Abstract
Steatotic liver disease has been shown to associate with cardiovascular disease independently of other risk factors. Lipoproteins have been shown to mediate some of this relationship but there remains unexplained variance. Here we investigate the plasma lipidomic changes associated with liver steatosis and the mediating effect of these lipids on coronary artery disease (CAD). In a population of 2579 Swedish participants of ages 50 to 65 years, lipids were measured by mass spectrometry, liver fat was measured using computed tomography (CT), and CAD status was defined as the presence of coronary artery calcification (CAC score > 0). Lipids associated with liver steatosis and CAD were identified and their mediating effects between the two conditions were investigated. Out of 458 lipids, 284 were found to associate with liver steatosis and 19 of them were found to also associate with CAD. Two fatty acids, docosatrienoate (22:3n6) and 2-hydroxyarachidate, presented the highest mediating effect between steatotic liver disease and CAD. Other mediators were also identified among sphingolipids and glycerophospholipids, although their mediating effects were attenuated when adjusting for circulating lipoproteins. Further research should investigate the role of docosatrienoate (22:3n6) and 2-hydroxyarachidate as mediators between steatotic liver disease and CAD alongside known risk factors.
Similar content being viewed by others
Introduction
Steatotic liver disease is recognized as the most prevalent liver disease, affecting 17–51% of adults worldwide1. It is commonly associated with the metabolic syndrome, which is characterized by obesity, insulin resistance, diabetes, hypertension, and dyslipidemia. Several studies, based on imaging-defined atherosclerosis as well as cardiovascular events, have reported that individuals with liver steatosis have a significantly increased risk of developing cardiovascular disease2,3,4,5. We have shown that the association between liver steatosis and coronary artery calcification score (CACS), which is a common measurement for the estimation of coronary artery disease (CAD), remains strong even after adjusting for other metabolic syndrome factors6. Despite this established association, there is a lack of knowledge regarding the mechanisms connecting these conditions.
Dyslipidemia is a significant risk factor for CAD and has been consistently associated with liver steatosis, even after adjusting for other metabolic risk factors which underscores the profound influence of the liver’s condition on lipid levels7. Changes in lipid metabolism induced by liver steatosis have been demonstrated to promote atherosclerosis through lipoproteins8. More specifically, triglyceride-rich lipoproteins have been found to mediate approximately 15% of the association between liver steatosis and CACS, while LDL cholesterol to mediate about 5%9. However, these lipoproteins do not encompass the entire impact of liver steatosis on circulating lipids, and a significant portion of the association between liver steatosis and CAD remains to be elucidated. Hence, the aim of this study is to identify potential lipids linking steatotic liver disease to CAD using plasma lipidomics.
Methods
Study population
This investigation includes two populations: the Impaired Glucose Tolerance and Microbiota Study (IGT-Microbiota study) and a sub-population of the Swedish CArdioPulmonary bioImage Study (SCAPIS). IGT-Microbiota is an observational study of randomly selected Swedish individuals aged 50 to 65, with 1965 participants selected from over 5191 screened individuals, categorized by glycemic status so that they reflect a wide range of glucose levels10.Participants were recruited if they had dysglycemia (diabetes, impaired fasting glucose and/or impaired glucose tolerance) based on fasting glucose values and an oral glucose tolerance test (OGTT). Individuals were also included if they showed increased risk for future diabetes according to the Finnish Diabetes Risk Score (FINDRISC, score > 14) or had 2 first-degree relatives with diabetes. Individuals with normal glucose tolerance and FINDRISC score ≤ 14 were randomized (1:4) to inclusion. Exclusion criteria were diabetes, serious illness such as inflammatory bowel disease, rheumatic diseases, treatment with steroids, immune-modulating drug use, malignancy, antibiotic use within the last 3 months, and major cognitive dysfunction. SCAPIS is an observational study of 30,154 people aged 50 to 65 examined at six different sites in Sweden. Only the inability to comprehend spoken and written Swedish required for informed consent constituted an exclusion criterion in SCAPIS. The current study involves a subcohort consisting of 1111 participants who were selected from the SCAPIS Gothenburg site to match the traits of the IGT-Microbiota study population11 by applying the same inclusion/exclusion criteria as for the IGT-Microbiota study and selection based on glucose measurements to obtain similar proportions of individuals with dysglycemia and normal glucose tolerance. Cross-sectional data at baseline from these two populations were combined, resulting in a total study population of 2579 after excluding individuals on lipid-lowering therapy or with missing CACS. All participants provided written informed consent. The IGT-Microbiota study was approved by the ethics committee at Gothenburg University (Dnr 560 − 13), and SCAPIS was approved as a multicenter study by the ethics committee at Umeå University (Dnr 2010-228-31 M). The use of their data for analysis was approved by the Swedish ethical review authority in Uppsala (Dnr 2021–04030). All procedures performed were in accordance with the ethical standards of the Declaration of Helsinki.
Measurements
Liver steatosis measurements
Computer tomography (CT) was used for estimating liver steatosis. Subjects fasted at least 4 h before the CT visit. To standardize the liver glycogen levels the participants were given a standardized meal (Modifast, Nutriton&Santé) calculated based on body mass index (BMI) two hours prior to CT examination12. A dedicated dual-source CT scanner equipped with a Stellar Detector (Somatom Definition Flash, Siemens Medical Solution, Forchheim, Germany) was used for measuring CACS and fat deposits11. Liver fat estimation in CT scans is based on lower brightness due to fat’s reduced radiation absorption compared to liver tissue, quantified as mean attenuation in Hounsfield units (HU). A single CT scan slice with thickness of 5 mm in the level of the fourth lumbar vertebra (L4) depicting both liver lobes and the spleen was used to estimate a mean liver attenuation value12. Algorithms were used for automated image analysis, providing a range from 2 to 74 HU in the population of this study. Liver attenuation was used as a continuous variable in our study with its values subtracted from 100 to have a value in positive relation to the degree of hepatic steatosis and then logged transformed to achieve normal distribution. This variable was used as an indicator of liver steatosis.
Coronary artery calcium score measurements
Calcium content in the coronary arteries was measured using CT as previously described11. In brief, electrocardiogram-gated noncontrast CT imaging at 120 kV was used to get calcium content images. These images were reconstructed by using B35f HeartView medium CaScore, while CACS was estimated by the use of syngo.via calcium scoring software (Volume Wizard; Siemens)13. The amount of calcified content found in coronary arteries was summed to create the total CACS according to international standards14. CACS was used as a binary variable, categorizing participants into two groups: those with a CACS of zero and those with a CACS > 0 indicating atherosclerotic plaque presence.
Clinical covariates
Data on age, sex, and alcohol consumption were collected using standardized questionnaires. Alcohol consumption (ordinal-scale variable) was based on frequency using the question “How often do you have a drink containing alcohol?”, corresponding to the first question in the Alcohol Use Disorders Identification Test (AUDIT). Body weight was measured in light clothing without shoes. Systolic and diastolic blood pressure were measured in the supine position with an automatic device (Omron M10-IT, Omron Health care Co, Kyoto, Japan). Clinical chemistry analyses included HDL-C, TG, LDL-C, plasma glucose, hemoglobin A1c (HbA1c), insulin, and C-reactive protein (CRP)11.
Lipidomics analysis
Venous blood samples were collected after an overnight fast of at least 8 h and were centrifuged and stored at − 80 °C. Maximum time from needle-to-freeze was 2 h. Plasma samples from the participants were analyzed by Metabolon, Inc (Durham, NC) using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS)15. Samples were prepared using the automated MicroLab STAR® system from Hamilton Company where proteins were removed. Raw data was extracted, peak-identified and quality control processed using Metabolon’s hardware and software. The retrieved values were normalized by dividing each metabolite by its median value across all samples in each batch. The imputation process conducted by Metabolon was to replace missing values with the observed minimum value for each metabolite after batch normalization when the missing values were not due to random processing error. The total number of metabolites that were assigned to the lipids category by Metabolon, Inc in the dataset were 458 and were thus included in this study.
Statistical methods
Lipids’ association with liver steatosis and CACS
Associations between plasma lipids and liver steatosis were determined in linear regression models adjusted for age, sex, alcohol consumption, and cohort (IGT-Microbiota study or SCAPIS). To test for associations between plasma lipids and CACS = 0 compared to CACS > 0, we used logistic regression models adjusted for age, sex, and cohort. P-values were adjusted for multiple comparisons using the false discovery rate method and the lipids significantly associated with both liver steatosis and CACS group (p-value < 0.05) constituted the dataset used in the mediation analysis.
Mediation analysis
Mediation analysis assesses relationships between variables using mediators. In this study, we used the package mediation in R. Briefly, the analysis involves two regression models: one for the independent variable’s effect on mediators and one for mediators’ effects on the dependent variable16. The covariates used in the mediation model were age, sex, study cohort and alcohol consumption and liver attenuation was logged and coded as a continuous variable, as opposed to using a cut-off value. The output of this process includes the value for the average causal mediation effect (ACME), namely the effect of the independent variable (i.e., liver fat) on the dependent variable (i.e., CACs) moving indirectly through the mediator (i.e., the plasma lipid), as well as the value for the average proportion mediated. Confidence intervals for the ACME values were obtained using the bootstrapping method provided by the mediation package. Sensitivity analysis was performed to examine how various metabolic risk factors affected the lipid-mediated effects by including body mass index (BMI), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), systolic blood pressure (SBP), C-reactive protein (CRP), LDL-cholesterol (LDL-C), and plasma triglycerides (TG), one at a time in the mediation model.
Results
284 lipids associated with steatotic liver disease, 19 of them also with CACS > 0
Of the 2579 individuals of Swedish descent, 46,6% were male and the mean age was approximately 58 years. Half of the population had liver attenuation > 57 HU denoting no liver steatosis, while 1426 individuals had a CACS of zero and 1153 individuals had CACS > 0 (Table 1). Out of 458 lipids, 284 (62%) were significantly associated with liver steatosis (Fig. 1). We also categorized the lipids into groups and subgroups to give an overall view of the plasma lipidomic changes in steatotic liver disease (Fig. 2). The model included adjustments for age, gender, cohort, and alcohol consumption, while the additional inclusion of BMI did not alter the overall profile (Supplementary Fig. 1).
Out of 284 lipids associated with liver attenuation, the plasma levels of 19 lipids were found to also be associated with CACS (Fig. 1). These 19 lipids were increased in relation to both higher liver steatosis and higher CACS. Dihydroceramides and dihydrosphingomyelins were among the groups of lipids with the most pronounced associations. The same lipid that was found to have the strongest association with liver steatosis, N-stearoyl-sphinganine (d18:0/18:0), a dihydroceramide, also had the most significant association with CACS > 0. The lipid 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) had the largest association to CACS.
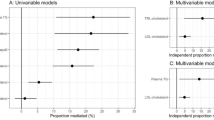
Two FFAs show high mediating effect between steatotic liver disease and CAD
To estimate the total impact of steatotic liver disease on CACS in our cohort, the odds ratio per 1-standard deviation increase in liver steatosis was first calculated (Supplementary Table 1), showing that liver fat was independently associated with CACS. We next performed a mediation analysis to investigate the relative importance of these 19 lipids in terms of mediating the association between liver steatosis and CACS. It was found that the two lipids that exhibited the highest ACME between steatotic liver disease and CACS were two FFAs (Fig. 3). The first FFA is docosatrienoate (22:3n6) with an ACME of 0.0197 (95% CI: 0.0025–0.0523). The second is 2-hydroxyarachidate with an ACME of 0.0175 (95% CI: 0.002–0.0475). The correlation matrix of these 19 lipids including TG and LDL-C indicates that these two fatty acids are not closely correlated (Supplementary Fig. 2). The groups of sphingolipids and GPLs followed the two FFAs based on their ranked ACME. Sphingolipids and GPLs were the most represented groups, with 8 and 5 lipids belonging to each group respectively, while docosatrienoate (22:3n6) and 2-hydroxyarachidate exhibited at least twice as large ACME as most other lipids.
The mediating effect of these two FFAs were independent of other risk factors
To examine the robustness of our results, sensitivity analysis was conducted. Only HOMA-IR lowered the ACME and narrowed the confidence interval of most lipids, while the opposite happened in case of BMI, SBP, and CRP (Supplementary Fig. 3). Overall, the results were robust against including these covariates. On the other hand, the addition of LDL-C attenuated the mediating role of sphingolipids, while the addition of TG had the same impact on GPLs (Supplementary Fig. 4).
Discussion
In this study, we investigated the mediating role of the plasma lipidome between steatotic liver disease and CAD. We found that more than half of circulating lipids were associated with steatotic liver disease, with 284 out of 458 lipids having altered plasma levels. This provides a comprehensive assessment of plasma lipid alterations across various lipid groups in a large population in comparison to previous research17,18,19,20. Among these 284 lipids, 19 were found to have increased plasma levels in both steatotic liver disease and CACS > 0. Of these 19 lipids, we identified two free fatty acids that independently mediated the effects of steatotic liver disease on CAD.
The main finding of this article is that two free fatty acids were identified as top mediators between steatotic liver disease and CAD. Research on both docosatrienoate (22:3n6) and 2-hydroxyarachidate is limited. Regarding docosatrienoate (22:3n6), a previous study demonstrated that it exhibits the highest fold change among metabolites measured in liver cells of humanized mice with liver steatosis in comparison to mice in the control group21. In general, N-6 PUFAs are implicated in promoting inflammation as the precursors of pro-inflammatory molecules22. Another study in mice showed that exposure to an organic pollutant increased the amount of docosatrienoate (22:3n6) in the liver, while increasing inflammation and exacerbating atherosclerosis23. In relation to 2-hydroxyarachidate, the only existing information in connection to steatotic liver disease is, to our knowledge, an observed elevation in the plasma of individuals with severe insulin-resistant diabetes24.
The next two groups of lipids according to the ranked ACMEs were sphingolipids and GPLs. When adjusting for LDL-C the role of sphingolipids were attenuated, whereas adjusting for plasma TG attenuated the role of GPLs (Supplementary Fig. 4). Since both LDL-particles and triglyceride-rich lipoproteins cause CAD, sphingolipids and GPLs do not appear to serve as independent risk factors for CAD. Consequently, the mediating effect of these two lipid groups may be explained by the role of constituents of atherogenic lipoproteins. However, the inclusion of LDL-C and TG did not affect the role of docosatrienoate (22:3n6) and 2-hydroxyarachidate. This suggests that they could mediate increased CAD risk independently from their role as constituents of lipoproteins.
A major limitation of this study is its observational nature, which precludes the establishment of causality; hence, the mediation results should not be interpreted as definite evidence of a causal relationship from steatotic liver disease via the mediators to CAD. Another limitation of the study is that the complexity of the relationships between liver steatosis, lipids, and CACS makes it challenging to precisely quantify the mediating role of these lipids since some of these lipids may further aggravate steatotic liver disease, for example, through inflammatory processes. Moreover, calculating the proportion mediated is based on the estimations of the ACME and total effect and their standard errors. Therefore, the mediation analysis primarily aids in identifying mediating lipids and ranking their importance, rather than providing precise mediation estimates. Another limitation may be that CT was used for estimating liver steatosis. MRI serves as the standard noninvasive method for assessing liver steatosis, non-contrast CT is a reliable method for quantifying liver fat given its strong linear correlation with MRI-derived values25, however CT suffers from lower sensitivity and hence there is a risk that some individual’s liver fat was not detected. The major cause of liver steatosis in this cohort is likely to be metabolic-dysfunction. However, while alcohol consumption could be adjusted for in our analyses, the potential influence of rarer causes such as viral and autoimmune liver diseases cannot be completely ruled out. Future studies are needed to establish whether the findings reported in this study are reflected in individuals with confirmed MASLD where other causes are ruled out. Furthermore, it is difficult to exclude the possibility that the increase in plasma levels of these lipids precedes liver steatosis using observational data, although the mediating effects of docosatrienoate (22:3n6) and 2-hydroxyarachidate are not affected by BMI and HOMA-IR, which are known risk factors for liver steatosis. Nevertheless, their significant associations with liver steatosis and atherosclerosis underscore their importance as common denominators of these conditions. Finally, the absence of a validation cohort with definitive cause of SLD impedes our ability to validate our findings and establish their robustness. Future research in this area may strengthen the validity of the conclusions drawn.
Conclusion
Our findings suggest that steatotic liver disease is associated with changes in the plasma lipidome that may have implications for coronary artery disease. The lipidomic profiling revealed that a substantial proportion (62%) of the 458 lipids examined were associated with liver steatosis. Importantly, two specific fatty acids, namely docosatrienoate (22:3n6) and 2-hydroxyarachidate, were identified as mediators between steatotic liver disease and coronary artery calcification, distinct from other known atherosclerotic risk factors. Further research is needed to elucidate any potential causal pathways and confirm these associations in longitudinal studies.
Data availability
Data may be shared upon request to the corresponding author.
Abbreviations
- SLD:
-
Steatotic liver disease
- CACS:
-
Coronary artery calcium score
- IGT-study :
-
Microbiota, development of type 2 diabetes and cardiovascular disease study
- SCAPIS:
-
Swedish CArdioPulmonary bioImage Study
- CT:
-
Computed tomography
- HU:
-
Hounsfield units
- CRP:
-
C-reactive protein
- HbA1c:
-
Hemoglobin A1c
- TG:
-
Triglycerides
- SBP:
-
Systolic blood pressure
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- GPL:
-
Glycerophospholipid
References
Baratta, F. et al. Heterogeneity of non-alcoholic fatty liver disease (NAFLD): implication for cardiovascular risk stratification. Atherosclerosis. 357, 51–59 (2022).
Liu, J. et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African americans: the Jackson Heart Study. Atherosclerosis. 224 (2), 521–525 (2012).
Mellinger, J. L. et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J. Hepatol.63 (2), 470–476 (2015).
Al Rifai, M. et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 239 (2), 629–633 (2015).
VanWagner, L. B. et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the coronary artery risk development in young adults study. Atherosclerosis. 235 (2), 599–605 (2014).
Gummesson, A. et al. Non-alcoholic fatty liver disease is a strong predictor of coronary artery calcification in metabolically healthy subjects: a cross-sectional, population-based study in middle-aged subjects. PLoS One. 13 (8), e0202666 (2018).
DeFilippis, A. P. et al. Nonalcoholic fatty liver disease and serum lipoproteins: the multi-ethnic study of atherosclerosis. Atherosclerosis. 227 (2), 429–436 (2013).
Deprince, A., Haas, J. T. & Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab.42, 101092 (2020).
Björnson, E. et al. Mediating role of atherogenic lipoproteins in the relationship between liver fat and coronary artery calcification. Sci. Rep.13 (1), 13217 (2023).
Schmidt, C., Gummesson, A., Bäckhed, F., Bergström, G. & Söderberg, M. Psychosocial work conditions and prediabetes risks: a cross-sectional study in middle-aged men and women. Sci. Rep.13 (1), 1198 (2023).
Bergström, G. et al. The Swedish CArdioPulmonary BioImage Study: objectives and design. J. Intern. Med.278 (6), 645–659 (2015).
Kullberg, J. et al. Automated analysis of liver fat, muscle and adipose tissue distribution from CT suitable for large-scale studies. Sci. Rep.7 (1), 10425 (2017).
Bergström, G. et al. Prevalence of subclinical coronary artery atherosclerosis in the General Population. Circulation. 144, 916–929 (2021).
McCollough, C. H. et al. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 243 (2), 527–538 (2007).
Evans, A. M. et al. Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the identification and relative quantification of the small-molecule complement of Biological systems. Anal. Chem.81 (16), 6656–6667 (2009).
Imai, K., Keele, L., Tingley, D. & Yamamoto, T. (eds) Causal mediation analysis using R. in Advances in Social Science Research Using R. (Springer, New York, NY, 2010).
Puri, P. et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 50 (6), 1827–1838 (2009).
Gorden, D. L. et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res.56 (3), 722–736 (2015).
Kalhan, S. C. et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 60 (3), 404–413 (2011).
Barr, J. et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J. Proteome Res.11 (4), 2521–2532 (2012).
Bissig-Choisat, B. et al. A human liver chimeric mouse model for non-alcoholic fatty liver disease. JHEP Rep.3 (3), 100281 (2021).
Hliwa, A., Ramos-Molina, B., Laski, D., Mika, A. & Sledzinski, T. The role of fatty acids in non-alcoholic fatty liver disease progression: An update. Int. J. Mol. Sci.22(13), 6900 (2021).
Petriello, M. C. et al. Dioxin-like PCB 126 increases systemic inflammation and accelerates atherosclerosis in lean LDL receptor-deficient mice. Toxicol. Sci.162 (2), 548–558 (2018).
Zaghlool, S. B. et al. Metabolic and proteomic signatures of type 2 diabetes subtypes in an arab population. Nat. Commun.13 (1), 7121 (2022).
Starekova, J., Hernando, D., Pickhardt, P. J. & Reeder, S. B. Quantification of Liver Fat content with CT and MRI: state of the art. Radiology. 301 (2), 250–262 (2021).
Acknowledgements
We thank the staff at the SCAPIS test center in Gothenburg and members of the physiology group at the Wallenberg Laboratory, University of Gothenburg, for technical assistance. We also thank Rosie Perkins for her contribution as scientific editor. Further, we thank all study participants for making the studies possible.
Funding
Work by Göran Bergström was supported by Forte (17-01964), Heart and Lung foundation (20180324), the Swedish Research Council (2019 − 01140) and LUA/ALF: ALFGBG-718851 and by the Knut and Alice Wallenberg Foundation, the Swedish Research Council and VINNOVA (Sweden’s innovation agency). Work by Fredrik Bäckhed (FB) was supported by AFA insurances, Knut and Alice Wallenberg Foundation (2017.0026), HLF Swedish Heart Lung Foundation (20210366) and Grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-718101). FB is Wallenberg Scholar and Torsten Soderberg Professor in Medicine. Work by Anders Gummesson was supported by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-929989, ALFGBG-991828) and the Sahlgrenska University Hospital Fund C4A for research in Laboratory Medicine and Imaging. Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
EB and DS performed statistical analyses, constructed the manuscript and edited the manuscript. ML critiqued and edited the manuscript. AG supervised all parts of the work, including the statistical analyses and writing of the manuscript. FB and GB supervised parts of the work and critiqued the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Björnson, E., Samaras, D., Levin, M. et al. The impact of steatotic liver disease on coronary artery disease through changes in the plasma lipidome. Sci Rep 14, 22307 (2024). https://doi.org/10.1038/s41598-024-73406-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73406-8
Keywords
This article is cited by
-
Towards sustainable lipidomics: computational screening and experimental validation of chloroform-free alternatives for lipid extraction
Analytical and Bioanalytical Chemistry (2025)