q1. Negative values indicated a delay in the onset of CMM, while positive values indicated an earlier onset. In this study, we selected a flexible “Weibull distribution” to adapt to the right-skewed shape of time to CMM (Figs. S2 and S3).
In the sensitivity analyses, the robustness of the results was evaluated using four strategies. First, missing categorical variables were handled by creating missing indicator categories, and missing continuous variables were imputed with their mean values. Additionally, a five-repeat predictive mean matching algorithm combined with the Markov chain Monte Carlo method was used to impute missing variables, followed by pooled analyses of the Cox regression model32. Deaths prior to CMM onset were considered as competing events, and their impact on outcomes was analysed using the Fine & Gray competing risk model33. Additionally, the Cox regression model was adjusted for the Townsend Deprivation Index, educational qualifications, annual household income before tax and HbA1c. Finally, the definition of CMM excluded the occurrence of hypertension and included only CAD, T2DM and stroke.
Statistical analyses were performed using R software (version 4.2.0) and GraphPad (version 9.0), with a significance level of P < 0.05 (two-sided) considered statistically significant.
Results
Among the 273,804 participants, the average age was 54.71 years, with 43.3% being men. Participants were categorised into quartile levels based on INFLA-score distribution. Missing values were minima, with only HDL and physical activity exhibiting higher rates at 8.5% and 21%, respectively (Table S1). All baseline characteristics significantly differed across INFLA-score quartile groups (all P < 0.001), as detailed in Table 1. During variable selection, total cholesterol exhibited strong collinearity with sleep behaviour traits (VIF ≥ 10), leading to its exclusion from subsequent regression models.
INFLA-score quartiles and CMM
Over a median follow-up of 166.37 months (interquartile range 24.02–195.38 months), 13,755 cases of CMM were recorded. Kaplan–Meier curves demonstrated a consistent positive association between increasing INFLA-score quartiles and cumulative hazard rates for both CMM and specific CMDs over the study period (all P < 0.001 by log-rank test; Fig. S4). The incidence of CMM increased significantly with higher INFLA-score quartile groups, registering rates of 2.48, 3.14, 3.98 and 5.44 per 1,000 person-years for the first, second, third and fourth quartiles, respectively (P < 0.001; Table S2 and Fig. 2). Moreover, the proportion of individuals with ≥ 3 coexisting CMDs increased significantly with higher INFLA-score quartiles, with values in the first, second, third and fourth quartiles as 0.35%, 0.47%, 0.67% and 0.98%, respectively (P < 0.001; Table S2 and Fig. 2).
In Cox regression model 1, a 1-point increase in INFLA-score corresponded to a 5.2% increase in HR and a 0.25% increase in AR for CMM (Table 2). In the quartiles with increasing INFLA-score levels, AR was 3.5%, 4.24%, 5.41% and 7.67%, respectively. Compared with the lowest quartile, the HR of CMM increased sequentially by 21%, 55%, and 1.21-fold, respectively. Similar associations were observed for specific CMDs, irrespective of whether the INFLA-score increased per unit or quartile level. Similarly, in model 2, for each 1-point increase in the INFLA-score, the HR and AR for CMM increased by 2.2% and 0.105%, respectively. The AR of CMM in quartiles with increasing INFLA-score was 4.41%, 4.49%, 5.04% and 6.01%, respectively, while HR increased by 2%, 15% and 36%, respectively, compared with the lowest quartile. Both CMM and CMDs demonstrated an increasing trend as the INFLA-score quartile level increased (all P for trend < 0.05).
In the subgroup analyses, the positive association between INFLA-score levels and CMM risk was consistent across all strata (P for interaction > 0.05; Fig. 3). However, an interaction was observed in the age group (P for interaction < 0.001), indicating higher CMM risk with increasing INFLA-score among those < 55 years. Participants < 55 years of age had a higher risk of CMM as the INFLA-score increased (Fig. S5). Additionally, the highest quartile INFLA-score levels in all strata were associated with a higher risk of CAD, T2DM and hypertension compared with the lower quartile group (all P < 0.05; Figs. S6–S8). The risk tended to increase as the INFLA-score quartile level increased in most strata, with no significant increase in the highest group (all P < 0.05; Fig. S9).
Subgroup analyses were conducted to examine the associations (hazard ratios, 95% CIs) between INFLA-score quartiles and the risk of cardiometabolic multimorbidity. The first quartile is the reference group. HRs and 95% CIs have been adjusted by age, sex, race, body mass index, diastolic blood pressure, systolic blood pressure, HDL, LDL, TG, physical activity, hypercholesterolemia, current use of lipid lowering drugs, current use of aspirin, drinker, and smoker.
Dose-dependent relationship of INFLA-score and CMM
The relationship between the INFLA-score and CMM risk demonstrated a nonlinear pattern (P for nonlinear < 0.001), with increasing CMM risk as the INFLA-score increased (Fig. 4). INFLA-score demonstrated a linear association with the risks of CAD (P for nonlinear = 0.197), stroke (P for nonlinear = 0.187) and T2DM (P for nonlinear = 0.072) while the associations with hypertension exhibited a nonlinear trend (P for nonlinear = 0.033). Threshold effect analyses revealed that when the INFLA-score was < 9, a 1-unit increase was associated with a 1.9% increased hazard of CMM; when the INFLA-score was ≥ 9, a 1-unit increase was associated with a 5.9% increased hazard of CMM (Table S3).
Restricted cubic splines of INFLA-score and risk of cardiometabolic multimorbidity and specific cardiometabolic diseases. HRs and 95% CIs have been adjusted by age, sex, race, body mass index, diastolic blood pressure, systolic blood pressure, HDL, LDL, TG, physical activity, hypercholesterolemia, current use of lipid lowering drugs, current use of aspirin, drinker, and smoker.
INFLA-score levels and time to CMM onset
In the INFLA-score quartiles, the follow-up times for CMM and CMD differed between groups (all P < 0.05), but the time to CMM onset exhibited no significant variance between quartiles (P = 0.728; Table S4). In Fig. 5 and Table S3, the AFT model demonstrated a significantly earlier onset of CMM in higher quartile groups compared to the first INFLA-score quartile. This translated into adjusted median times to CMM occurrence of 2.92, 6.1 and 13.19 months earlier in the second, third and fourth quartile groups, respectively, compared with the first INFLA-score quartile. Additionally, compared with the lowest quartile group, the median time to CAD increased by 1.01, 1.61 and 3.07 months with escalating INFLA-score quartiles, while the median time to T2DM increased by 2.07, 4.36 and 7.67 months; the median time to hypertension increased by 1.02, 2.44, and 4.48 months; and the median time to stroke also tended to be increased (P for trend = 0.003).
Adjusted median time difference for new-onset cardiometabolic multimorbidity and specific cardiometabolic diseases in the second, third and fourth quartile groups compared to the first quartile INFLA-score group. Median difference = median occurrence time in reference group (Q1)—median occurrence time in comparison group. Negative values indicate a delay in the onset of events, while positive values indicate an earlier onset. In the AFT model, adjustments were made for age, sex, race, body mass index, diastolic blood pressure, systolic blood pressure, HDL, LDL, TG, physical activity, hypercholesterolemia, current use of lipid lowering drugs, current use of aspirin, drinker, and smoker.
Sensitivity analyses
To account for death as a competing risk before CMM onset, a competing risk model was employed. In this model, death was considered an independent event, and the results indicated that with every 1-point increase in the INFLA-score, there was a 2.4% increase in the HR of CMM (Table S6). The highest quartile INFLA-score group exhibited a 39% increased HR of CMM compared to the lowest first quartile group, consistent with the primary analysis. When participants who died prematurely before CMM onset were excluded, the AR in quartiles with increased INFLA-score levels was 4.36%, 4.44%, 5.02% and 6.06%, respectively, mirroring the main analysis results (Table S6). Moreover, stability persisted even after additional adjustments for the Townsend Deprivation Index, educational qualifications, annual household income before tax and HbA1c (Table S7). Additionally, the pooled results from multiple imputations aligned closely with the main analysis outcomes (Table S8). In multivariate Cox regression, the association of the INFLA-score with the risk of CMM and CMDs remained consistent when additional adjustments for the Townsend Deprivation Index, educational qualifications, annual household income before tax and HbA1c were incorporated (Table S9). The AFT model, irrespective of the exclusion of participants who died prematurely or additional adjustment for Townsend Deprivation Index, educational qualifications, annual household income before tax and HbA1c, consistently indicated that higher INFLA-score levels were associated with an earlier onset of CMM and CMDs (Figs. S10–S11 and Tables S10–S11). When redefining CAD, T2DM and stroke as criteria for CMM and observing the occurrence of ≥ 2 diseases, a total of 2495 events were recorded. With an increasing INFLA-score, both AR and HR for CMM demonstrated an upward trend, aligning with the primary study findings (Table S12). In the AFT model, CMM occurred 23.06 months earlier in the highest quartile compared with the lowest quartile of the INFLA-score, and this earlier effect remained significant across specific CMDs (Table S13).
Discussion
In this prospective cohort of 273,804 initially CMD-free adults, elevated INFLA-score emerged as a robust predictor of HR and AR for the occurrence of CMD. Intriguingly, a nonlinear positive association with CMM hazard was observed. These associations endured robustly even after meticulous adjustments for potential confounders. Notably, irrespective of the sequence in which CMDs occurred, an elevated INFLA-score not only accelerated the onset of CMDs but also significantly advanced the onset of CMM. Furthermore, the impact of the INFLA-score on CMM was significant among middle-aged participants compared to their older counterparts.
Herein, we defined multimorbidity with specificity, focusing on CMM alongside hypertension, CAD, diabetes and stroke5 as these four chronic diseases exhibited similar overall pathophysiologic risk profiles34. The prevalence of CMM in our study was notably higher at 5.02%, surpassing rates reported in the UK (1.21%) and China (3.07%)35,36. This divergence could stem from the omission of hypertension in the latter two studies. Many studies have focused on the association between chronic inflammation and specific CMDs, yielding inconsistent results. For instance, Kansui et al., in a study involving 2991 Japanese men, reported that baseline elevated levels of WBC count were not associated with the future incidence of hypertension37. Conversely, a retrospective cohort study based on the entire population of 96,606 Koreans reported a correlation between WBC and CRP quartiles and the incidence of hypertension38. In a meta-analysis by Danish et al., baseline CRP levels and WBC count had similar effects on CVD risk39. Similarly, Bao X, et al. reported that total WBC counts, along with CRP, were associated with increased risks of diabetes and CVD40. Furthermore, a prospective, nested case–control study in women revealed that an elevated CRP level could predict the occurrence of T2DM; however, these results were not observed in Korean women38,41. A prospective longitudinal study with an average follow-up of 8.4 years indicated that baseline high levels of platelet count (even within the normal range) were positively correlated with the risk of T2DM and the occurrence of CVD42,43. Additionally, neutrophil-to-lymphocyte ratio (NLR) was correlated to the risk of hypertension, total CVD, myocardial infarction and ischaemic stroke, but not T2DM44,45,46. Overall, current epidemiological evidence supports a strong link between chronic low-grade inflammation and CMD, primarily owing to its role in the development of CMD by promoting thrombosis, exacerbating vascular damage and contributing to atherosclerosis47. However, different inflammatory markers may exhibit differential but complementary characteristics, which could be influenced by various factors such as age, gender and study sample size. Therefore, when assessing the role of chronic inflammation in CMD, a wider variety of inflammatory markers need to be included for a holistic understanding.
Unlike conventional inflammatory markers, our study introduced the INFLA-score, a composite inflammatory index based on four circulating biomarkers (CRP, platelet count, white blood cell count and Glr). The INFLA-score demonstrated a dose–response relationship with CMM, as evidenced by an increasing HR with ascending INFLA-score quartiles (first quartile, 4.41%; second quartile, 4.49%; third quartile, 5.04%; fourth quartile, 6.01%). Higher INFLA-score levels were significantly and positively associated with elevated hazards of CAD, hypertension, T2DM and stroke, consistent with previous studies38,40,45. Furthermore, the association between INFLA-score and stroke risk was comparatively weaker than in other CMDs, suggesting a potential dependence on blood pressure levels. Blood pressure is an important determinant of stroke, with approximately 64% of patients with stroke presenting with concomitant hypertension48. Notably, hypertensive patients with concomitant elevated CRP levels have twice the risk of stroke compared to those with only high blood pressure49. Furthermore, the risk of stroke in hypertensive women is significantly higher than that in normotensive women, regardless of CPR levels, suggesting that inflammatory markers may play a mediating role between hypertension and stroke creation50. Therefore, in previous inflammation-related studies, increases or decreases in a single inflammatory marker could have been misinterpreted as an increase or decrease in the risk of certain diseases. Hence, a comprehensive assessment of the INFLA-score, encompassing multiple inflammatory markers, offers a more comprehensive assessment of chronic inflammation and may serve as a stable metric for identifying adverse cardiometabolic features, surpassing individual inflammatory markers in diagnostic51. The INFLA-score thus emerges as a valuable tool for assessing chronic inflammation levels.
A recent study by Liu et al. examined the association between the INFLA-score and CMD in an obese population. The findings indicated that for each standard deviation increase in the INFLA-score, which includes hs-CRP, white blood cell count and GrL, the risk of CMD increased by 9.9%, 7.3%, 9.6% and 2.7%, respectively17. However, a one standard deviation increase in platelet count was not associated with an increased risk of CMD17. These results suggest that the INFLA-score provides a more comprehensive assessment of an individual’s overall inflammatory status by integrating multiple inflammatory markers. It captures the synergistic effects of various inflammatory pathways and offers a more accurate prediction of disease risk than any single inflammatory marker. Furthermore, the INFLA-score is more practical and reliable in clinical settings as it minimises the fluctuations and uncertainties associated with relying on individual biomarkers.
The AFT analysis illuminated the disparity in the acceleration of CMM onset based on INFLA-score levels. When grouping the INFLA-score into quartiles, the highest quartile group exhibited a significantly increased time interval for CMM onset compared to the lowest quartile, diverging significantly from the developmental trajectory of other specific CMDs. Patients with the highest INFLA-score experienced an accelerated time to CMM onset of 13.19 months compared to patients with the lowest score. Analysing the four CMDs facilitated a direct comparison of INFLA-score estimates for the time to onset of these diseases. Since CMM was defined as the co-occurrence of two CMDs, it was expected to share a similar pattern of development with other specific CMDs if the same intensity of inflammation-induced both CMDs alike. However, our findings suggested that higher INFLA-score levels may have accelerated the onset of the second CMD, thus significantly advancing the appearance of CMM. Threshold effect analyses corroborated this observation, revealing a significantly increased risk of CMM occurrence when the INFLA-score exceeded 9, with the highest INFLA-score fully encompassing this interval. Previous studies have underscored the heightened risk of cardiovascular complications and death associated with early-onset CMDs (such as diabetes and hypertension)52,53,54. Additionally, to exclude the potential influence of follow-up factors on time to onset, we compared the follow-up duration and time to onset for CMM and CMD in the entire cohort, revealing that follow-up time has a negligible impact on the observed outcomes, reinforcing the robustness of our findings.
In subgroup analyses, significant heterogeneity in the effect of INFLA-score on the incidence of CMM across different groups was observed. Notably, adults < 55 years exhibited a relatively higher risk of CMM with increasing INFLA-score compared to older participants. Our results are consistent with previous studies, such as a UK cross-sectional study revealing that elevated CRP levels were not associated with increased blood pressure in women aged 60–7955. Among participants under the age of 60, a positive correlation was observed between the systemic inflammation response index and the risk of myocardial infarction (MI), while no significant correlation was observed in those aged 60 and above56. Additionally, a 20-year follow-up study in the Honolulu Heart Program indicated a stronger association between CRP levels and MI and thrombotic stroke in men aged 48–55 compared to those aged 56–7057,58. The weaker association in older age groups could be attributed to the presence of other risk factors overshadowing the effects of inflammation. With increasing age, CMDs can occur even in the absence of significant cardiovascular diseases. Therefore, inflammation may have a more pronounced role in relatively isolated disease development among middle-aged populations. The incidence of CMM in the middle-aged population may be relatively low; however, most individuals are not aware of their higher CMM risk, leading to a significant disease burden. Therefore, early assessment of INFLA-score levels holds clinical significance.
Consistent with previous studies, our observations revealed lower physical activity and higher BMI and smoking rates among individuals with elevated inflammation levels59,60. Increasing evidence suggests high-risk lifestyles play a crucial and distinctive role in the progression from health to the onset of CMDs, further advancing to CMM, and ultimately leading to death61. The correlation between higher BMI and a tenfold increase in CMM risk compared to healthy individuals is supported by evidence from 16 cohort studies62. The Moli-sani cross-sectional study further illustrates a positive correlation between the Dietary Inflammatory Index and the INFLA-score20. Importantly, an unhealthy lifestyle also contributes to chronic inflammation. Moderate to vigorous leisure-time physical activity and a diet rich in natural fibres effectively reduce low-grade inflammation in high-risk individuals for CMDs, a correlation that persists even after adjusting for baseline BMI. Therefore, adopting a healthy lifestyle, encompassing dietary patterns, physical activity and smoking cessation in the era of multimorbidity might mitigate CMM risk by lowering low-grade inflammation. Nevertheless, even after accounting for BMI, alcohol consumption and smoking, our study found that the associations of the INFLA-score with CMM remained robust. Future investigations should carefully explore the intricate interplay between lifestyle, chronic inflammation and CMM.
Strengths and limitations
This study represents the pioneering exploration into the impact of baseline INFLA-score levels on the risk of CMM alongside its constituent diseases. Several strengths underscore the significance of our findings. Firstly, as a prospective cohort study with a large sample size and extensive follow-up, it spans diverse age groups, ensuring broad applicability. Secondly, the utilisation of both the AFT model and the Cox model imparts robustness to the study results. Furthermore, the incorporation of extensive sensitivity analyses bolsters the internal validity of our findings.
However, certain limitations warrant consideration. Firstly, the INFLA-score relies on baseline data, introducing the potential impact of fluctuations over time. Secondly, the absence of data on additional inflammatory markers such as tumour necrosis factor-alpha, interleukin-6 (IL-6) and IL-8 limits the comprehensiveness of our analysis. Additionally, this study draws from the UK Biobank database, with 95% of the included population being Caucasian, thereby affecting the generalizability of our results. Lastly, the exclusion criteria for hypertension may not encompass all asymptomatic individuals, potentially leading to an underestimation of hypertension prevalence. Additionally, the current use of cholesterol-lowering drugs and aspirin, possessing anti-inflammatory effects, were adjusted for during the analysis, mitigating potential confounding effects.
Conclusion
This study demonstrates that chronic low-grade inflammation, as measured by the INFLA-score, is not only associated with an increased risk of CMM but also significantly accelerates its onset, particularly among middle-aged individuals. These findings highlight the importance of proactive INFLA-score screening in adults free of CMDs to prevent the risk of CMM.
Data availability
This study was conducted using the UK Biobank Resource, Application Number: 107335. The data that support the findings of this study are available from UK Biobank (https://www.ukbiobank.ac.uk/.), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The first (Cheng, wenke; e-mail: cwk2517@163.com) author should be contacted if someone wants to request the data from this study.
Abbreviations
- AFT:
-
Accelerated failure time
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CMM:
-
Cardiometabolic multimorbidity
- CMDs:
-
Cardiometabolic diseases
- CVD:
-
Cardiovascular diseases
- CRP:
-
C-reactive protein
- GrL:
-
Granulocyte to lymphocyte ratio
- HbA1c:
-
Glycated hemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- ICD:
-
International classification of diseases
- INFLA-score:
-
The aggregated inflammation score
- IL-6:
-
Interleukin-6
- LDL-C:
-
Low-density lipoprotein cholesterol
- NLR:
-
Neutrophil-to-lymphocyte ratio
- MET:
-
Metabolic equivalent task
- MI:
-
Myocardial infarction
- VIF:
-
Variance inflation factor
- RCS:
-
Restricted cubic spline
- TC:
-
Total cholesterol
- T2DM:
-
Type 2 diabetes mellitus
- TG:
-
Triglyceride
References
Smith, S. M., Soubhi, H., Fortin, M., Hudon, C. & O’Dowd, T. Managing patients with multimorbidity: Systematic review of interventions in primary care and community settings. BMJ345, e5205 (2012).
Organization WH. The Top 10 Causes of Death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (09 Dec 2020).
Mensah, G. A., Fuster, V. & Roth, G. A. A heart-healthy and stroke-free world: Using data to inform global action. J. Am. Coll. Cardiol.82(25), 2343–2349 (2023).
Zhang, D. et al. Multimorbidity of cardiometabolic diseases: Prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open.9(3), e024476 (2019).
Cheng, X., Ma, T., Ouyang, F., Zhang, G. & Bai, Y. Trends in the prevalence of cardiometabolic multimorbidity in the United States, 1999–2018. Int. J. Environ. Res. Public Health.19(8), 4726 (2022).
Di Angelantonio, E. et al. Association of cardiometabolic multimorbidity with mortality. Jama.314(1), 52–60 (2015).
Ganjali, S. et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol.233(12), 9237–9246 (2018).
Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med.11, 117 (2013).
Dinh, Q. N., Drummond, G. R., Sobey, C. G. & Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int.2014, 406960 (2014).
Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Cook, N. R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med.347(20), 1557–1565 (2002).
Freeman, D. J. et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland coronary prevention study. Diabetes.51(5), 1596–1600 (2002).
Cheng, W. et al. Higher systemic immune-inflammation index and systemic inflammation response index levels are associated with stroke prevalence in the asthmatic population: A cross-sectional analysis of the NHANES 1999–2018. Front. Immunol.14, 1191130 (2023).
Cai, X. et al. Systemic inflammation response index as a predictor of stroke risk in elderly patients with hypertension: A cohort study. J. Inflamm. Res.16, 4821–4832 (2023).
Singh-Manoux, A. et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: A cohort study. PLoS Med.15(5), e1002571 (2018).
Chudasama, Y. V. et al. Healthy lifestyle and life expectancy in people with multimorbidity in the UK Biobank: A longitudinal cohort study. PLoS Med.17(9), e1003332 (2020).
Andreis, A. et al. INFLA-score: A new diagnostic paradigm to identify pericarditis. Hellenic J.Cardiol.https://doi.org/10.1016/j.hjc.2024.03.010 (2024).
Liu, S. & Gu, Y. INFLA score: A novel inflammatory marker for assessing cardiometabolic disease risk in obese individuals. Diabetol. Metab. Syndr.16(1), 151 (2024).
Han, H. et al. Association of a healthy lifestyle with all-cause and cause-specific mortality among individuals with type 2 diabetes: A prospective study in UK Biobank. Diabetes Care.45(2), 319–329 (2022).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12(3), e1001779 (2015).
Shivappa, N. et al. Association of proinflammatory diet with low-grade inflammation: Results from the Moli–sani study. Nutrition.54, 182–188 (2018).
Shi, H. et al. Low-grade inflammation as mediator between diet and behavioral disinhibition: A UK Biobank study. Brain Behav. Immun.106, 100–110 (2022).
Pounis, G. et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli–sani study. Thromb. Haemost.115(2), 344–352 (2016).
Wang, X. et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern. Med.183(4), 340–349 (2023).
Lin, L. Z., Chen, J. H. & Dong, G. H. Air pollution and trajectory of cardiometabolic multimorbidity. EBioMedicine.86, 104346 (2022).
Yousaf S, Bonsall A. UK Townsend Deprivation Scores from 2011 Census Data. 2017.
Ma, T. et al. Use of fish oil and mortality of patients with cardiometabolic multimorbidity: A prospective study of UK biobank. Nutr. Metab. Cardiovasc. Dis.32(12), 2751–2759 (2022).
Liao, Y. et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: A cohort of 3026 patients. Cardiovasc. Diabetol.21(1), 128 (2022).
Liu, Y. et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population: A secondary analysis based on a cross-sectional study. Lipids Health Dis.17(1), 275 (2018).
Lambert, P., Collett, D., Kimber, A. & Johnson, R. Parametric accelerated failure time models with random effects and an application to kidney transplant survival. Stat. Med.23(20), 3177–3192 (2004).
Kay, R. & Kinnersley, N. On the use of the accelerated failure time model as an alternative to the proportional hazards model in the treatment of time to event data: A case study in influenza. Drug Inform. J.36(3), 571–579 (2002).
Crowther, M. J., Royston, P. & Clements, M. A flexible parametric accelerated failure time model and the extension to time-dependent acceleration factors. Biostatistics.24(3), 811–831 (2023).
Park, S. Y. et al. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann. Intern. Med.167(4), 228–235 (2017).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc.94(446), 496–509 (1999).
Piette, J. D. & Kerr, E. A. The impact of comorbid chronic conditions on ciabetes care. Diabetes Care.29(3), 725–731 (2006).
Liu, B. P. et al. The impact of physical activity intensity on the dynamic progression of cardiometabolic multimorbidity: Prospective cohort study using UK Biobank data. JMIR Public Health Surveill.9, e46991 (2023).
Han, Y. et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J.42(34), 3374–3384 (2021).
Kansui, Y. et al. C-reactive protein and incident hypertension in a worksite population of Japanese men. J. Clin. Hypertens.21(4), 524–532 (2019).
Sung, K.-C. et al. Inflammation in the prediction of type 2 diabetes and hypertension in healthy adults. Arch. Med. Res.48(6), 535–45 (2017).
Danesh, J., Collins, R., Appleby, P. & Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. Jama.279(18), 1477–1482 (1998).
Bao, X. et al. Comparing the inflammatory profiles for incidence of diabetes mellitus and cardiovascular diseases: A prospective study exploring the ‘common soil’ hypothesis. Cardiovasc Diabetol.17(1), 87 (2018).
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama.286(3), 327–334 (2001).
Hwang, J. Y., Kwon, Y. J., Choi, W. J. & Jung, D. H. Platelet count and 8-year incidence of diabetes: The Korean genome and epidemiology study. Diabetes Res. Clin. Pract.143, 301–309 (2018).
Vinholt, P. J. et al. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb. Res.148, 136–142 (2016).
Liu, X. et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am. J. Hyperten..28(11), 1339–1346 (2015).
Zhao, Y. et al. Neutrophil-to-lymphocyte ratio as a predictor for cardiovascular diseases: A cohort study in Tianjin. China J. Hum. Hypertens.37(7), 576–583 (2023).
Kashima, S., Inoue, K., Matsumoto, M. & Akimoto, K. White blood cell count and C-reactive protein independently predicted incident diabetes: Yuport Medical checkup center study. Endocr. Res.44(4), 127–137 (2019).
Libby, P. Inflammation in atherosclerosis. Nature.420(6917), 868–874 (2002).
Wajngarten, M. & Silva, G. S. Hypertension and stroke: Update on treatment. Eur. Cardiol.14(2), 111–115 (2019).
Chen, X. et al. Joint effect of elevated-c-reactive protein level and hypertension on new-onset stroke: A nationwide prospective cohort study of CHARLS. Front. Public Health.10, 919506 (2022).
Jiménez, M. C. et al. Association between markers of inflammation and total stroke by hypertensive status among women. Am. J. Hypertens.29(9), 1117–1124 (2016).
Agostinis-Sobrinho, C. et al. Ability of nontraditional risk factors and inflammatory biomarkers for cardiovascular disease to identify high cardiometabolic risk in adolescents: Results from the labMed physical activity study. J. Adolesc. Health.62(3), 320–326 (2018).
Chen, F. et al. Cardiovascular disease risk in early-onset vs late-onset type 2 diabetes in China: A population-based cross-sectional study. J. Diabetes.16(2), e13493 (2024).
Wang, C. et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J. Am. Coll. Cardiol.75(23), 2921–2930 (2020).
Huo, X. et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: A cross-sectional study. Lancet Diabetes Endocrinol.4(2), 115–124 (2016).
Davey Smith, G. et al. Association of C-reactive protein with blood pressure and hypertension: Life course confounding and mendelian randomization tests of causality. Arterioscler. Thromb. Vasc. Biol.25(5), 1051–1056 (2005).
Jin, Z. et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: A ten-year yollow-up study in 85,154 individuals. J. Inflamm. Res.14, 131–140 (2021).
Curb, J. D. et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation.107(15), 2016–2020 (2003).
Sakkinen, P. et al. C-reactive protein and myocardial infarction. J. Clin. Epidemiol.55(5), 445–451 (2002).
van Greevenbroek, M. M., Schalkwijk, C. G. & Stehouwer, C. D. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: Causes and consequences. Neth. J. Med.71(4), 174–187 (2013).
Fröhlich, M. et al. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg survey 1994/95). Eur. Heart J.24(14), 1365–72 (2003).
Han, Y. et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J.42(34), 3374–3384 (2021).
Kivimäki, M. et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health.2(6), e277–e285 (2017).
Acknowledgements
WKC is funded by China Scholarship Council (CSC No.202009370095). We extend our deepest gratitude to the study participants and the members of the UK Biobank cohort. The establishment of the UK Biobank was made possible through the efforts of the Wellcome Trust, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. We thank my colleague Dr. Chuang Yang for censoring the data. Besides, we thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This study was supported by the Program of Shanghai Municipal Health Commission (202240053), and Shanghai Collaborative Innovation Center of Industrial Transformation of Hospital TCM Preparation.
Author information
Authors and Affiliations
Contributions
BL designed the study topic; WKC performed all the statistical analysis; WKC and BL first drafted the manuscript; WKC, ZYD and BL read and revised the manuscript. WKC, ZYD and BL finally approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
North West Multicentre Research Ethics Committee, Manchester, UK (REC reference: 11/NW/0382), and all participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, W., Du, Z. & Lu, B. Chronic low-grade inflammation associated with higher risk and earlier onset of cardiometabolic multimorbidity in middle-aged and older adults: a population-based cohort study. Sci Rep 14, 22635 (2024). https://doi.org/10.1038/s41598-024-72988-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72988-7
Keywords
This article is cited by
-
Association between sensory impairment and cardiometabolic multimorbidity among older Chinese adults: a cross-sectional study based on the China Health and Retirement Longitudinal Study
BMC Geriatrics (2025)
-
Low-grade inflammation score (INFLA- score) associated with metabolic syndrome and its components in shift workers
Diabetology & Metabolic Syndrome (2025)
-
Estimated glucose disposal rate and the dynamic transitions of cardiometabolic multimorbidity and all-cause mortality in prediabetes: a multistate trajectory analysis
Lipids in Health and Disease (2025)
-
Adipose tissue as target of environmental toxicants: focus on mitochondrial dysfunction and oxidative inflammation in metabolic dysfunction-associated steatotic liver disease
Molecular and Cellular Biochemistry (2025)