Abstract
Heart failure (HF) is closely linked to platelet counts and lipid levels. The platelet-to-high-density lipoprotein cholesterol ratio (PHR) is a novel marker for assessing cardiovascular disease. This study investigates the relationship between PHR and HF. We analyzed data from ten consecutive NHANES survey cycles (1998–2018), focusing on self-reported HF diagnoses with complete PHR information. PHR was calculated as platelet count divided by HDL-C (mmol/L). A multivariate logistic regression model was used to examine the association between PHR and HF, with subgroup analyses to identify potential influencing factors. RCS curve plotting and threshold effect analysis were employed to describe non-linear relationships. The study included 31,410 adults aged 20–85 years. The multivariate logistic regression indicated that individuals with the highest PHR had 82% increased likelihood of HF compared to those with the lowest PHR (OR = 1.82; 95% CI, 1.37–2.40, P < 0.001). Subgroup analyses revealed no significant interactions between PHR and specific subgroups (P > 0.05), except in those with alcohol consumption (yes/no) and BMI subgroups (P < 0.05). The association between PHR and HF was non-linear, with a notable turning point at 281.53. Elevated PHR is significantly associated with HF, suggesting it may serve as an effective clinical indicator for monitoring HF risk. Larger prospective cohort studies are needed to validate these findings and further assess the clinical utility of PHR in cardiovascular risk assessment.
Similar content being viewed by others
Introduction
Heart Failure (HF) is characterized by abnormal changes in the structure and function of the heart, leading to increased intracardiac pressure or inadequate cardiac output during rest or exertion. These changes result in a complex clinical syndrome manifesting as dyspnea, edema, fatigue, and elevated jugular venous pressure, representing the end stage of various cardiovascular diseases1. In Europe, the current prevalence of HF in the general population is approximately 0.3%, and about 5% among adults2. In recent years, thanks to better management of cardiovascular diseases, the incidence of HF appears to be declining. However, with the increasing global aging population, the overall prevalence of HF is indeed rising3,4. The incidence of HF is approximately 1% among people under 55 years of age, but about 10% in the population around 70 years old5,6. Therefore, the risk and management of HF have become significant public health concerns.
Platelets, which are fragments of megakaryocytes in the bone marrow, are enucleated small cellular fragments that play a key role in blood coagulation and hemostasis. Currently, numerous studies have shown an association between platelets and heart failure. Some studies have indicated that heart failure patients exhibit abnormal activation of platelets and thrombin, which increases with the severity of heart failure7,8. A clinical study also pointed out that increased levels of circulating platelets exacerbate oxidative stress in heart failure patients9, which may be a reason for the progression of HF. Moreover, while the association between lipoproteins and HF has been widely explored, there is still certain controversy. Some studies suggest that high-density lipoprotein cholesterol (HDL-C), with its anti-inflammatory, antioxidant, and anti-platelet properties, can provide protective effects on the cardiovascular system by reducing oxidative stress and inflammation10,11. However, other studies have found no significant relationship between high HDL-C levels and survival rates or risk of incidence in HF patients, with some even showing a negative correlation12,13. Therefore, developing more reliable and simpler parameters as indicators for HF risk assessment, and investigating their potential application in HF risk evaluation and preventive decision-making, may significantly benefit a large number of HF patients.
The platelet-to-High-Density Lipoprotein Cholesterol Ratio (PHR) is a novel and effective indicator for predicting metabolic syndrome, proposed by Jialal14. Previous studies have shown that PHR has higher predictive value in alcoholic liver disease, chronic kidney disease, etc., compared to traditional lipid parameters15,16. Recent studies have further revealed the potential predictive value of PHR in various cardiovascular diseases, including stroke and cardiovascular mortality17.To date, there have been no reports on the relationship between PHR and HF. This study hypothesizes that there may be a correlation between PHR and HF. Exploring the association between PHR levels and HF could aid in HF risk assessment, and since PHR only requires lipid profile and platelet count for calculation, it offers a more convenient means of disease evaluation. Based on this, our study conducted a large-scale cross-sectional study based on the NHANES database from 1999 to 2018 to investigate the association between PHR and HF risk.
Materials and methods
Study population
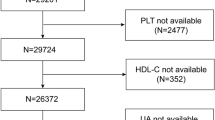
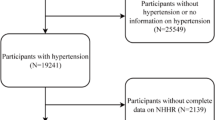
This study utilized data from the NHANES dataset, which spans a period of twenty years from 1999 to 2018. All research data can be accessed at https://www.cdc.gov/nchs/nhanes/index.htm. A total of 101,316 participants were included in this study, covering 10 consecutive survey cycles from 1999 to 2018. The participants included in this study had complete demographic data, standard body measurements, lipid profile indicators, blood cell counts, and medical conditions information. The exclusion criteria were as follows: (1) age < 20 years; (2) lack of data required for the diagnosis of heart failure or the calculation of PHR; (3) The participants who used lipid-lowering medication; (4) Missing covariate data including BMI, marital status, education level, Poverty Income Ratio (PIR), alcohol consumption, smoking status, hypertension, diabetes, coronary heart disease, angina, stroke, and heart attack. The flowchart of this process is shown in Fig. 1.
Exposure variable: PHR
The PHR was calculated using the lipid profile and blood cell count from the NHANES laboratory examination list. The formula was: PHR = platelet count (1000 cells/uL)/HDL-C(mmol/L)14.
Outcome variable: HF
In the NHANES study, information regarding heart failure (HF) was obtained through respondents’ health questionnaires, specifically based on their responses to “Has a doctor or other healthcare professional ever told you that you have heart failure?” An affirmative answer classified the individual as an HF patient. Although using questionnaire feedback to define the core study outcome may introduce some degree of uncertainty, given the lack of direct diagnostic evidence such as myocardial troponin, B-type natriuretic peptide (BNP), N-terminal pro B-type natriuretic peptide (NT-proBNP) levels, or cardiac imaging examinations in the NHANES database, accurately identifying HF cases is significantly challenging. Previous studies have demonstrated that using questionnaires to determine the heart failure status of NHANES participants is feasible and accepted18,19,20.
Covariates
To illustrate the independent association between PHR and HF, potential covariates that might affect the association between PHR and HF were adjusted based on clinical relevance, including sociodemographic, lifestyle, and health status factors. Sociodemographic and lifestyle-related variables included age (years), sex (male/female), race (Mexican American/Other Hispanic/Non-Hispanic White/Non-Hispanic Black/Other race), marital status (married/unmarried), education level (Less than 9th, 9–11th, High school, Some college, College graduate), PIR, smoking, and alcohol consumption. Alcohol consumption was categorized as No (participants had < 12 drinks containing alcohol in the past 12 months) and Yes (participants had at least 12 drinks containing alcohol in the past 12 months). Smoking was categorized as No (participants had smoked < 100 cigarettes in their lifetime) and Yes (participants had smoked at least 100 cigarettes in their lifetime).
Body mass index (BMI, kg/m²), hypertension, diabetes, coronary heart disease, angina, heart attack, platelet count, and HDL-C were considered important indicators of individual health status. BMI was directly measured at the Mobile Examination Center (MEC) by professionals, calculated by dividing the individual’s weight (kg) by the square of their height (m) (kg/m²). For the blood platelet count and HDL-C measurement, samples were sent to the University of Minnesota for specialized handling and analysis. Details of this analysis process are comprehensively described in the NHANES laboratory operation manuals. Hypertension was defined as systolic blood pressure ≥ 140 mmHg/diastolic blood pressure ≥ 90 mmHg and self-reported hypertension, diabetes was defined as fasting blood glucose ≥ 6.1 mmol/L and self-reported diabetes, while data on coronary heart disease, angina, heart attack, and stroke were obtained based on information self-reported by the participants through questionnaires.
Statistical analysis
In this study, all statistical procedures were analyzed using NHANES sampling weights. According to the heart failure (HF) status of the subjects, the basic characteristics of the survey population were categorized into two categories. Continuous measurement data were reported as mean ± standard deviation, while categorical variables were presented as percentages to detail the characteristics of each group. Weighted linear regression and weighted chi-square tests were used to compare differences between baseline continuous and categorical variables, respectively. The relationship between PHR and HF was studied using multivariable logistic regression equations, with three models: Model 1 (no covariates were adjusted), Model 2 (age, sex, race, marital status, PIR and education level were adjusted) and Model 3 (sex, age, race, education level, marital status, PIR, BMI, hypertension, smoking, alcohol consumption, coronary heart disease, angina, heart attack, stroke and diabetes were adjusted). For Model 1, Model 2, and Model 3, draw the RCS curves. A threshold effect analysis model was used to test the relationship and inflection point between PHR and HF. Finally, a subgroup analysis method was used to classify the population into different levels, including sex, race, age, PIR, education level, BMI, hypertension, smoking, alcohol consumption, diabetes, coronary heart disease, angina, heart attack, and stroke status, adding interaction terms to test the heterogeneity between subgroups. Considering the high proportion of the excluded population leading to bias, we performed multiple imputation for missing covariates and conducted sensitivity analysis on the complete cases. All statistical analyses were performed using R (version 4.2.1) and EmpowerStats (version 2.0). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the population
According to the inclusion and exclusion criteria, a total of 39,990 adults participated in this study. The average age of the subjects was 45.53 ± 17.44 years, including 15,075 (47.99%) males and 16,335 (52.01%) females, of which 18.22% were Mexican Americans, 45.28% were non-Hispanic whites, 20.15% were non-Hispanic blacks, 7.69% were other Hispanics, and 8.65% were from other races. The average BMI of the population was 28.66 ± 6.8 (kg/m²).
All clinical characteristics of the participants are listed in Table 1, where heart failure status was used as a column stratification variable to classify the population into Heart failure and Non-Heart failure categories. This study showed significant differences in demographic and baseline clinical characteristics between patients with or without HF. Compared to non-HF subjects, HF subjects were more likely to be male, unmarried, elderly, non-Hispanic black and white, drink alcohol, smoke, and have a lower PIR. Notably, compared to non-HF subjects, HF subjects had higher BMI levels and lower HDL-C levels and platelet counts. Additionally, the prevalence of hypertension, diabetes, coronary heart disease, angina, heart attack, and stroke was significantly higher in HF patients than in non-HF subjects.
Association between PHR and HF
The results indicate that higher PHR is associated with an increased likelihood of HF prevalence (Table 2). Model 1 (unadjusted for any variables) (OR = 1.12; 95% CI, 1.04–1.21, P = 0.003), Model 2 (adjusted for sex, age, race, education level, marital status, and PIR variables) (OR = 1.15; 95% CI, 1.07–1.24, P < 0.001), and Model 3 (further adjusted for BMI, hypertension, smoking, alcohol consumption, coronary heart disease, angina, heart attack, stroke and diabetes based on Model 2) (OR = 1.24; 95% CI, 1.13–1.35, P < 0.001) all demonstrate that this association is significant. Each unit increase in PHR is associated with a 12%, 15%, and 24% increase in the risk of HF prevalence, respectively. When PHR is used as a categorical variable for sensitivity analysis (quartiles), participants with the highest PHR had 82% increased risk of HF compared to those with the lowest PHR (OR = 1.82; 95% CI, 1.37–2.40, P < 0.001) (Table 2).
To avoid exclusion bias caused by missing covariates, a sensitivity analysis was conducted after multiple imputation of missing covariates. The baseline characters distribution was depicted in Supplementary Table S1. The results showed that following multiple imputation, the outputs of both the unadjusted Model 1, partially adjust Model 2 and fully adjusted Model 3 were similar to those of the models without multiple imputation. (Table 3)
RCS curve plotting and threshold effect analysis
To further elucidate the relationship between PHR and heart failure, RCS curve plotting and threshold effect analysis were conducted on data after excluding covariates and after multiple imputation of covariates. (Fig. 2, Figure S1, Table 4 and Table S2). The results indicated a nonlinear relationship between PHR and HF, with a breakpoint at 281.53. When PHR < 281.53, the risk of HF increased with rising PHR (OR = 1.24; 95% CI, 1.13–1.35, P < 0.001). When PHR > 281.53, there was no statistically significant relationship between HF and PHR (OR = 1.10; 95% CI, 0.97-0.24, P = 0.144) (Fig. 2, and Table 4). Furthermore, the analysis of the data after multiple imputation also displayed similar results, demonstrating the stability of the study findings (Figure S1 and Table S2).
(A) RCS curve plotting results with no covariates adjusted; (B) RCS curve plotting results adjusted for sex, age, race, education level, marital status, and PIR; (C) RCS curve plotting results adjusted for sex, age, race, education level, marital status, PIR, BMI, hypertension, smoking, alcohol consumption, coronary heart disease, angina, heart attack, stroke and diabetes.
Subgroup analyses
In order to determine whether the association between PHR and HF is stable among subgroups, a subgroup analysis was conducted. The interaction test showed that there were no statistically significant differences in the association between PHR and HF among the subgroups (Fig. 3), indicating that gender (male/female), ethnicity (Mexican American/other Hispanic/non-Hispanic white/non-Hispanic Black/other races), age, marital status (married/unmarried), education level (less than 9th/9–11th/high school/some college/college graduate), PIR, coronary heart disease (yes/no), smoking (yes/no), hypertension (yes/no), diabetes (yes/no), angina (yes/no), heart attack (yes/no), and stroke (yes/no) did not significantly influence this positive correlation (all interaction P > 0.05). However, the interaction between subgroups was significant for alcohol consumption (yes/no) and BMI (P < 0.05). To further validate this result, an interaction test was conducted on the data after multiple imputation. The results were similar to those mentioned above, with no significant interaction observed in any subgroups except for alcohol consumption (yes/no) and BMI subgroups (Figure S2). This suggests that the correlation between PHR and HF is similar among the subgroups, with high stability and reliability.
Discussion
In this cross-sectional study sample of 31,410 participants, we observed a significant association between PHR levels and the prevalence of HF. Further subgroup analyses and interaction tests showed similar trends in different population environments. After smooth curve fitting and threshold effect analysis, a nonlinear association between PHR and HF was identified, with a breakpoint of 281.53. When PHR < 281.53, PHR is an independent risk factor for increased prevalence of HF. These findings validate and deepen the initial hypothesis of this study, highlighting that PHR comprehensively reflects the interaction of individual lipid metabolism, blood coagulation function, and heart failure, which helps assess overall cardiovascular health status.
In recent years, studies using the NHANES database on HF primarily focused on the association between physical activity, inflammation indices, and insulin levels with HF18,21,22. This study, however, applied platelet count and HDL-C that form the PHR, providing a more comprehensive and straightforward assessment method. Additionally, this study demonstrated through subgroup analysis that alcohol consumption and BMI are significant factors for PHR and HF, which deepens the understanding of the impact of obesity and alcohol consumption on lipid metabolism and cardiovascular disease risk. Therefore, emphasizing and managing platelet and lipoprotein levels become particularly important in preventing and early intervening in HF.
Existing studies have extensively explored the association between HDL-C and platelets with cardiovascular disease and HF. Early studies showed that high HDL-C could reduce the incidence of cardiovascular disease. For instance, a prospective study involving 104,961 individuals suggested that HDL could prevent cardiovascular disease across all age groups with various risk factors23. Consistent with these findings, some studies investigating the association between HDL-C levels and cardiovascular event risk have shown a negative correlation between HDL-C levels and the occurrence of cardiovascular events24,25,26. A Mendelian randomization study also indicated that low plasma HDL-C levels were associated with an increased risk of myocardial infarction27. This aligns with the conclusion of this study that PHR (which includes HDL-C) is negatively correlated with HF risk. Several studies have consistently demonstrated the negative correlation between HDL-C levels and the occurrence of cardiovascular events.
Moreover, while there is debate regarding the prognosis of HF and platelet function, most studies indicate that platelet dysfunction is relatively common in HF patients. Acute heart failure patients often show significant platelet hyperactivation28, suggesting the importance of platelets in the pathogenesis of HF. However, similar to HDL-C, some studies provide an opposing perspective regarding the association between platelets and heart failure. For example, a large meta-analysis involving 113,000 patients showed that therapies to increase HDL-C did not significantly impact the incidence of cardiovascular events29. Moreover, regarding platelets, some studies suggest that both thrombocytopenia and thrombocytosis may be associated with poor prognosis in HF patients30,31. The inconsistency in these results may stem from differences in study design, sample sizes, and characteristics of study populations. Our study adds a new perspective to understanding the connection between HDL-C, platelets, and HF.
The study shows that the association between PHR and HF is statistically significant (P < 0.001) without any adjustments. Furthermore, after including a series of covariates, the significant association between PHR and HF increases (P < 0.001). The reason for this outcome may be that some covariates, which could potentially influence the relationship, act as mediators between PHR and HF. For example, smoking, BMI, and alcohol consumption may affect both PHR and HF, and adjusting for these factors can highlight the direct association between PHR and HF. To validate whether the association between PHR and HF is stable, this study conducted subgroup analyses considering potential confounding covariates affecting the relationship between PHR and HF. We found that regardless of whether missing covariates were addressed through multiple imputations, the association between PHR and HF consistently showed a significant interaction with alcohol consumption and BMI. Specifically, individuals with higher levels of alcohol consumption and those with a BMI > 31.8 demonstrated a more pronounced impact on the relationship between PHR and HF. Several factors may contribute to this phenomenon. Firstly, BMI is an essential indicator for assessing obesity and metabolic abnormalities. Some studies have indicated that when the body is in a state of obesity and metabolic dysfunction, platelets, which are vital components of PHR, can become hyperactivated, and HDL-C levels may decrease32,33. HDL-C plays a crucial role in inhibiting platelet activation; when it fails to suppress platelet hyperactivation, platelets can release various inflammatory factors such as IL-6 and TNF-α34. These inflammatory factors initiate and sustain a chronic inflammatory state in the body, which subsequently affects the structure and function of the heart, thereby increasing the risk of heart failure35.Secondly, alcohol consumption is a known risk factor for cardiovascular diseases, which may also influence the interaction between PHR and HF. Research indicates that among patients hospitalized for heart failure, individuals who consume alcohol have poorer prognoses and higher all-cause mortality rates36,37.Overall, these findings underscore the necessity for detailed adjustment strategies and in-depth clinical stratification analyses when comprehensively assessing the relationship between PHR and HF. However, further exploration of this relationship and its underlying mechanisms will require more carefully designed large-sample basic and clinical studies.
Study strengths and limitations
This study utilizes cross-sectional data from the NHANES database. Previous research has not explored the relationship between PHR and HF, making this study the first to identify PHR as a potential predictor of HF. By employing multivariate logistic regression analysis and subgroup analysis, this study aims to elucidate the association between PHR and HF and verify the reliability of the findings. These results are significant for advancing strategies for the prevention and early intervention of HF. However, it is important to note that the limitations of cross-sectional studies, including the inability to determine whether PHR precedes HF or vice versa, necessitate cautious interpretation of the results. Due to the inherent temporal relationship uncertainty, the study findings need careful consideration. Additionally, in dynamic social environments or changing population dynamics, such data might influence the outcomes. Furthermore, limited sample sizes in some subgroups may affect the statistical power of subgroup analysis and interaction tests, potentially impacting the regression analysis results and increasing the instability of the study findings. Therefore, to further explore the relationship between PHR and HF, future research should focus on large-scale cohort studies to longitudinally observe PHR trends and investigate their potential temporal relationship with the occurrence and progression of HF. This approach will enhance the credibility of causal interpretation. Moreover, future studies should examine the complex biological mechanisms underlying the interaction between PHR and HF. This may include experimental studies or clinical trials to elucidate the pathways and underlying principles of their interaction in detail. Comprehensive studies of this nature will not only deepen the understanding of this complex relationship but also introduce innovative methods and strategies for preventing and managing HF and its related complications. By addressing these challenges and opportunities, future research is expected to provide more comprehensive and profound insights, significantly advancing the understanding of the relationship between PHR and HF.
Conclusion
PHR levels are closely associated with HF, and PHR can serve as an effective indicator for monitoring HF risk in a clinical setting. However, to validate these results and further explore PHR’s potential as a clinical tool for cardiovascular risk assessment, larger-scale prospective cohort studies are needed. These studies will help uncover the specific causal relationship between PHR and HF and advance the practical application of this indicator in the clinic.
Data availability
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at [https://www.cdc.gov/nchs/nhanes].
References
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J. Am. Coll. Cardiol. 79, e263–e421 (2022).
Members:, A. T. F. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure 24, 4-131 (2022). (2021).
Conrad, N. et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 391, 572–580 (2018).
Roth, G. A. et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 372, 1333–1341 (2015).
van Riet, E. E. et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 18, 242–252 (2016).
Benjamin, E. J. et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137, e67-e492 (2018).
Jafri, S. et al. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur. Heart J. 14, 205–212 (1993).
Shah, A., Passacquale, G., Gkaliagkousi, E., Ritter, J. & Ferro, A. Platelet nitric oxide signalling in heart failure: role of oxidative stress. Cardiovascular. Res. 91, 625–631 (2011).
IJsselmuiden, A. J. et al. Circulating white blood cells and platelets amplify oxidative stress in heart failure. Nat. Clin. Pract. Cardiovasc. Med. 5, 811–820 (2008).
Jia, C. et al. High-density lipoprotein anti-inflammatory capacity and incident cardiovascular events. Circulation. 143, 1935–1945 (2021).
Zhang, Q., Jiang, Z. & Xu, Y. in HDL Metabolism and Diseases 63–77 (Springer, 2022).
Potočnjak, I. et al. Serum concentration of HDL particles predicts mortality in acute heart failure patients. Sci. Rep. 7, 46642 (2017).
Dhingra, R., Sesso, H. D., Kenchaiah, S. & Gaziano, J. M. Differential effects of lipids on the risk of heart failure and coronary heart disease: the Physicians’ Health Study. Am. Heart J. 155, 869–875 (2008).
Jialal, I., Jialal, G. & Adams-Huet, B. The platelet to high density lipoprotein‐cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diab./Metab. Res. Rev. 37, e3403 (2021).
Lu, C. et al. Association between the platelet/high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: results from NHANES 2017–2020. Lipids Health Dis. 22, 130 (2023).
Yan, L., Hu, X., Wu, S. & Zhao, S. Association of platelet to high-density lipoprotein cholesterol ratio with hyperuricemia. Sci. Rep. 14, 15641 (2024).
Zhang, H., Xu, Y. & Xu, Y. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: a population-based observational study. Lipids Health Dis. 23, 121 (2024).
Zheng, H. et al. Associations between systemic immunity-inflammation index and heart failure: evidence from the NHANES 1999–2018. Int. J. Cardiol. 395, 131400 (2024).
Wu, Z., Tian, T., Ma, W., Gao, W. & Song, N. Higher urinary nitrate was associated with lower prevalence of congestive heart failure: results from NHANES. BMC Cardiovasc. Disord. 20, 1–9 (2020).
Sattler, E. L. et al. Association between the prognostic nutritional index and dietary intake in community-dwelling older adults with heart failure: findings from NHANES III. Nutrients. 11, 2608 (2019).
Zhu, X. F., Mo, Y. T., Hu, Y. Q., Feng, Y. X. & Liu, E. H. Association between single-point insulin sensitivity estimator and heart failure in older adults: a cross-sectional study. Exp. Gerontol. 196, 112578 (2024).
Geller, J. in International Journal of Exercise Science: Conference Proceedings. 260.
Cooney, M. et al. HDL cholesterol protects against cardiovascular disease in both genders, at all ages and at all levels of risk. Atherosclerosis. 206, 611–616 (2009).
Laitinen, D. L., Manthena, S. & Webb, S. Association between HDL-C concentration and risk for a major cardiovascular event. Curr. Med. Res. Opin. 26, 933–941 (2010).
Pekkanen, J. et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N. Engl. J. Med. 322, 1700–1707 (1990).
Mehra, M. R. et al. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J. Heart lung Transplantation. 28, 876–880 (2009).
Haase, C. L. et al. HDL cholesterol and ischemic cardiovascular disease: a mendelian randomization study of HDL cholesterol in 54,500 individuals. J. Clin. Endocrinol. Metabolism. 97, E248–E256 (2012).
O’Connor, C. M., Gurbel, P. A. & Serebruany, V. L. Usefulness of soluble and surface-bound P-selectin in detecting heightened platelet activity in patients with congestive heart failure. Am. J. Cardiol. 83, 1345–1349 (1999).
Keene, D., Price, C., Shun-Shin, M. J. & Francis, D. P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117 411 patients. Bmj 349 (2014).
Dahlen, B. et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. ESC Heart Fail. 8, 2991–3001 (2021).
Yamaguchi, S., Abe, M., Arakaki, T., Arasaki, O. & Shimabukuro, M. Incremental prognostic value of platelet count in patients with Acute Heart Failure―A Retrospective Observational Study―. Circ. J. 83, 576–583 (2019).
Santilli, F., Vazzana, N., Liani, R., Guagnano, M. T. & Davi, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 13, 27–42 (2012).
Bora, K., Pathak, M. S., Borah, P. & Das, D. Association of decreased high-density lipoprotein cholesterol (HDL-C) with obesity and risk estimates for decreased HDL-C attributable to obesity: preliminary findings from a hospital-based study in a city from Northeast India. J. Prim. care Community Health. 8, 26–30 (2017).
Ferroni, P., Basili, S. & Davi, G. Platelet activation, inflammatory mediators and hypercholesterolemia. Curr. Vasc. Pharmacol. 1, 157–169 (2003).
Markousis-Mavrogenis, G. et al. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur. J. Heart Fail. 21, 965–973 (2019).
Whitman, I. R. et al. Alcohol abuse and cardiac disease. J. Am. Coll. Cardiol. 69, 13–24 (2017).
Zhao, J. et al. Association between daily alcohol intake and risk of all-cause mortality: a systematic review and meta-analyses. JAMA Netw. open. 6, e236185–e236185 (2023).
Acknowledgements
We would like to express our thanks to the personnel at the National Center for Health Statistics of the Centers for Disease Control for their work in developing, gathering, and organizing the NHANES data, as well as for establishing the publicly accessible database.
Funding
Heilongjiang Provincial Traditional Chinese Medicine Research Project (ZHY2022-113).
Author information
Authors and Affiliations
Contributions
WBY: Conceptualization, Methodology, Formal Analysis, Writing - Original Draft, Writing - Review & Editing. WJM: Formal Analysis, Validation, Investigation, Data curation. LCX: Methodology, Formal Analysis, Investigation. HXY: Supervision, Project Management, Writing - Review Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involving human participants were reviewed and approved by the Research Ethics Review Board of the NCHS, and all participants provided written informed consent during the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, B., Wang, J., Liu, C. et al. The potential of platelet to high-density lipoprotein cholesterol ratio (PHR) as a novel biomarker for heart failure. Sci Rep 14, 23283 (2024). https://doi.org/10.1038/s41598-024-75453-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75453-7
Keywords
This article is cited by
-
Association between platelet/high-density lipoprotein cholesterol ratio and blood eosinophil counts in American adults with asthma: a population-based study
Lipids in Health and Disease (2025)
-
Association of platelet to high density lipoprotein cholesterol ratio with coronary lesion severity in middle aged and elderly adults
Scientific Reports (2025)
-
Platelet to high density lipoprotein cholesterol ratio is associated with diabetes and prediabetes in NHANES 2005 to 2018
Scientific Reports (2024)