Abstract
Focal segmental glomerulosclerosis (FSGS) is a major cause of pediatric kidney failure. Most cases of FSGS in children are idiopathic and have a high risk of post-transplantation recurrence and graft loss. Common treatments for recurrent FSGS (rFSGS) post-transplantation include plasmapheresis, immunoadsorption, and/or immunomodulatory therapy. This study retrospectively evaluated the efficacy and safety of early plasmapheresis followed by rituximab for inducing and maintaining remission in rFSGS. Between 2014 and 2023, 8 of 65 pediatric kidney transplant recipients at our center were diagnosed with idiopathic FSGS. rFSGS was diagnosed based on nephrotic range proteinuria with no other cause and managed with plasmapheresis. Rituximab therapy was used for those who did not achieve complete remission with prolonged plasmapheresis or remained plasmapheresis dependent. 6 of 8 (75%) transplant recipients with idiopathic FSGS experienced rFSGS. All patients achieved partial or complete remission with plasmapheresis, with response times ranging from 8 to 379 days (median 13 days). Rituximab therapy was introduced for 5 plasmapheresis-dependent patients, leading to sustained remission and cessation of plasmapheresis in 3 patients, while 2 showed improved proteinuria and reduced plasmapheresis frequency. Adverse effects included rituximab-induced serum sickness in one patient and one mild allergic reaction. One patient experienced graft loss due to humoral rejection, but no grafts were lost to rFSGS, and all other grafts remained functional over an average follow-up of 50 months. Early plasmapheresis followed by rituximab therapy effectively induces remission in most post-transplantation rFSGS cases, is well tolerated, and prevents graft loss. Larger studies are needed to confirm these findings.
Similar content being viewed by others
Introduction
Focal segmental glomerulosclerosis (FSGS) is a leading cause of pediatric kidney failure, accounting for approximately 11.5% of transplanted patients in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTC) reports over the past two decades. This condition remains a leading cause of pediatric kidney failure1,2,3Data from Israel show a similar prevalence4. While approximately 30% of pediatric FSGS cases are attributed to monogenic disorders, the majority are idiopathic, presumably caused by currently unidentified circulating factor(s)3,5,6.
Idiopathic FSGS of presumed immunologic etiology poses a significant risk of post-transplant recurrence and subsequent graft loss. Due to its rarity, comprehensive data on post-transplantation recurrent FSGS (rFSGS) is limited. In adults, the reported recurrence rate is approximately 30%, with graft loss observed in around 40% of cases7. However, pediatric FSGS has a higher recurrence rate, ranging from 20% to as high as 86% in first kidney transplantation, eventually reaching nearly 100% in subsequent transplantations3,8,9. A recent systematic review and meta-analysis reported an overall pediatric rFSGS rate of 39% (95% confidence interval), ranging between 33 and 44%10.
Identifying patients at higher risk for rFSGS is challenging, and has been a topic of debate, due to conflicting evidence. According to the above-mentioned systematic review and meta-analysis by Morello at al, initial steroid sensitivity and non-genetic steroid-resistant nephrotic syndrome (SRNS) were associated with a higher recurrence risk, while histology and gender did not significantly affect recurrence rate10. Other potential risk factors, such as age at presentation, time from presentation to kidney failure, and donor type, were not fully analyzed, due to lack of data10. In contrast, other studies have indicated, albeit with conflicting results, that histology showing minimal change/mesangial proliferation, both young and old age at presentation, rapid progression to kidney failure, and living donation may serve as potential risk factors for rFSGS3,9. These discrepancies highlight the need for more comprehensive research to better understand the predictors of rFSGS and improve patient outcomes.
Currently available data on prevention and management of rFSGS are mostly derived from small case series characterized by substantial inconsistency between study protocols (e.g., treatment timing, protocol duration, dosage variation, etc.)9. This is further complicated by limited understanding of the pathophysiology underlying both primary and rFSGS. Therefore, there is a substantial need for consensus recommendations regarding prevention and treatment of rFSGS. This necessity, recognized by the CERTAIN study group8, and subsequently summarized by Harshman et al.3, has led to publication of a consensus statement9. The main conclusion derived from this work indicates that available evidence is insufficient to endorse specific intervention strategies for preventing rFSGS, such as unilateral/bilateral native kidney nephrectomy, prophylactic plasmapheresis (PP), specific immunomodulatory induction therapy, or donor selection. The only exception, supported by a weak recommendation from the CERTAIN group, pertains to the use of prophylactic PP/immunoadsorption (IA) in children who have already experienced previous allograft loss due to rFSGS8. All mentioned studies, however, recommend close surveillance with early initiation of PP/IA in case of overt rFSGS3,8,9. Additional therapeutic strategies may involve administration of anti-CD20 depleting antibodies, LDL apheresis, high-dose cyclosporine, and RAAS blockade3,8,9. PP/IA has demonstrated notable benefits, and when supplemented with anti-CD20 depleting antibody agents, led to approximately 75% partial or complete remission rate3,8,9. Importantly, all these recommendations rely on limited available data and on a low level of supporting evidence3,8,9, highlighting the need for a larger body of evidence to reach more conclusive recommendations.
In this single-center, retrospective study, we share our experience regarding the occurrence, management, and long-term outcome of rFSGS in a cohort of pediatric kidney transplant recipients. As a tertiary medical center caring for diverse ethnic communities, our insights reflect a wide spectrum of patient backgrounds and clinical scenarios.
Methods
We retrospectively summarized the clinical course of eight pediatric kidney transplant recipients with idiopathic FSGS, out of a total of 65 kidney transplants performed at our center over a decade, from January 2014 and December 2023. Five of the eight FSGS patients underwent preventive PP initiated between day seven pre-transplantation and one day post-transplantation, depending on donor source (living/deceased).
Close surveillance for rFSGS was performed through frequent urinalysis. During the first week post-transplant, the urine protein/creatinine (uP/Cr) ratio was measured daily. Subsequently, uP/Cr was monitored twice weekly for the first month, with a gradual reduction in frequency to weekly, biweekly, and finally monthly after six months. Any rise in the uP/Cr ratio above 0.2 mg/mg was further evaluated by a 24-h urine collection. rFSGS was diagnosed based on the presence of nephrotic range proteinuria of no other cause, following exclusion of clinical and laboratory evidence for transplant rejection, infection or other post-transplant potential complications.
Upon recurrence of FSGS, PP was initiated either daily or trice weekly, depending on clinical severity at presentation. IVIG replacement (0.4 g/kg/dose) was given to patients undergoing extensive PP (more than twice weekly). Fibrinogen levels were monitored and fibrinogen concentrate was given when levels were below 100 mg/dl. Close monitoring guided the pace of tapering down PP frequency, upon achievement of complete remission, which was defined as a urine protein-to-creatinine ratio (uP/Cr) < 0.2 mg/mg. Partial but persistent remission, with a uP/Cr ratio between 0.2 mg/mg and 2 mg/mg, prompted a slower tapering of PP. Initial management included daily or trice weekly PP, with a gradual decrease in frequency. Failure to achieve complete remission while on prolonged PP, or development of PP dependency precluding PP cessation, was managed with rituximab therapy. Rituximab was administered in four weekly doses of 375 mg/m² body surface area (BSA). This study was conducted in accordance with ethical standards and received approval from the Institutional Review Board (IRB) of Rambam Health Care Campus (IRB Number: 0254 − 24). Given that this was a retrospective study utilizing anonymized data, informed consent was waived by the respective IRB. All patient data were fully anonymized and unidentifiable, ensuring compliance with the relevant data protection and privacy regulations. All methods were performed in accordance with relevant guidelines and regulations.
Results
Between 2014 and 2023, 65 pediatric kidney transplantations were performed at our center. Of these, 15 were diagnosed with steroid Resistant Nephrotic Syndrome (23% of total). Among them, seven presented with congenital/infantile nephrotic syndrome, and eight patients (three females and five males) were diagnosed with idiopathic FSGS, representing 12.5% of the total (see Table 1 for detailed demographic and baseline clinical characteristics). The average age at presentation with FSGS was 7 years (median 6 years ± 4.75), and at transplantation 13.5 years (median 14 years ± 3). Most patients were of Arab-Muslim origin. Five out of eight patients presented with SRNS at initial presentation, while three patients presented with steroid sensitive nephrotic syndrome, which later transformed into steroid resistance.
Histopathology of native kidney biopsy at presentation showed FSGS in four patients, minimal change disease (MCD) in three (as can be seen in early FSGS), and no available data in one patient. Genetic analysis using whole exome sequencing was performed in seven patients, excluding potential disease-causing variants in genes known to be associated with FSGS. One patient displayed a homozygous variant of unknown significance (VUS) in the ARHGAP24 gene, which has been associated with FSGS. However, this VUS was categorized as “likely benign” by computational predicting software. This patient also had an additional heterozygous risk allele in APOL1 gene.
Three out of eight patients (37.5%), received living-related kidney donation, while five (62.5%) underwent deceased-donor kidney transplantation. Five patients (62.5%) were managed with preemptive PP, initiated between seven days pre-transplantation and one day one post-transplantation, depending on donor source (living/deceased), for at least six consecutive sessions. Most patients received induction therapy with basiliximab (an anti-IL-2R antibody) and glucocorticoids. One highly sensitized patient, exhibiting a positive panel reactive antibody (PRA) test, received thymoglobulin induction.
Three patients (37.5%) developed delayed graft function, with two requiring short-term post-transplantation hemodialysis. All patients were routinely maintained on triple immunosuppressive therapy, including glucocorticoids, tacrolimus and mycophenolate mofetil (MMF). Six patients (75%) experienced rFSGS, presenting between 1 and 60 days post-transplant (median 2.5 days, average 12.5 days).
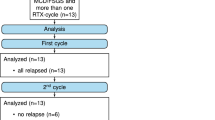
rFSGS was treated with prompt initiation of PP as described above. The overall duration of PP therapy during the first episode of rFSGS varied widely among patients, ranging from 22 to over 2000 days, as some patients remained PP-dependent until the end of the follow up period. We did not encounter any severe complications with PP. Two patients (#6) who underwent daily PP required several doses of fibrinogen concentrate due to severe hypofibrogenemia. However, they did not present any episodes of bleeding. Additionally, patients had several episodes of central line-related bacteremia, which were treated with systemic antibiotics, and two cases necessitating central line replacement due to malfunction. All patients achieved either partial or complete remission while on PP therapy. However, five of the six patients with rFSGS who demonstrated prolonged PP-dependence received rituximab therapy, as per protocol. The average and median time on PP prior to rituximab treatment was 169 and 185 days, respectively, with SD of 157 days. Among these patients, three achieved complete and sustained remission, enabling cessation of PP, while two achieved partial remission, allowing the reduction of plasmapheresis frequency to once weekly (Fig. 1).
Adverse effects included rituximab-induced serum sickness in one patient, necessitating discontinuation of therapy after two doses.However, there were no additional episodes of rFSGS during two-year follow-up in this patient. Another patient experienced a suspected mild allergic reaction after the first dose of rituximab, and subsequent doses were successfully administered under a desensitizing protocol.
Other treatment modalities used prior to rituximab therapy during the earlier phase of this longitudinal study included short-term LDL apheresis in two patients, and temporarily transitioning two other patients from tacrolimus to high dose cyclosporine. However, three out of four patients managed with those measures eventually required rituximab therapy to achieve complete remission.
In our patients, as mentioned above, rituximab was administered in four weekly doses of 375mg/m2 BSA each. All patients, except one, have completed the full protocol. In all patients, C19/CD20 or IgG levels measured within a month of treatment were suppressed, indicating effective B cell depletion.One patient (# 6) developed severe serum sickness four days after the second rituximab dose, manifested with general malaise, arthralgia, myalgia, fever, and anemia. An infectious and immunologic workup were unremarkable. She was successfully treated with glucocorticoids, analgesia, and discontinuation of following rituximab doses. Her CD19/CD20 counts were suppressed to 0% following the second rituximab dose. As she achieved complete remission and CD19/CD20 levels were fully suppressed, alternative treatments were not necessary at that time and during the next two-year follow up. Another patient (# 8), developed a mild allergic reaction to rituximab, characterized by rash, itching, and subjective shortness of breath (with no objective evidence of pulmonary involvement). Subsequently, all remaining doses were administered with premedication, using a desensitization protocol, without further complications. We have not encountered any serious infectious complications within our cohort.
One patient (#5) experienced three episodes of rFSGS. While the first two episodes responded to PP alone, allowing for PP withdrawal for several months, the third episode required rituximab therapy, resulting in subsequent complete sustained remission and enabling treatment cessation. One patient (#4) did not achieve complete remission and remained PP dependent throughout the follow-up period, experiencing exacerbations in proteinuria following attempted decrease in PP frequency, but without full-blown recurrent relapses. All other patients did not experience recurrent relapses during the follow-up period.
During follow-up, two patients developed acute transplant humoral rejection managed with PP, IVIG, and one with Bortezomib.
One patient suffered from graft loss due to humoral rejection, but no graft was lost to rFSGS. All other grafts remained functional with normal GFR, over an average follow-up period of 50 months for all patients, and for 42 months for those with rFSGS. Tables 2, 3 and 4 provide comprehensive clinical data regarding each patient. Figure 2 illustrate representative examples depicting the response to treatment over time.
Discussion
In our cohort of pediatric transplant recipients, idiopathic FSGS accounted for 12.5% of patients, consistent with previous reports2,4. We observed a 75% recurrence rate, predominantly occurring within the first week post-transplantation, aligning with previous findings3,8,9. Despite the high incidence of recurrence and the associated risk of graft loss, well-established evidence-based guidelines regarding preventive and treatment measures for rFSGS are limited, hence, complicating clinical decision-making during the pre-, peri- and post transplantation periods.
Prevention of rFSGS
Insufficient data exists to endorse a specific immunosuppressive induction regimen for preventing rFSGS3,8,9. Additionally, current evidence does not support refraining from living kidney donation or performing of native kidney nephrectomies as preventive measures8.
Several small-scale studies suggest the potential effectiveness of preemptive PP during the perioperative period11,12. However, contrasting findings indicate no discernible benefit from this approach13,14. These conflicting outcomes have led to the publication of various consensus guidelines, including the aforementioned CERTAIN consensus, which refrain from advocating for the routine use of preemptive PP8,9. Our own findings align with this uncertainty, underscoring the lack of consistent evidence supporting routine use of preemptive PP for preventing rFSGS.
Of note, a recent randomized controlled trial on preemptive rituximab therapy for prevention of rFSGS post-kidney transplantation (PRI-VENT FSGS) in both pediatric and adult patients is currently underway. This study aims to provide evidence-based data regarding measures for preventing rFSGS, which could significantly enhance clinical decision-making15.
Treatment of rFSGS
It is recommended to meticulously monitor and promptly treat rFSGS, with particular emphasis on the early post-transplant period8,9. Given the hypothesis suggesting an unidentified circulating permeability factor underlying primary FSGS, the use of an extracorporeal clearance mechanism, such as PP or IA to remove this factor, is compelling. Indeed, previous case series have shown favorable results using either PP or IA in the treatment of rFSGS3,16. In adult cohorts, remission rates with PP/IA varied widely, ranging from 20 to 100%7,16,17, while outcomes in children appeared to be even more promising, with remission rates between 83 and 100%18,19,20. Common duration of reported PP protocols in adults is ~ 2 weeks9. In our cohort, all patients achieved either partial or complete remission with PP therapy alone. However, unlike most published protocols, that typically prescribe a limited number of PP/IA sessions9,21, we observed a high rate of PP dependence, requiring significantly extended periods of PP therapy for achieving sustained remission (average time on PP 645 days, median 400 days). Uffing et al.21 documented a subset of adult patients who remained PP-dependent for extended periods, up to several years, even with the addition of rituximab (78%) and RAAS blockade (85%). To the best of our knowledge, there are no similar long-term reports on pediatric patients. However, in a short-term follow-up study, Alhasan et al.18. , reported that some pediatric patients treated with PP/IA remained PP-dependent for up to five months, with many also receiving rituximab and/or abatacept. In our hands, 5 out of 6 of patients remained PP dependent, experiencing worsening proteinuria upon PP withdrawal. Unfortunately, long-term PP dependency, although enabling sustained partial or complete remission and preservation of graft function, is associated with increased risk of bleeding, central line-related infections, aggravated immunosuppressive state, the burden of recurrent hospital visits, and an over reduction of quality of life for both patients and caregivers. The high rate of PP dependence in our cohort, compared to previous studies conducted in adults, may stem from several factors. These include differences in the natural history of the disease in children, who experience higher relapse rates, may experience a more aggressive course—our goal of achieving complete rather than incomplete remission, and potential changes in the biological features of the disease over time. Rituximab, an anti-CD20 monoclonal antibody, was initially proposed as a potential therapeutic avenue for rFSGS in 200622. Subsequent studies, primarily involving adult patients, have demonstrated its efficacy, particularly when used in combination with PP/IA16. While case reports and small case series in pediatric patients have indicated some benefits, the results have been inconsistent23,24,25,26. Regimens of 1–6 doses have been reported3,9. Major side effects include risk of allergic reaction and infectious complications secondary to severe immunosuppression3,16.
In our cohort, rituximab therapy proved to be a highly effective intervention for managing PP-dependence in rFSGS. Specifically, three out of five patients who received rituximab due to PP dependence achieved complete remission, enabling subsequent PP withdrawal. The remaining two patients exhibited markedly improved proteinuria, enabling reduction in PP frequency to once weekly. Additionally, in one patient who experienced multiple episodes of rFSGS, rituximab therapy initiated during the third relapse led to sustained remission. This patient’s clinical course highlights the potential of rituximab as a valuable treatment option in recurrent cases of rFSGS.
Our protocol yielded excellent outcomes during an average follow-up of 42 months (mean 40.5 months), with a 100% remission rate. While only one patient experienced graft loss due to uncontrolled humoral rejection, all other grafts remained well-functioning. However, it is important to note that two patients continued to require persistent PP, reflecting a balance between effective disease control and the challenges of long-term therapy.
As detailed in the results section, alternative therapies such as LDL apheresis27 and transitioning from tacrolimus to high-dose cyclosporine28, were occasionally administered, yielding mixed outcomes. However, sustained remission was achieved only following rituximab treatment.
Considering the constraints and methodological challenges inherent in studies addressing the management of rFSGS, including of our current report, there is a substantial need for innovative, precisely targeted, and more efficacious therapeutic strategies. Such advancements may hold the potential to prevent, or at least improve the management of rFSGS. With ongoing progress in understanding the pathophysiology underlying SRNS, novel targeted and specific therapies are constantly being developed and evaluated9,29, including, among others:
-
1.
Novel anti-CD20 agents, including fully humanized anti-CD20 monoclonal antibodies, such as ofatumumab or obinutuzumab, which have shown potential superiority over rituximab concerning both safety and efficacy against rFSGS30,31.
-
2.
Global anti-B cell strategy, involving the utilization of daratumumab (targeting CD38) in conjunction with an anti-CD20 monoclonal antibody aimed at long-lived plasma cells, emerges as a potential therapeutic approach for rFSGS30,32,33.
-
3.
Abatacept, a CTLA-4 inhibitor, designed to hinder the co-stimulation of antibody presenting cells crucial for downstream T cell activation. However, recent trials present conflicting results regarding the efficacy of abatacept in treating rFSGS34,35.
-
4.
Ongoing research dedicated to exploring optimal extracorporeal therapy methods and preventive measures for rFSGS (clinicaltrials.gov).
In summary, managing rFSGS in pediatric kidney transplantation presents a significant challenge, marked by the lack of clear etiology, preventative strategies, or robust evidence-based treatment guidelines. In our outlined approach, we advocate for early initiation of PP therapy, emphasizing the importance of prolonged PP when necessary, in conjucntion with rituximab. In our hands, this combined treatment has demonstrated promising results, achieving high remission rate with minimal side effects. Nevertheless, given the relatively small number of patients in our retrospective cohort, further trials, especially larger-scale randomized and controlled studies involving pediatric patients from multiple centers are crucial to validate and refine our approach. Furthermore, robust basic science research is essential to deepen our understanding of the molecular mechanisms underlying both primary and rFSGS, thereby hopefully enhancing treatment modalities and ultimately improving patient outcomes.
Data availability
All data generated or analysed during this study are included in this published article.
References
Smith, J. M., Martz, K. & Blydt-Hansen, T. D. Pediatric kidney transplant practice patterns and outcome benchmarks, 1987–2010: A report of the North American Pediatric renal trials and collaborative studies. Pediatr Transplant 17(2), 149–157. https://doi.org/10.1111/petr.12034 (2013).
Chua, A. et al. Kidney transplant practice patterns and outcome benchmarks over 30 years: The 2018 report of the NAPRTCS. Pediatr Transplant 23, 8. https://doi.org/10.1111/petr.13597 (2019).
Harshman, L. A., Bartosh, S., Engen, R. M. Focal segmental glomerulosclerosis: Risk for recurrence and interventions to optimize outcomes following recurrence. 2022. https://doi.org/10.1111/petr.14307.
Cleper, R. et al. Focal segmental glomerulosclerosis in pediatric kidney transplantation: 30 years’ experience. Clin Transplant 30(10), 1324–1331. https://doi.org/10.1111/ctr.12825 (2016).
Trautmann, A. et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatric Nephrol. 35(8), 1529–1561. https://doi.org/10.1007/s00467-020-04519-1 (2020).
Sharma, M., Sharma, R., Mccarthy, E. T., and Savin, V. J. ‘The FSGS factor: Enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma, 1999. http://journals.lww.com/jasn
Uffing, A. et al. Recurrence of FSGS after kidney transplantation in adults. Clin J. Am. Soc. Nephrol. 15(2), 247–256. https://doi.org/10.2215/CJN.08970719 (2020).
Weber, L. T. et al. Clinical practice recommendations for recurrence of focal and segmental glomerulosclerosis/steroid-resistant nephrotic syndrome. Pediatr Transpl. 25(3), (2021). https://doi.org/10.1111/petr.13955.
Raina, R. et al. Post-transplant recurrence of focal segmental glomerular sclerosis: Consensus statements. Kidney Int 105(3), 450–463. https://doi.org/10.1016/j.kint.2023.10.017 (2024).
Morello, W., Proverbio, E., Puccio, G. & Montini, G. A systematic review and meta-analysis of the rate and risk factors for post-transplant disease recurrence in children with steroid resistant nephrotic syndrome. Kidney Int Rep 8(2), 254–264. https://doi.org/10.1016/j.ekir.2022.10.030 (2023).
Gohh, R. Y. et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am. J. Transplant. 5(12), 2907–2912. https://doi.org/10.1111/j.1600-6143.2005.01112.x (2005).
T. Ohta et al. Effect of pre-and postoperative plasmapheresis on posttransplant recurrence of focal segmental glomerulosclerosis in children, 2001. Available: http://journals.lww.com/transplantjournal
P. S. Verghese, M. N. Rheault, S. Jackson, A. J. Matas, S. Chinnakotla, and B. Chavers, The effect of peri-transplant plasmapheresis in the prevention of recurrent FSGS. Pediatr Transplant 22(3), (2018). https://doi.org/10.1111/petr.13154.
Gonzalez, E., Ettenger, R., Rianthavorn, P., Tsai, E. & Malekzadeh, M. Preemptive plasmapheresis and recurrence of focal segmental glomerulosclerosis in pediatric renal transplantation. Pediatr Transplant 15(5), 495–501. https://doi.org/10.1111/j.1399-3046.2011.01478.x (2011).
Rheault, M. N. et al. A randomized controlled trial of preemptive rituximab to prevent recurrent focal segmental glomerulosclerosis post-kidney transplant (PRI-VENT FSGS): protocol and study design. Front. Nephrol. 3 (2023). https://doi.org/10.3389/fneph.2023.1181076.
Alasfar, S. et al. Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation. Transplantation 102(3), e115–e120. https://doi.org/10.1097/TP.0000000000002008 (2018).
Al Shamsi, H. R., Shaheen, I., and Aziz, D. Management of recurrent focal segmental glomerulosclerosis (FSGS) post renal transplantation. W.B. Saunders (2022). https://doi.org/10.1016/j.trre.2021.100675.
Alhasan, K. A., Alherbish, A., Osman, A., Kari, J. A. & Almojalli, H. Successful treatment of recurrent focal segmental glomerulosclerosis after transplantation in children: A single-center experience. Transplant Proc 51(2), 517–521. https://doi.org/10.1016/j.transproceed.2019.01.004 (2019).
Runowski, D., Prokurat, S., Rubik, J. & Grenda, R. Therapeutic plasma exchange in pediatric renal transplantation experience of one decade and 389 sessions. Transplant Proc 50(10), 3483–3486. https://doi.org/10.1016/j.transproceed.2018.07.015 (2018).
Straatmann, C. et al. Success with plasmapheresis treatment for recurrent focal segmental glomerulosclerosis in pediatric renal transplant recipients. Pediatr Transplant 18(1), 29–34. https://doi.org/10.1111/petr.12185 (2014).
Uffing, A. et al. Long-term apheresis in the management of patients with recurrent focal segmental glomerulosclerosis after kidney transplantation. Kidney Int Rep 7(6), 1424–1427. https://doi.org/10.1016/j.ekir.2022.03.002 (2022).
Pescovitz, M. D., Book, B. K. & Sidner, R. A. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med 354(18), 1961–1963. https://doi.org/10.1056/NEJMc055495 (2006).
Grenda, R., Jarmuzek, W., Piatosa, B., and Rubik, J. Long-term effect of rituximab in maintaining remission of recurrent and plasmapheresis-dependent nephrotic syndrome post-renal transplantation: Case report. Pediatr Transplant, 15(6) (2011). https://doi.org/10.1111/j.1399-3046.2010.01303.x.
Grenda, R., Jarmużek, W., Rubik, J., Piątosa, B. & Prokurat, S. Rituximab is not a ‘magic drug’ in post-transplant recurrence of nephrotic syndrome. Eur J Pediatr 175(9), 1133–1137. https://doi.org/10.1007/s00431-016-2747-1 (2016).
Rodríguez-Ferrero, M., Ampuero, J. & Anaya, F. Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transplant Proc 41(6), 2406–2408. https://doi.org/10.1016/j.transproceed.2009.06.044 (2009).
Dello Strologo, L. et al. Use of rituximab in focal glomerulosclerosis relapses after renal transplantation. Transplantation 88(3), 417–420. https://doi.org/10.1097/TP.0b013e3181aed9d7 (2009).
Shah, L. et al. LDL-apheresis-induced remission of focal segmental glomerulosclerosis recurrence in pediatric renal transplant recipients. Pediatric Nephrol. 34(11), 2343–2350. https://doi.org/10.1007/s00467-019-04296-6 (2019).
Raafat, R. H., Kalia, A., Travis, L. B. & Diven, S. C. High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am. J. Kidney Dis. 44(1), 50–56. https://doi.org/10.1053/j.ajkd.2004.03.028 (2004).
Angeletti, A. et al. Biologics in steroid resistant nephrotic syndrome in childhood: review and new hypothesis-driven treatment, 2023, Frontiers Media SA. https://doi.org/10.3389/fimmu.2023.1213203.
Delbet, J. D., Hogan, J., Parmentier, C., Ulinski, T., and Dossier, C. Successful global anti-B-cell strategy with daratumumab in a patient with post-transplant nephrotic syndrome recurrence unresponsive to immunoadsorption and obinutuzumab. Pediatr Transplant, 27(5) (2023). https://doi.org/10.1111/petr.14544.
Reynolds, B. C. et al. UK experience of ofatumumab in recurrence of focal segmental glomerulosclerosis post-kidney transplant. Pediatric Nephrol. 37(1), 199–207. https://doi.org/10.1007/s00467-021-05248-9 (2022).
Angeletti, A. et al. Combined rituximab and daratumumab treatment in difficult-to-treat nephrotic syndrome cases. Kidney Int Rep 9(6), 1892–1896. https://doi.org/10.1016/j.ekir.2024.04.006 (2024).
Angeletti, A. et al. Efficacy of combined rituximab and daratumumab treatment in posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant 24(4), 688–692. https://doi.org/10.1016/j.ajt.2023.12.010 (2024).
Burke, G. W. et al. Benefit of B7–1 staining and abatacept for treatment-resistant post-transplant focal segmental glomerulosclerosis in a predominantly pediatric cohort: time for a reappraisal. Pediatric Nephrol. 38(1), 145–159. https://doi.org/10.1007/s00467-022-05549-7 (2023).
Delville, M. et al. B7–1 blockade does not improve post-transplant nephrotic syndrome caused by recurrent FSGS. J. Am. Soc. Nephrol. 27(8), 2520–2527. https://doi.org/10.1681/ASN.2015091002 (2016).
Acknowledgements
This study was conducted in accordance with ethical standards and received approval from the Institutional Review Board (IRB) of Rambam Health Care Campus (IRB Number: 0254 − 24). Given that this was a retrospective study utilizing anonymized data, informed consent was waived by the respective IRB. All patient data were fully anonymized and unidentifiable, ensuring compliance with the relevant data protection and privacy regulations.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to the work. The authors declare no conflicts of interest.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Plonsky-Toder, M., Pollack, S., Tibi, R. et al. Management and long-term outcome of recurrent idiopathic FSGS in pediatric kidney transplant recipients. Sci Rep 14, 25493 (2024). https://doi.org/10.1038/s41598-024-74184-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74184-z
Keywords
This article is cited by
-
Nephrotic syndrome caused by recurrent podocytopathy after living donor renal transplantation with elevated anti-nephrin antibody levels: a case report and a review
Renal Replacement Therapy (2025)
-
Ciclosporin/rituximab
Reactions Weekly (2024)