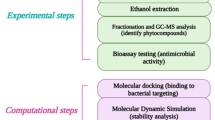
Abstract
Salmonella Typhi is a major global concern in many low- and middle-income countries. In addition, the emergence and persistence of drug resistant strains has increased the impact of this disease. Plant metabolites have been explored traditionally and scientifically as antimicrobial agents. Thus, this study was designed to investigate the antimicrobial potential of acetone leaf fractions of H. indica against S. Typhi. Dried pulverized leaves of H. indica were extracted using cold maceration with acetone after defatting with n-hexane. The leaf extract was concentrated and subjected to column chromatography and eight bioactive fractions were identified. The fractions were characterized using gas chromatography-mass spectrometry. The fractions were evaluated for antibacterial activity against Salmonella Typhi in-vitro and in-silico. The lowest MIC was observed in fractions 20 and 21 (0.375 mg/mL) while the lowest MBC was observed in all fractions except 7, 17 and 18 (0.375 mg/mL). A ligand from fraction 8 had the highest binding affinity to Type I dehydroquinase (-3.4) and a ligand from fraction 7 had the highest binding affinity to Gyrase B (-11.2). This study concludes that the overall antimicrobial activity of the acetone leaf extract of H. indica provided evidence that it contains drug-like compounds that can be further explored as a drug candidate against S. Typhi.
Similar content being viewed by others
Introduction
Records dating as far back as 2600 BCE have documented the use of plant material in traditional medicine, highlighting the historical significance of natural resources in medicine. Phytochemical constituents of plants have been explored therapeutic uses1. Modern medicine has benefited immensely from drugs obtained from natural resources, while in most developing countries, 70 to 95% of the population still relies on plant-based medicine, either as alternative or complementary therapies2,3,4,5. However, the emergence and reemergence of drug-resistant microbial pathogens continue to pose a global challenge to the management of infectious diseases.
Typhoid fever is a severe systemic infectious disease, caused by Salmonella Typhi, which is vectored by contaminated food and water. This is the most sever Salmonella infection and the causative agent is host specific6. It is a significant public health burden in most low and middle-income nations in Asia and Africa, where poor sanitation and hygiene contribute to the spread of the disease, resulting in estimated 11–18 million cases and 100,000-200,000 deaths annually7. The emergence of extensively drug-resistant (XDR) strains of S. Typhi has further complicated the management of the disease, as some first-line antibiotics traditionally used for the management of typhoid fever, such as ceftriaxone and fluoroquinolones, have become less effective due to the emergence of drug-resistant cases8,9. Similarly, outbreaks of extensively drug-resistant typhoid have been reported in Europe and Asia, and the presence of such strains in Africa cannot be ruled out10. Therefore, the continuous exploration of alternative treatments and optimization of medicinal plants found to possess antimicrobial properties as potential sources of new drug candidates are vital.
Africa has been reported to host a large variety of plants with about 60% of them being indigenous and most are reported to be used for the treatment of diverse diseases11,12. The medicinal plant, Hippocratea indica, is frequently found in tropical areas of Africa and Asia. Different parts of the plant are known for various ethnomedicinal properties, such as treatment of respiratory and guinea worm infections13. Specifically, the plant leaves have a long history of traditional use in treating various ailments with anti-inflammatory and antimicrobial properties. For example, macerated leaf decoction of the plant has been reportedly used for treating wounds and sores in Liberia14. Although reports have revealed the antimicrobial activity of H. indica against various bacteria, including S. Typhi, but the specific bioactive compounds responsible for this activity are not yet identified. Therefore, the aim of this study was to investigate the antimicrobial potential of acetone leaf fractions of H. indica against S. Typhi and identify the bioactive compounds responsible for the observed activity.
Materials and methods
Plant collection and identification
Fresh leaves of Hippocratea indica were collected from the wild in the forest of Omu-Aran, Kwara State (Nigeria) (8°07’17.8"N 5°04’27.7"E, 8°07’30.0"N 5°05’18.6"E and 8°07’33.0"N 5°05’50.3"E). The leaves were collected and used in tandem with extant ethical guidelines on plant sample experimentation. The plant part was identified at the Herbarium of the Forest Research Institute of Nigeria (FRIN), Ibadan, Nigeria by Odewo S.A. Following identification, the specimen was deposited and voucher number FHI/113,234 was obtained. Also ethical clearance was gotten for this work (LUAC/2021/0020A).
Preparation of extracts
The fresh leaves were air-dried at 16–18 °C and pulverized to fine powder, using a mechanical grinder. One thousand two hundred grams (1.2 kg) of the dried, pulverized plant leaves was defatted with n-hexane15. The defatted leaves were then extracted with acetone (ratio 1:10) for 72 h while shaking at 100 rpm (HY-2 speed adjusting multipurpose-vibrator) to enhance the rate of extraction. Residual extracting solvent after each extraction was removed from the leaf by vacuum filtration and the residue was discarded. The extracting solvent containing the extract was collected in conical flasks and concentrated using a rotary evaporator at 40 °C. The concentrated extract was then collected in a pre-weighed specimen tube and labeled appropriately. The extracts were stored in airtight containers at 4 °C until further use.
Bioassay-guided fractionation and identification of active fractions
Seventy grams of the solvent-free acetone extract was fractionated using column chromatography technique. The extract was packed on silica gel (100–200 mesh – Merck) being the static phase and eluted with 10 solvent systems of n-hexane /ethyl acetate (100:0 → 0:100, v/v) in a glass column. The column was later washed with methanol after all fractions had been collected. The collected fractions were merged based on similarity in retention factor (Rf) of thin layer chromatography assay and then concentrated to a solvent-free state16.
Antibacterial assays
Salmonella Typhi strains (ATCC 20971) were cultured overnight and adjusted to a concentration of 1.5 × 10^8 CFU/mL. The minimum inhibitory concentration (MIC) of the respective fractions was assessed against the freshly cultures microorganism using the INT colorimetric microbroth dilution assay procedure17,18. A series of twofold dilutions of the plant extract fractions (6 mg/mL) were prepared in Mueller-Hinton broth in 96 wells of microtiter plates. Twenty microliters (20 µL) of broth containing the test organism was introduced into each well. The plates were covered and incubated at 37 °C for 16–18 h. The assay was done in replicates with dimethylsulfoxide (DMSO) and ciprofloxacin serving as controls. Ten microlitre (10 µL) of 0.2 mg/mL p-iodonitrotetrazolium chloride (INT) was added to all the wells after 18 h followed by further incubation for another 30 min. The MIC was recorded as the least concentration in wells that could not reduce the yellow INT solution to pink19.
The minimum bactericidal concentration (MBC) of the bioactive fractions were established by adding 50 µL aliquot from MIC assay wells with a concentration of extract equal to or greater than the observed MIC, into labeled wells in a new microtiter plate. 150 µL of Mueller Hinton broth was added to the wells holding the 50 µL aliquot. These setups were incubated at 37 °C for 48 h. The MBC was taken to be the lowest concentration of bioactive fraction without color change upon the addition of INT as described above in the MIC assay20.
Characterization of bioactive fractions
Components of the bioactive fractions which exhibited the lowest MICs and MBCs were identified using GC-MS analysis (Agilent 7890-A GC-MS system with an MS detector 5975-C). The mass analyzer used was Quadrupole, and the ionization was achieved by electron impact ionization. The chromatogram was reviewed, and the peaks were analyzed using the National Institute for Standard and Technology-2008 (NIST-2008) database.
In silico docking studies
The protein targets for S. Typhi DNA gyrase subunit B (6J90) and Type I Dehydroquinase (4CNO) were prepared for molecular docking using Chimera 1.1.4 software. The compounds identified in the bioactive fractions were documented, and their corresponding canonical SMILES (Simplified molecular-input line-entry system) and SDF files were obtained from the Pubchem database. The SDF files were converted to PDB file format using OPEN BABEL software. The protein-ligand docking was executed using Autodock Vina Software, and the docking modes of the ligands and protein pairs were displayed in a hierarchy based on their binding affinities21,22. Docking studies were conducted on 110 identified compounds, with two pre-established proteins as the target. The compounds with the best binding affinity were selected and analyzed further in UCHF chimera and Discovery studios.
Lead-likeness properties
The SWISSADME server was used to analyze the drug likeness and physicochemical properties of the compounds. The Rule of Five (RO5), also known as Lipinski’s rule, Ghose’s rule, Veber’s rule, Egan’s rule, Muegge’s rule, and lead likeness, were used to determine if a chemical compound with certain pharmacological or biological activity has properties that may be active peroral23,24,25.
Molecular dynamics
To verify the efficacy of the docking results, the reference drug (2764) and a selected hit candidate (8671) were subjected to molecular dynamics (MD) simulations. Using the CHARMM-GUI pipeline (https://www.charmm-gui.org/), input files were generated for the protein-ligand complex. The uploaded structures are complexes of the docked compounds (protein structure, in PDB format, and the ligand, in MOL2 format). A 10 Å rectangular box was employed to ensure precise interactions between each molecule and its neighbors. The system was solvated by adding water molecules and ions (0.15 M, K + and Cl-) to neutralize the charge. Input files were prepared using the AMBER force field (GAFF2), compatible with the AMBER ff19SB protein force field26. The complexes underwent 100,000 steps of energy minimization and were equilibrated for 5 nanoseconds (ns) before a 100 ns production run27. Specifically, the system was equilibrated in an NVT ensemble for the equilibration step, followed by an NPT ensemble for the production step at a constant temperature of 310.15 K28. Molecular dynamics trajectories were analyzed using the CPPTRAJ module. To validate and assess the interactions and binding energies (ΔGbind) of the protein-ligand complexes, Molecular Mechanics Generalized Born Surface Area (MMGBSA) calculation was performed.
Results
Bioassay guided fractionation
H. indica acetone extract column chromatography resulted in 218 fractions. The review of the fractions with thin layer chromatography resulted in combination based on similar Rfs (retention or retardation factor), which produced 21 different fractions.
MIC and MBC of the extract fractions
The MIC values ranged from 0.375 mg/mL to 3 mg/mL. Samples 1, 3, 5, 7, 8, 9, 13, 19, 20 and 21 exhibited MIC values of 0.75 mg/mL or lower. The MBC values ranged from 3 mg/mL to greater than 3 mg/mL. The result is in comparison to the control drug (Ciprofloxacin) with MIC and MBC less than 3 ug/mL (Table 1).
Characterization of bioactive fractions by GC–MS
The GC-MS analysis of H. indica acetone extract compounds are presented in Table 2. Eight samples were analyzed to identify constituent compounds, and the results are presented in Table 2. The major compounds identified by characterization using Gas chromatography-mass spectrometry (GC-MS) include silane, dimethyl(2,2,2-trichloroethoxy)nonyloxy- (28.12%) in fraction 1; heptadecane, 9-octyl- and androstan-17-one, 3-[(triethylsilyl)oxy]-, (3α,5β)- (15.58%) in fraction 3; and 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4 H)-one (11.21%) and dl-α-Tocopherol (23.01%) in fraction 5. In fraction 9, there was 1-Bromo-11-iodoundecane (34.88%), fraction 13: n-Hexadecanoic acid (20.42%), 7-Methylenebicyclo[4.2.0]octane (26.79%) and γ-Sitosterol (13.09%), while Vitamin E (37.09%) in fraction 20 and 21.
In silico studies
Molecular docking studies
The docking study revealed that some of the ligands expressed better docking scores than ciprofloxacin (Table 3). The 2D and 3D analysis of interactions between the ligands and the drug target proteins of S. Typhi showed that the four top scoring compounds interacted with the active site amino acid residues of the proteins (Figs. 1–4). The best binding affinity was observed with sample 8 (D-erythro-Pentose, 2-deoxy) for Type I dehydroquinase with a binding affinity of -3.4. However, for Gyrase B, the best binding affinity was observed with fraction 7 (β-Sitosterol) with binding affinity of -11.2, which was also found in fraction 9, 13, and 19. Control compound Ciprofloxacin showed binding affinity of 81.9 for Type I dehydroquinase and − 8.6 for Gyrase B. The RMSD values for all compounds were 0, indicating a good fit.
Lead-likeness properties
Compounds with binding affinity better than ciprofloxacin were analyzed for medicinal drug-likeness and physicochemical properties using the SWISS ADME web platform. Ciprofloxacin and 1,8-Dioxacyclohexadecane-2,10-dione, 5,6:12,13-diepoxy-8,16-dimethyl- have no Lead-likeness violations but show some ADME properties that could limit their efficacy. Whereas, β-Sitosterol had the highest molecular weight, while D-erythro-Pentose, 2-deoxy- had the most Lipinski and Muegge violations. None of the compounds tested had any PAINS alerts (Table 4).
Molecular dynamics
The MD simulation methods are frequently used to examine the binding kinetics of a ligand to a protein target, investigate issues related to protein unfolding, explore the conformational and compactness properties of molecular systems29. The root-mean-square deviation (RMSD) of MD simulation reflects the motion of the complex, with larger RMSDs and more intense fluctuations indicating more intense motion30. Analysis showed that reference drug (Ciprofloxacin) is stable between 15 and 45 ns but fluctuated continuously during the simulations between 46 and 60 ns as revealed on Figure YA. Afterwards, stability was notice with some minor fluctuations. In contrast, the RMSD of hit candidate (Pentacene) showed stable fluctuation within 5 Å throughout the stage of the simulations. Obviously, pentacene is more stably bound to protein compared to ciprofloxacin.
Discussion
Several medicinal plants have been explored in traditional medicines for the treatment of typhoid fever among which research on H. indica has been shown to have antimicrobial properties. Despite this report, there is little or no information on the activity of the plant leaf extract or its metabolites against S. Typhi. This study respectively explored the antimicrobial potential of the plant leaf acetone extract and its bioactive constituents against S. Typhi in vitro and in silico. This was achieved by using microbroth dilution techniques and computational techniques such as molecular docking and ADMET profiling for the purpose of drug discovery.
It has earlier been reported that removal of the fatty constituent of plant materials with n-hexane before extraction of its phytocontent for antimicrobial susceptibility testing could increase the potency of the extracts31. Hence, in this study, the dried pulverized leaves of H. indica were defatted before extraction with acetone to focus on the phytocontent that are available in the non-lipid component of the plant leaf. Other literatures have also reported a wide-range of benefits for acetone extracts32,33,34. These benefits are direct effects of the pharmacological constituents of H. indica such as saponins, phenolic compounds and glycosides.
In the search for new and novel antibiotics, screening of natural products holds a promising potential because compounds of this origin naturally interact with biological systems and can be considered drug candidates35. Our results revealed that the fractions from acetone extract of H. indica have a moderate inhibitory effect on the growth of S. Typhi, with the least MIC of 0.375 mg/mL and MBC of 3 mg/mL. This may be the first time the antimicrobial potential of fractions of acetone leaf extracts of H. indica against S. Typhi will be reported. There are scanty works done on other parts of the plant against other organisms where the plant has been recorded to have antimicrobial activities. The activity of the acetone extract further supports the claim that acetone is a good solvent for the extraction of antimicrobial secondary metabolites from plants36,37,38.
The more recent improvement in accessibility of plant natural products is due to enhanced compatibility with high throughput screening (HTS) and advances in lead optimization, compound isolation and identification38,39,40. The antimicrobial activities of medicinal plants are a function of their phytochemical constituents, therefore the samples were subjected to the characterization of the most active fraction using GC-MS. The GC-MS identified eight likely constituent compounds, which were further subjected to in silico docking studies. The analysis of bioactive fractions of H. indica acetone leaf fraction revealed 110 compounds.
The secondary metabolites of plants are known to have some functions which include defense in hostile environs. These metabolites have been harnessed and applied against the spread of pathogenic organisms35. Due to the failure of several drug candidates during clinical trials, the use of computational prediction tools in the testing of new drug leads has been generally adopted so as to prevent wastage of time and other resources. Molecular docking is an in silico technique used to virtually predict the complex of two binding molecules which could be a small molecule (e.g., drugs, ligands) and a macromolecule (e.g., protein, peptide). The stronger the interaction between the drug target and the ligand, the lower the docking score which is reported as binding affinity. This is a prediction of the success of the drug or ligand in vivo. The results of the docking studies as shown in Table 3 and displayed in plates 1–4 indicated that some of the compounds identified in the bioactive acetone fractions of H. indica had better binding affinity (-9.2 to -11.2 and − 1.9 to -3.4) to gyrase B and Type I dehydroquinase when compared to ciprofloxacin (-8.6 and 81.9), suggesting their potential as lead compounds for the development of new drugs against S. Typhi infections. The S. Typhi gyrase B is a type II topoisomerase responsible for the un-coiling of supercoiled closed circular double-stranded (ds) DNA of S. Typhi to enable separation of DNA strands, transcription, recombination, replication and repair of the DNA. It also participates in the interconversion of some dsDNA ring topological isomers, which includes knotted rings and catenanes (“gyrB - DNA gyrase subunit B - Salmonella Typhi - gyrB gene & protein,” n.d.). Our ligands have the potential to inhibit the activity of the gyrase B enzyme, which is ATP-dependent and shares the same active site as ciprofloxacin. Additionally, our ligands may also target the Type 1 dehydroquinase enzyme involved in the catabolic quinate pathway and shikimate pathway in microorganisms (Arcuri et al., 2010). The inhibition of these enzymes can limit the growth and replication of pathogens, making them potential targets for antimicrobial agents. Furthermore, β-Sitosterol is commonly used for reducing cholesterol levels and improving clinical manifestations of benign prostatic hyperplasia, according to previous studies41,42,43.
Simulating the drug-likeness of compounds reported to have potential antimicrobial activities saves time and cost. Two of which (1,8-Dioxacyclohexadecane-2,10-dione, 5,6:12,13-diepoxy-8,16-dimethyl- and 2 H-Isoquinolin-1-one, 3-(4-acetylphenylamino)-) were found to have potentials similar to that of ciprofloxacin in terms of drug-likeness and other parameters investigated. Some of these compounds were found in multiple fractions, suggesting that they may contribute to the observed antimicrobial activity. The study did not find any PAINS alerts for the compounds tested, indicating that they are not likely to interfere with biological assays or produce misleading results. Compounds that perform lower in some of the parameters can be structurally optimized to qualify as potential drug candidates for the treatment of S. Typhi.
The mobility of backbone atoms during MD simulation can be determined by the root mean square fluctuation (RMSF). This parameter measures the fluctuation of the average position for each atom44. To investigate the impact of ligand binding on protein dynamics and understand conformational changes, the RMSF of the studied systems was calculated from MD trajectories. RMSF analysis for both the pentacene and ciprofloxacin complexes was conducted over a 100 ns simulation period. Notably, pentacene and ciprofloxacin atoms maintained a stable RMSF of less than 5 Å, as depicted in the Fig. 1B. Interestingly, the RMSF values for both ligand complexes were elevated for the same set of atoms, suggesting similar binding patterns and indicating that both ligands interact with the same protein residues.
The radius of gyration (Rg) is a valuable metric that reveals important information about the compactness of a molecule over a given time period. It measures the average expansion of the atoms in relation to the molecule’s center of mass, providing valuable insights into its overall structure and arrangement45. A clear distinction can be observed when comparing the Rg characteristics of the pentacene and ciprofloxacin complexes. From just before 15 ns to 45 ns (Fig. 1C), there is a significant increase in gyration, indicating greater fluctuations and reduced compactness until the profile stabilizes. This trend aligns with the RMSD pattern, which also displays noticeable fluctuations during the same timeframe. The positioning of the ligand within the active site of the complex is considered to be the determining factor.
MMGBSA Analysis is pivotal in drug design because it accurately predicts ΔGbind and offers detailed insights into the quantitative strength of interactions between small molecules and a target protein46. In this study, this method was employed to determine the ΔGbind of pentacene and ciprofloxacin compounds. The binding energy of pentacene complex was found to be -42.81 ± 1.60 kcal/mol while complex ciprofloxacin was found to be -30 ± 0.51 kcal/mol as shown in Table 5. MMGBSA calculations, based on 100 ns MD trajectories, consistently supported the observed trends, confirming a significantly greater binding affinity of docking results and validating experimental data.
Conclusions
Overall, the present study provides valuable insights into the potential of H. indica leaf extract as a source of bioactive compounds against S. Typhi infections. The bioassay-guided fractionation approach used in this study enabled the identification of 21 different fractions with varying degrees of activity against S. Typhi. The results of the in silico docking studies further validated the potential of these fractions as sources of lead compounds for the development of new drugs against S. Typhi infections. The ADME result obtained in this study revealed the potential of the similarity and superiority of some compounds to ciprofloxacin in silico. These compounds could be viable replacement or complementary drugs for the treatment of typhoid fever and a backup or replacement for ciprofloxacin as health workers battle the emergence ciprofloxacin and multidrug resistant strain of S. Typhi. In addition, the success of this work has provided valuable data towards the realization of the WHO Traditional Medicine (TM) Strategy 2014–2023 establishing the antimicrobial properties of the three indigenous plants and the in vivo toxicity of the most promising extract; acetone extract of H. indica leaves. However, further studies are required to validate in vivo activity of these compounds and to optimize their pharmacokinetic properties.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chassagne, F., Samarakoon, T., Porras, G., Lyles, J. T., Dettweiler, M., Marquez, L., Salam, A. M., Shabih, S., Farrokhi, D. R., & Quave, C. L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Frontiers in Pharmacology, 11. https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2020.586548 (2021).
Adedeji, A. A., Talabi, I. E., & Oladoja, F. Alternative Medicine in Health Care: Is the Time not Now to Standardize African Phytomedicine to Indigenize Health Care and Create Entrepreneurial Opportunities? In L. Raimi & I. A. Oreagba (Eds.), Medical Entrepreneurship: Trends and Prospects in the Digital Age (pp. 259–273). Springer Nature. https://doi.org/10.1007/978-981-19-6696-5_17 (2023).
Lawani, B.T., Bayode, M.T., Sadibo, M.E., Awodire E.F., Aro O.P. & Akindele, A.A. Antibiotic Resistance Microbes’ (ARM) Mechanisms and Management: A Phytomedicinal Approach. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. https://doi.org/10.1007/s40011-023-01525-9 (2023).
Ahmad Khan, M. S., & Ahmad, I. Chapter 1 - Herbal Medicine: Current Trends and Future Prospects. In M. S. Ahmad Khan, I. Ahmad, & D. Chattopadhyay (Eds.), New Look to Phytomedicine (pp. 3–13). Academic Press. https://doi.org/10.1016/B978-0-12-814619-4.00001-X (2019).
Yuan, H., Ma, Q., Ye, L., & Piao, G. The Traditional Medicine and Modern Medicine from Natural products. Molecules, 21(5), Article 5. https://doi.org/10.3390/molecules21050559 (2016).
Syed, K. A., Saluja, T., Cho, H., Hsiao, A., Shaikh, H., Wartel, T. A., Mogasale, V., Lynch, J., Kim, J. H., Excler, J.-L., & Sahastrabuddhe, S. Review on the recent advances on Typhoid Vaccine Development and challenges ahead. Clinical Infectious Diseases, 71(Supplement_2), S141–S150. https://doi.org/10.1093/cid/ciaa504 (2020).
Meiring, J. E., Shakya, M., Khanam, F., Voysey, M., Phillips, M. T., Tonks, S., Thindwa,D., Darton, T. C., Dongol, S., Karkey, A., Zaman, K., Baker, S., Dolecek, C., Dunstan,S. J., Dougan, G., Holt, K. E., Heyderman, R. S., Qadri, F., Pitzer, V. E., … Pollard,A. J. Burden of enteric fever at three urban sites in Africa and Asia: A multicentre population-based study. The Lancet Global Health, 9(12), e1688–e1696. https://doi.org/10.1016/S2214-109X(21)00370-3 (2021).
Saeed, M., Rasool, M. H., Rasheed, F., Saqalein, M., Nisar, M. A., Imran, A. A., Tariq, S., Amir, A., Ikram, A., & Khurshid, M. Extended-spectrum beta-lactamases producing extensively drug-resistant Salmonella Typhi in Punjab, Pakistan. The Journal of Infection in Developing Countries, 14(02), Article 02. https://doi.org/10.3855/jidc.12049 (2020).
Shah, S. A. A., Nadeem, M., Syed, S. A., Abidi, S. T. F., Khan, N., Bano, N., Shah, S. A. A., Nadeem, M., Syed, S. A., Abidi, S. T. F., Khan, N., & Bano, N. Antimicrobial sensitivity pattern of Salmonella Typhi: emergence of resistant strains. Cureus, 12(11). https://doi.org/10.7759/cureus.11778 (2020).
Procaccianti, M., Motta, A., Giordani, S., Riscassi, S., Guidi, B., Ruffini, M., Maffini, V., Esposito, S., & Dodi, I. First Case of Typhoid Fever due to extensively drug-resistant Salmonella enterica serovar Typhi in Italy. Pathogens, 9(2), 151. https://doi.org/10.3390/pathogens9020151 (2020).
El-Saadony, M. T., Zabermawi, N. M., Zabermawi, N. M., Burollus, M. A., Shafi, M. E., Alagawany, M., Yehia, N., Askar, A. M., Alsafy, S. A., Noreldin, A. E., Khafaga, A. F., Dhama, K., Elnesr, S. S., Elwan, H. A. M., Cerbo, A. D., El-Tarabily, K. A., & Abd El-Hack, M. E. Nutritional aspects and health benefits of Bioactive Plant compounds against Infectious diseases: a review. Food Reviews International, 39(4), 2138–2160. https://doi.org/10.1080/87559129.2021.1944183 (2023).
Mbuni, Y. M., Wang, S., Mwangi, B. N., Mbari, N. J., Musili, P. M., Walter, N. O., Hu, G., Zhou, Y., & Wang, Q. Medicinal plants and their traditional uses in Local communities around Cherangani Hills, Western Kenya. Plants, 9(3), Article 3. https://doi.org/10.3390/plants9030331 (2020).
Sojinu, O. S., Akinloye, D. I., Mosaku, A. M., Adebusuyi, A. T., Oduntan, D., Afolabi, A. E., & Arifalod, M. K. O. CHEMICAL CONSTITUENTS AND AN ANTIMICROBIAL ASSAY OF METHANOL ROOT AND LEAVES EXTRACTS OF Hippocratea indica Willd. Journal of Chemical Society of Nigeria, 47(3), Article 3. https://doi.org/10.46602/jcsn.v67i3.753 (2022).
Ogbole, O. O., Ekor, M. N., Oluremi, B. B., Ajaiyeoba, E. O., Gbolade, A. A., Ayoola, M. A., & Adeyemi, A. A. Anti-inflammatory and antimicrobial activities of Hippocratea indica Root Bark and Poga Oleosa Fruits. African Journal of Traditional, Complementary and Alternative Medicines, 4(3), Article 3. https://doi.org/10.4314/ajtcam.v4i3 (2007).
Njanje, I., Bagla, V. P., Beseni, B. K., Mbazima, V., Lebogo, K. W., Mampuru, L., & Mokgotho, M. P. Defatting of acetone leaf extract of Acacia karroo (Hayne) enhances its hypoglycaemic potential. BMC Complementary and Alternative Medicine, 17(1), 482. https://doi.org/10.1186/s12906-017-1987-6 (2017).
Oluyori, A., Olatunji, G. A., Shaw, A. K., Rastogi, P., Sanjeev, M., & Dipak, D. Chromatographic fractionation of an ethanolic extract of peels from Ipomoea batatas Lam for improved anticancer activity. Oncogen Journal, 2(1), 2. https://doi.org/10.35702/onc.10002 (2019).
Mombeshora, M., Chi, G. F., & Mukanganyama, S. Antibiofilm Activity of Extract and a compound isolated from Triumfetta welwitschii against Pseudomonas aeruginosa. Biochemistry Research International, 2021, e9946183. https://doi.org/10.1155/2021/9946183 (2021).
Omosa, L. K., Nchiozem-Ngnitedem, V.-A., Guefack, M.-G. F., Mbaveng, A. T., & Kuete, V. Antibacterial activities of thirteen naturally occuring compounds from two Kenyan medicinal plants: Zanthoxylum Paracanthum (Mildbr). Kokwaro (Rutaceae) and Dracaena usambarensis Engl. (Asparagaceae) against MDR phenotypes. South African Journal of Botany, 151, 756–762. https://doi.org/10.1016/j.sajb.2022.10.050 (2022).
Owolabi, A., Ndako, J., Owa, S., Oluyori, A., Oludipe, E., & Akinsanola, B. Antibacterial and Phytochemical Potentials of Ficus capensis Leaf Extracts Against Some Pathogenic Bacteria. https://doi.org/10.26538/tjnpr/v6i3.14 (2022).
Nigussie, D., Davey, G., Legesse, B. A., Fekadu, A., & Makonnen, E. Antibacterial activity of methanol extracts of the leaves of three medicinal plants against selected bacteria isolated from wounds of lymphoedema patients. BMC Complementary Medicine and Therapies, 21(1), 2. https://doi.org/10.1186/s12906-020-03183-0 (2021).
Ismail, N. Z., Mohamed, W. A. S., Ab. Rahim, N., Hashim, N. M., Adebayo, I. A., Mohamad Zain, N. N., & Arsad, H. Molecular docking and molecular dynamic simulations of apoptosis proteins with potential anticancer compounds present in Clinacanthus nutans extract using gas chromatography–mass spectrometry. Journal of Biomolecular Structure and Dynamics, 0(0), 1–17. https://doi.org/10.1080/07391102.2022.2101530 (2022).
Obuotor, T. M., Kolawole, A. O., Apalowo, O. E., & Akamo, A. J. Metabolic profiling, ADME pharmacokinetics, molecular docking studies and antibacterial potential of Phyllantus muellerianus leaves. Advances in Traditional Medicine. https://doi.org/10.1007/s13596-021-00611-5 (2021).
Daina, A., Michielin, O., & Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7, 42717. https://doi.org/10.1038/srep42717 (2017).
Lakhera, S., Devlal, K., Ghosh, A., & Rana, M. In silico investigation of phytoconstituents of medicinal herb ‘Piper Longum’ against SARS-CoV-2 by molecular docking and molecular dynamics analysis. Results in Chemistry, 3, 100199. https://doi.org/10.1016/j.rechem.2021.100199 (2021).
Yadav, A. R., & Mohite, S. K. ADME Analysis of Phytochemical Constituents of Psidium guajava. Asian Journal of Research in Chemistry, 13(5), 373–375. https://doi.org/10.5958/0974-4150.2020.00070.X (2020).
He X, Man VH, Yang W, Lee TS, Wang J. A fast and high-quality charge model for the next generation general AMBER force field. J Chem Phys. 21; 153(11):114502. doi: https://doi.org/10.1063/5.0019056. PMID: 32962378; PMCID: PMC7728379 (2020).
Verkhivker GM. Simulating Molecular mechanisms of the MDM2-Mediated Regulatory interactions: a conformational selection model of the MDM2 Lid Dynamics. PLoS ONE 7(7): e40897. doi:https://doi.org/10.1371/journal.pone.0040897 (2012).
Gao Y, Wei C, Luo L, Tang Y, Yu Y, Li Y, Xing J, Pan X. Membrane-assisted tariquidar access and binding mechanisms of human ATP-binding cassette transporter P-glycoprotein. Frontiers in Molecular Biosciences. 15; https://doi.org/10.3389/fmolb.2024.1364494 (2024).
Ghahremanian S, Rashidi MM, Raeisi K, Toghraie D. Molecular dynamics simulation approach for discovering potential inhibitors against SARS-CoV-2: a structural review. J Mol Liq. 15;354:118901. doi: https://doi.org/10.1016/j.molliq.2022.118901. Epub 2022 Mar 9. PMID: 35309259; PMCID: PMC8916543 (2022).
Maruyama Y, Igarashi R, Ushiku Y, Mitsutake A. Analysis of protein folding simulation with moving root mean square deviation. Journal of Chemical Information and Modeling. 23;63(5):1529-41https://doi.org/10.1021/acs.jcim.2c01444 (2023).
Olajuyigbe, O. O., Adedayo, O., & Coopoosamy, R. M. Antibacterial Activity of Defatted and Nondefatted Methanolic Extracts of Aframomum melegueta K. Schum. Against Multidrug-Resistant Bacteria of Clinical Importance. The Scientific World Journal, 2020, e4808432. https://doi.org/10.1155/2020/4808432 (2020).
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G., & Lightfoot, D. A. Phytochemicals: Extraction, isolation, and identification of Bioactive compounds from Plant extracts. Plants, 6(4), Article 4. https://doi.org/10.3390/plants6040042 (2017).
McGaw, L. J., & Eloff, J. N. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. Journal of Ethnopharmacology, 119(3), 559–574. https://doi.org/10.1016/j.jep.2008.06.013 (2008).
Shuping, D. S. S., & Eloff, J. N. The use of plants to protect plants and food against fungal pathogens: a review. African Journal of Traditional, Complementary and Alternative Medicines, 14(4), Article 4. https://doi.org/10.4314/ajtcam.v14i4 (2017).
Porras, G., Chassagne, F., Lyles, J. T., Marquez, L., Dettweiler, M., Salam, A. M., Samarakoon, T., Shabih, S., Farrokhi, D. R., & Quave, C. L. Ethnobotany and the role of Plant Natural products in Antibiotic Drug Discovery. Chemical Reviews, 121(6), 3495–3560. https://doi.org/10.1021/acs.chemrev.0c00922 (2021).
Netshiluvhi, T. R., & Eloff, J. N. Temperature stress does not affect antimicrobial activity of some South African medicinal plants. South African Journal of Botany, 123, 93–97. https://doi.org/10.1016/j.sajb.2019.01.019 (2019).
Olusola-Makinde, O. O., & Bayode, M. T. Comparative antimicrobial study of Vernonia Amygdalina Del. and Lawsonia inermis L. against microorganisms from aqueous milieu. European Journal of Biological Research, 11(3), 283–293. https://doi.org/10.5281/zenodo.4742538 (2021).
Ongpipattanakul, C., Desormeaux, E. K., DiCaprio, A., van der Donk, W. A., Mitchell, D. A., & Nair, S. K. Mechanism of action of Ribosomally Synthesized and Post-translationally modified peptides. Chemical Reviews, 122(18), 14722–14814. https://doi.org/10.1021/acs.chemrev.2c00210 (2022).
Wang, J., Ansari, M. F., & Zhou, C.-H. Identification of Unique Quinazolone Thiazoles as Novel Structural scaffolds for potential gram-negative bacterial conquerors. Journal of Medicinal Chemistry, 64(11), 7630–7645. https://doi.org/10.1021/acs.jmedchem.1c00334 (2021).
Yao, L., Liao, M., Wang, J.-K., Wang, J., Liu, D., Tu, P.-F., & Zeng, K.-W. Gold nanoparticle-based Photo-Cross-linking Strategy for Cellular Target Identification of Supercomplex Molecular systems. Analytical Chemistry, 94(7), 3180–3187. https://doi.org/10.1021/acs.analchem.1c04652 (2022).
Dreikorn, K. The role of phytotherapy in treating lower urinary tract symptoms and benign prostatic hyperplasia. World Journal of Urology, 19(6), 426–435. https://doi.org/10.1007/s00345-002-0247-6 (2002).
Jena, A. K., Vasisht, K., Sharma, N., Kaur, R., Dhingra, M. S., & Karan, M. Amelioration of testosterone induced benign prostatic hyperplasia by Prunus species. Journal of Ethnopharmacology, 190, 33–45. https://doi.org/10.1016/j.jep.2016.05.052 (2016).
Sasidharan, S., KP, S., Bhaumik, A., Kanti Das, S., & Nair J, H. Administration of Caesalpinia bonduc seed extracts ameliorates Testosterone-Induced Benign Prostatic Hyperplasia (BPH) in male Wistar rats. Research and Reports in Urology, 14, 225–239. https://doi.org/10.2147/RRU.S365598 (2022).
Martínez L. Automatic identification of mobile and rigid substructures in molecular dynamics simulations and fractional structural fluctuation analysis. PLoS One. 27;10(3):e0119264. doi: https://doi.org/10.1371/journal.pone.0119264. PMID: 25816325; PMCID: PMC4376797 (2015).
Yann Vander Meersche, Gabriel Cretin, Aria Gheeraert, Jean-Christophe Gelly, Tatiana Galochkina, ATLAS: protein flexibility description from atomistic molecular dynamics simulations, nucleic acids Research, volume 52, issue D1, 5 January 2024, Pages D384–D392, https://doi.org/10.1093/nar/gkad1084.
Genheden, S. & Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5), 449–61. https://doi.org/10.1517/17460441.2015.1032936 (2015).
Acknowledgements
The authors appreciate the support of the staffs and faculty at the Department of Microbiology and the Department of Chemistry at the Landmark University, Omu-Aran, for providing an enabling environment throughout the course of this research.
Funding
This work did not receive any external funding.
Author information
Authors and Affiliations
Contributions
Methodology: A.O.O., O.B.A., J.A.N., S. O. A. and S.O.O.; Experiment, record data: A.O.O., A.P.O, E.O.O and R.M.A.; Software: A.O.O., S. O. A. and A.P.O.; Writing—original draft preparation: A.O.O., S. O. A. and E.O.O.; Writing—review and editing: A.O.O., O.B.A, R.M.A., A.P.O., S. O. A. and E.O.O. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Owolabi, A.O., Akpor, O.B., Ndako, J.A. et al. Antimicrobial potential of Hippocratea Indica Willd. Acetone Leaf fractions against Salmonella Typhi: an in vitro and in silico study. Sci Rep 14, 25222 (2024). https://doi.org/10.1038/s41598-024-75796-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75796-1