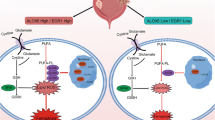
Abstract
Bladder cancer (BCa) is a lethal malignancy of the urinary system and exhibits a poor prognosis. Chaperonin-containing tailless complex polypeptide 1 subunit 3 (CCT3) acts as an oncogene in various tumors, whereas its effect on BCa remains unknown. We identified the ferroptosis-associated differentially expressed genes through bioinformatic analysis and selected CCT3 for further verification. The levels of cell viability, apoptosis, migration, invasion, and proliferation were measured to clarify the effect of silencing CCT3 on BCa cells. Then we evaluated the role of CCT3 knockdown in vivo. Ferroptosis was assessed by the expression detection of the ferroptosis-related proteins. The underlying mechanism was predicted by RNA sequencing and verified by an agonist for nucleotide-binding and oligomerization domain 1 (NOD1). Western blotting was conducted to detect the protein expression of NOD1, nuclear factor kappa B (NF-κB) inhibitor alpha (IκBα), and phospho-IκBα (p-IκBα). In vitro, down-regulation of CCT3 suppressed the cell viability, migration, invasion, and proliferation, as well as induced apoptosis of BCa cells. In vivo, silencing CCT3 elevated the body weight of mice and suppressed the BCa progression. In addition, CCT3 knockdown could induce ferroptosis in vitro and in vivo. CCT3 knockdown suppressed the expression of NOD1 and p-IκBα/IκBα and the NOD1 agonist could reverse the effect of CCT3 suppression on BCa in vitro and in vivo. In summary, our findings demonstrate that silencing CCT3 inhibits BCa via induction of ferroptosis and suppression of the NOD1-NF-κB pathway.
Similar content being viewed by others
Introduction
Bladder cancer (BCa) is the most prevailing kind of lethal malignancy of the urinary system1. By 2024, it is projected that 83,190 new BCa cases and 16,840 mortality are expected to emerge in the United States1. Based on tumor invasion depth, BCa can be divided into the non-muscle invasive BCa, with minimal effects on survival, and the muscle-invasive BCa, with serious impairments in survival2. Clinically, transurethral resection is the cornerstone treatment for non-muscle invasive BCa3, while platinum-based neoadjuvant chemotherapy is the standard of care for patients with muscle-invasive BCa4. Nevertheless, despite these treatments, the survival rates of BC patients have not improved significantly over the past two decades5. Hence, it is of great importance to find effective therapeutic targets for BCa.
Ferroptosis represents a unique form of programmed cell death driven by the accumulation of lipid peroxidation in an iron-dependent manner6. Among its primary positive regulators are malondialdehyde (MDA) and ferrous iron (Fe2+), whereas the negative include glutathione (GSH), superoxide dismutase (SOD), ferritin heavy polypeptide 1 (FTH1), recombinant solute carrier family 7, member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4)7. Recent research has increasingly illustrated the efficacy of enhancing ferroptosis in tumor cells as an anti-tumor strategy, due to its contribution to reversing drug resistance8, tumor microenvironment9, cancer metabolism10, etc. Abnormal expression levels of various ferroptosis-related genes have been observed in BCa specimens11, and inducing ferroptosis has been found to repress the progression of BCa and even lead to the elimination of BCa cells12, 13, indicating the pivotal role of ferroptosis in BCa. Therefore, it is of great importance to find new therapeutic targets for BCa based on the regulatory mechanism of ferroptosis in BCa.
Chaperonin-containing tailless complex polypeptide 1 subunit 3 (CCT3) has been implicated in a series of biological processes, as well as the initiation and progression of different malignant tumors14. Elevated expression levels of CCT3 have been positively correlated with carcinogenesis15. The knockdown of CCT3 has been shown to stimulate apoptotic pathways in breast and prostate cancers15, and to promote cervical cancer cell death via down-regulation of fibronectin 116. Knockdown of CCT3 represses the progression of lung adenocarcinoma by inhibiting the function of glycolysis17. In addition, Wang et al. have reported that the overexpression of CCT3 can inhibit ferroptosis in lung adenocarcinoma, leading to tumor growth18. Nevertheless, the role of CCT3 in the progression of BCa is unclear.
Herein, CCT3 was identified as a key ferroptosis-related differentially expressed gene (DEG) in BCa by bioinformatics analysis and its effect on BCa was explored both in vitro and in vivo by down-regulating its expression. Our research, for the first time, revealed the anti-BCa potential of silencing CCT3 via inducing ferroptosis through suppressing the NOD1-NF-κB signaling pathway, which could offer a novel and promising therapeutic target for BCa treatment.
Materials and methods
Data acquisition and processing
The original gene expression data was sourced from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nih.gov/geo/). The search was conducted using the term “carcinoma of bladder” as a query, with the organism limited to “Homo sapiens”. The inclusion criteria specified the follows: (1) the comparison involves human bladder tumor samples vs. normal bladder tissue samples; (2) the expression profiling method by array or high-throughput sequencing; (3) the sample size in each group must be at least 3; (4) raw data is available. Finally, the datasets GSE133624 and GSE42089 that met the above criteria were selected. GSE42089 comprised 18 samples (10 urothelial carcinoma samples and 8 para-carcinoma tissue samples) based on GPL9828 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. Meanwhile, GSE133624 contained 65 samples (36 urothelial carcinoma samples and 29 para-carcinoma tissue samples) based on the GPL20795 HiSeq X Ten (Homo sapiens) were chosen for further analysis. The DEGs between carcinoma samples and normal tissue samples (control) were identified by GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r) and all identified DEGs met adjusted p-value ≤ 0.05 and |log2(Fold-change)| ≥ 0.8.
The Genecards database (https://www.genecards.org/cgi-bin/carddisp) was used to retrieve ferroptosis-related genes. The FDEGs were then defined as DEGs from GSE42089 and GSE133624 that overlapped with the list of ferroptosis-related genes in the Venn diagram.
Functional and pathway enrichment analysis
We uploaded the above FDEGs to the Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/summary.jsp) for Gene Ontology (GO) function enrichment analysis, which consisted of biological process, cellular component, and molecular function, and the Kyoto Encyclopedia of Gene and Genome (KEGG) functional pathway enrichment analysis. The enriched GO terms and KEGG pathways with the minimum p-value, were shown with histograms and bubble plots.
Construction of the protein-protein interaction (PPI) network and acquisition of hub genes
The PPI analysis was performed by the Search Tool for the Retrieval of Interacting Genes (STRING, http://string-db.org/) with a confidence score of 0.4 and was visualized as a network in the Cytoscape (https://www.cytoscape.org/). Subsequently, we used the Cytoscape plug-in molecular complex detection (MCODE) to filter the significant modules (gene clusters) and selected innovative genes as hub genes.
Hub gene analysis
A principal component analysis was conducted using the expression levels of hub genes from the GSE133624 dataset. Then a ridgeline plot was generated to visually represent the abundance and distribution of expression data of hub genes within the GSE133624 dataset. Furthermore, correlation coefficients between the hub genes were calculated, based on which the matrix analysis diagram was plotted, revealing the internal correlation of hub genes in GSE133624. The Gene Expression Profile Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn/) was used for hub gene expression verification.
Cell culture and treatment
The human BCa cell lines (T24 and 5637) along with the immortal ureteral epithelium cell (SV-HUC-1) were purchased from iCell Bioscience Inc (Shanghai, China). T24 and 5637 cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Grand Island, NY, USA) and SV-HUC-1 was in F-12 K medium (Thermo Fisher Scientific, Inc.USA). Both RPMI-1640 and F-12 K mediums contained 10% fetal bovine serum (FBS, Gibco), 10 U/mL penicillin (Gibco), and 100 µg/mL streptomycin (Gibco).
To knock down the expression of CCT3, the T24 and 5637 cell lines were transfected with small interfering RNA (siRNA) targeting CCT3 (si-CCT3-1/2–3) using the Lipofectamine 3000 kit (Thermo Fisher Scientific). The si-NC was transfected into cells using the same methodology. The primer sequences were listed in Supplementary Table 1. In addition, T24 cells were exposed to 5 µg/mL N-acetyl-muramyl-L-ala-y-D-glutamyl-meso-diaminopimelic acid (M-TriDAP, InvivoGen, Toulouse, France), an agonist for nucleotide-binding and oligomerization domain 1 (NOD1), for 24 h to activate the NOD1-nuclear factor kappa B (NF-κB) signaling pathway19. Therefore, the cells were divided into the following 3 groups: si-NC, si-CCT3, and si-CCT3 + M-TriDAP groups.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Firstly, we extracted total RNAs from cells or tumor tissue samples of different groups by TRIZOL reagent (Invitrogen Carlsbad, CA, USA). RNAs with an optical density ratio at 260/280 nm ranging between 1.9 and 2.0 were selected to reverse transcribed into cDNAs. Then RT-qPCR was performed on StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), adhering to the instructions. The reaction conditions were set as follows: at 95℃ for 30 s initial denaturation, followed by 40 amplification cycles (95℃, 10 s, and 60℃, 30 s). The sequences of primers were supplied in Supplementary Tables 1 and the mRNA relative expression levels were calculated using the 2−∆∆Ct formula using GAPDH as an internal standard.
Cell viability determination
T24 and 5637 cells from different groups were seeded into 96-well plates (3 × 103 cells/well). The cell count kit-8 (CCK-8) reagents were added to each well after 0, 24, 48, and 72 h of culture, followed by an incubation period at 37℃ for 2 h. Then the optical density of each well was recorded at 450 nm wavelength.
Apoptosis assay
T24 and 5637 cells from different groups were inoculated into a six-well plate. After 24 h of incubation, the cells were exposed to fluorescein isothiocyanate-conjugated anti-annexin V antibody (Solarbio, Beijing, China) and propidium iodide (Solarbio) for 15 min. With a flow cytometer, we measured the fluorescence intensity which was quantified using FlowJo software (Becton Dickinson, Ashland, OR, USA).
Wound healing assay
T24 and 5637 cells from different groups were inoculated into a six-well plate. A scratch was then introduced on the surface of the cell layer once the cell confluence reached 80%, followed by 24 h of culture. At 0 h and 24 h, with a microscope (Olympus, Tokyo, Japan), we captured the cell migration status which was then processed by Image-J software.
Transwell assay
Briefly, T24 and 5637 cells (5 × 104/chamber) were seeded onto the upper chamber (Corning, Kennebunk, ME, USA), which has been pre-coated with a 1: 8 diluted Matrigel. The lower chamber was filled with 500 µL of RPMI-1640 medium supplemented containing 20% FBS. After 24 h, the invasive cells in the bottom of the upper chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, cells in the bottom of the upper chamber were counted under a microscope (Olympus).
Determination of MDA, SOD, GSH, and Fe2+
Measurements of GSH, SOD, and MDA were conducted with the help of corresponding Enzyme-linked immunosorbent assay (ELISA) kits (Esebio, Shanghai, China). Additionally, the iron assay kits (Abcam, Cambridge, USA) were applied to detect the ferrous iron levels. All procedures were conducted in strict accordance with the instructions in the manual.
Western blotting
Total proteins extracted from T24 and 5637 cells or tumor tissues were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Millipore, Danvers, MA, USA). The membrane with the proteins was blocked with 5% skim milk and then incubated with primary antibodies, including anti-FTH1 (ab65080; 1:1,000; Abcam), anti-SLC7A11 (ab307601; 1:1,000; Abcam), anti-GPX4 (ab125066; 1:2,000; Abcam), anti-NOD1 (ab189409; 1:1,000; Abcam), anti-NF-κB inhibitor alpha (IκBα; ab32518; 1:2,000; Abcam), anti-phospho-IκBα (p-IκBα; ab92700; 1:1,000; Abcam), and anti-GAPDH (ab181602; 1:10,000; Abcam) antibodies at 4℃ overnight. After washing, the membrane was incubated with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (ab288151; 1:5,000; Abcam) at room temperature. Finally, the protein bands were visualized using a Tanon 5200 chemiluminescence imaging system (Tanon, Shanghai, China).
Proliferation assay
A 5-ethynyl-2-deoxyuridine (Edu) staining proliferation kit (Abcam) was utilized to detect the proliferation level of T24 cells. Edu, incorporated into newly synthesized DNA, was subsequently combined with a green fluorescent called iFluor 488 azide. DAPI (Abcam) was also added to stain DNA blue. Images were captured using fluorescence microscopy, wherein an increased relative expression of green fluorescence indicated a heightened capacity for cell proliferation.
RNA- sequencing and data analysis
RNA samples extracted from cells of the si-NC and si-CCT3 groups underwent transcriptome sequencing, with the aim of identifying DEGs between the above groups. DEGs were defined as |log2(Fold-change)| > 1 with adjusted p-value ≤ 0.05. The heatmap of cluster analysis of DEGs was mapped using the R package “Pheatmap”, which relied on measures of similarity, complete linkage, and the Euclidean distance. Using the R language ggplots2 software package, a volcano plot of DEGs was drawn to show gene distribution and differences in gene expression levels using the R package “ggplots2”. Finally, to predict the downstream regulatory pathway of silencing CCT3, we subjected the DEGs to GO functions and KEGG pathways enrichment analysis.
Establishment of BCa mice model
A total of 18 female Balb/c nude mice (6-week-old, 18–20 g) were obtained from SPF (Beijing) Biotechnology Co., Ltd. (Beijing, China). Lentiviruses harboring either empty vectors (LV-NC) or interfering RNA lentiviral vectors (LV-CCT3) were purchased from GeneChem Co., Ltd. (Shanghai, China). A total of 4 × 107 T24 cells, either infected with corresponding lentiviral vectors or treated with M-TriDAP, were diluted in 80 µL of sterile PBS. These cells were then subcutaneously injected into the right flank of mice, which were divided into 3 groups, namely LV-NC, LV-CCT3, and LV-CCT3 + M-TriDAP groups (n = 6)20. The body weight of mice was detected every 7 days. After 4 weeks, all mice were anesthetized by inhalation of isoflurane and then sacrificed by cervical dislocation for collection of tumor tissues20. All mouse experiments were approved by the local Use and Care of Animals Committee.
Hematoxylin and Eosin (H&E) staining and immunohistochemical staining
The tumor tissue samples, which were initially fixed in 4% paraformaldehyde and then embedded in paraffin, were sliced into 4 μm sections. These sections underwent H&E staining to assess the extent of inflammatory infiltration of tumor tissues. In addition, the sections were exposed to the rabbit anti-Ki67 antibody (ab16667; 1:100; Abcam) at 37℃ overnight, followed by incubation with anti-rabbit IgG (ab150077; 1:200; Abcam). Then, the sections reacted with 3.3-diaminobenzidine. Finally, all the sections were observed by a microscope (Olympus).
Statistical analysis
Data were presented as multiple groups of repeated data or as means ± standard deviation and were analyzed using GraphPad Prism 7.0 statistical software (GraphPad, San Diego, CA, USA). For comparisons between two groups, Student’s t-test was employed, whereas for comparisons involving multiple groups, one-way ANOVA with Tukey’s post hoc analysis was conducted. A p-value less than 0.05 was deemed statistically significant.
Results
Identification of DEGs
We selected the original gene expression profiles from the GSE42089 and GSE133624 datasets for further screening. There were 5,944 DEGs in the GSE133624, including 2,793 up-regulated and 3,151 down-regulated genes in tumor samples compared to normal samples. Similarly, there were 2,159 DEGs in the GSE42089, including 1,035 up-regulated and 1,124 down-regulated genes. Volcano plots were conducted to visualize the DGEs (Fig. 1A). Additionally, the top 15 up-regulated and down-regulated DEGs in the GSE133624 and GSE42089 datasets were shown using heatmaps (Supplementary Fig. 1A). Then we achieved data correction and standardization for the selected samples from both datasets using boxplots (Supplementary Fig. 1B) to ensure data consistency.
Identification of differentially expressed genes (DEGs). (A) The volcano plots of DEGs in GSE42089 and GSE133624; red points presented up-regulated DEGs, and blue points presented down-regulated DEGs. (B) The top 3 pathways of gene set enrichment analysis of DEGs in GSE42089 and GSE133624. (C) The Veen diagrams of ferroptosis-related DEGs (FDEGs), which were defined as DEGs from GSE42089 and GSE13362 overlapped with the list of ferroptosis-related genes.
The results of GSEA were listed in Fig. 1B. In addition, a total of 30 up-regulated and 23 down-regulated FDEGs were obtained after Venn diagram analysis (Fig. 1C), which were selected for GO and KEGG analysis. The top 10 GO terms and KEGG pathways with the minimum p-value were shown with histograms and bubble plots (Supplementary Fig. 2A-2D).
PPI network construction and hub gene selection
The interaction information of 30 up-regulated and 23 down-regulated FDEGs was obtained by the STRING database (Supplementary Fig. 3A and 3 C). We imported the above FDEGs into Cytoscape and identified 2 gene clusters by the plug-in MCODE (Supplementary Fig. 3B and 3D). On the basis of innovation, we selected CCT3, cell division cycle 25 A (CDC25A), protein regulator of cytokinesis 1 (PRC1), diaphanous 3 (DIAPH3), and kruppel-like factor 2 (KLF2) as hub genes for the following analysis.
Hub gene analysis
The raw expression data of the 5 hub genes within the GSE133624 dataset were used for hub gene analysis. A total of 2 principal component axes (PC1 and PC2) were generated after principal component analysis, collectively explaining 87.7% of the variance between the Control group and the Tumor group (Fig. 2A). The ridge plot of hub gene expression demonstrated that CCT3 possessed the highest expression level and the most tightly distributed expression data (Fig. 2B). The correlation among hub genes was shown in Fig. 2C, with CCT3 exhibiting the lowest correlation with the remaining four genes. Then we validated the expression of CCT3 in tumor samples compared with normal samples using GEPIA2 and found that its expression level was higher in BCa tissues compared to normal tissues (p < 0.05, Fig. 2D). Subsequently, RT-qPCR displayed that CCT3, CDC25A, PRC1, and DIAPH3 had significantly upregulated expression whereas KLF2 had significantly downregulated expression in human BCa cell lines, T24 and 5637, compared to the normal bladder cell line SVHUC-1 (all p < 0.001, Fig. 3). Then based on our results, corroborated with existing literature, we chose CCT3 for experimental validation.
Hub gene analysis. (A) Principal component analysis of hub genes. The axes PC1 and PC2 in the figure were the first and second principal components. Dots represented samples, and different colors represented different groups. (B) A ridge diagram of hub genes. The horizontal axis represented the gene expression, the shape of the peak represented the dispersion among data, and the height stands for the number of samples corresponding to the gene expression. (C) Matrix correlation analysis chart. Red represented positive correlations, and blue represented negative correlations. (D) The validation of the expression of chaperonin-containing tailless complex polypeptide 1 subunit 3 (CCT3) in tumor samples compared with normal samples using the Gene Expression Profile Interactive Analysis database.
Silencing CCT3 inhibits cell growth, invasion, and migration and promotes apoptosis of BCa cells
The transfection efficiency of si-CCT3-1/2/3 in T24 and 5637 was assessed through RT-qPCR and the results showed that si-CCT3-1/2/3 transfection could significantly reduce the mRNA level of CCT3 in both T24 and 5637 cells (all p < 0.001, Fig. 4A). Given its superior transfection efficiency, si-CCT3-1 was selected for further experimentation. The cell viability, invasion ability, and migration rate of T24 and 5637 were significantly reduced whereas the apoptosis rate was significantly increased in the si-CCT3 group compared to the si-NC group (p < 0.001 or p < 0.01, Fig. 4B-4E).
Silencing CCT3 inhibited cell growth, invasion, and migration and promoted apoptosis of BCa cells. (A) The transfection efficiency of si-CCT3-1/2/3 was determined by RT-qPCR. (B) The viability of treated cells was detected by cell counting kit-8 assay. (C) The apoptosis rate of treated cells was detected by a flow cytometer. (D) The invasion of treated cells was measured by transwell assay. (E) The migration of treated cells was detected by wound healing assay. ***p < 0.001, **p < 0.01 vs. si-NC group.
Silencing CCT3 induces ferroptosis in BCa cells
Compared with the si-NC group, increased levels of MDA and Fe2+ alongside decreased levels of GSH and SOD were found in both T24 and 5637 cells of the si-CCT3 group (all p < 0.001, Fig. 5A and B). The results of western blotting revealed that the protein expression levels of FTH1, GPX4, and SLC7A11 in T24 and 5637 were significantly reduced by down-regulation of CCT3 (all p < 0.001, Fig. 5C).
Silencing CCT3 induced ferroptosis in BCa cells. (A) Content detection of malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) by corresponding Enzyme-linked immunosorbent assay (ELISA) kits. (B) The iron assay kits were applied to detect the ferrous iron (Fe2+) levels. (C) The protein expression levels of ferritin heavy polypeptide 1, recombinant solute carrier family 7, member 11 (SLC7A11), and glutathione peroxidase 4 (GPX4). ***p < 0.001 vs. si-NC group.
Selection of the NOD-like receptor signaling pathway
The DEGs between the si-NC and si-CCT3 groups were identified through transcriptome sequencing, and a heatmap displaying the cluster analysis of these DEGs was present in Fig. 6A. As evident from the volcano plot, cells with silenced CCT3 expression exhibited 77 up-regulated DEGs and 273 significantly down-regulated DEGs (Fig. 6B). The top 10 up-regulated and down-regulated DEGs were listed in Supplementary Table 2. Then GO and KEGG enrichment analyses were conducted to calculate the distribution of these DEGs. The top 10 GO terms with the minimum p-value were shown in Fig. 6C, demonstrating that silencing of CCT3 mostly influenced the extracellular region, carboxylic acid transmembrane transporter activity, and positive regulation of cholesterol transport. Furthermore, the top 20 KEGG pathways with the minimum p-value were present im Fig. 6D, from which the NOD-like receptor signaling pathway was selected for further validation.
Selection of the nucleotide-binding and oligomerization domain (NOD)-like receptor signaling pathway. (A) The heatmap of cluster analysis of DEGs between the si-NC and si-CCT3 groups identified by transcriptome sequencing. (B) The volcano plot of DEGs between the si-NC and si-CCT3 groups. (C) The GO enrichment analysis and (D) The KEGG pathway analysis of DEGs between the si-NC and si-CCT3 groups.
Activating the NOD1-NF-κBsignaling pathway reverses the effect of CCT3 knockdown on BCa cells
The NOD-like receptor pathway was validated using the NOD1 agonist M-TriDAP. Compared with the si-CCT3 group, the mRNA expression level of CCT3 in T24 was significantly elevated in the si-CCT3 + M-TriDAP group (p < 0.001, Fig. 7A). Based on CCK-8 assay results, we found that M-TriDAP promoted the cell viability of T24, which had been suppressed by CCT3 silencing(p < 0.05 or p < 0.001, Fig. 7B). The cells in the si-CCT3 + M-TriDAP group exhibited a lower apoptosis rate and higher proliferation, migration, and invasion rates compared to the si-CCT3 group (p < 0.001 or p < 0.01, Fig. 7C-7F). In addition, compared to the si-CCT3 group, reduced levels of MDA and Fe2+, alongside elevated levels of GSH, SOD, GPX4, SLC7A11, and NOD1, were noted in the si-CCT3 + M-TriDAP group (p < 0.001 or p < 0.01, Fig. 7G-7I), indicating that NOD1 activation suppressed ferroptosis which had been induced by silencing CCT3. In addition, we detected the expression levels of IκBα and p-IκBα, two key proteins of the NF-κB signaling pathway mediated by NOD1. Compared to the si-NC group, the p-IκBα/IκBα ratio in the si-CCT3 group was significantly down-regulated (p < 0.001, Fig. 7I), consistent with NOD1 expression level, which indicated the inhibition of the NOD1-NF-κB signaling pathway. However, upon M-TriDAP treatment, the p-IκBα/IκBα ratio was up-regulated (p < 0.001, Fig. 7I). Therefore, we suggested that silencing CCT3 promoted ferroptosis by inhibiting the NOD1-NF-κB signaling pathway, which suppressed cell growth, invasion, and migration and promoted apoptosis of BCa cells.
Activating the NOD1-nuclear factor kappa B (NF-κB) signaling pathway reversed the effect of CCT3 knockdown on BCa cells. (A) The mRNA relative expression level of CCT3 was determined by RT-qPCR. (B) The viability of treated cells was detected by cell counting kit-8 assay. (C) The apoptosis rate of treated cells was detected by a flow cytometer. (D) The proliferation ability of treated cells was detected by a 5-ethynyl-2-deoxyuridine staining proliferation kit. (E) The migration of treated cells was detected by wound healing assay. (F) The invasion of treated cells was measured by transwell assay. (G) Content detection of MDA, GSH, and SOD by corresponding ELISA kits. (H) The iron assay kits were applied to detect the Fe2+ levels. (I)The protein expression levels of NOD1, NF-κB inhibitor alpha (IκBα), phospho-IκBα (p-IκBα), SLC7A11, and GPX4. ***p < 0.001 vs. si-NC group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. si-CCT3 group.
Silencing CCT3 induces ferroptosis to suppress BCa progression in mice by inhibiting the NOD1-NF-κB signaling pathway
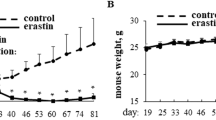
To further confirm the effect and regulatory mechanism of CCT3 in BCa, we established a subcutaneous xenograft tumor model. Compared to the LV-NC group, the LV-CCT3 group displayed an obvious increase in body weight, with a substantial decrease in tumor weight and volume (p < 0.001, Fig. 8A-8C). Notably, these effects were reversed by M-TriDAP activating the NOD1-NF-κB signaling pathway (p < 0.05 or p < 0.001, Fig. 8A-8C). RT-qPCR analysis showed that the mRNA level of CCT3 was reduced in the LV-CCT3 group compared to the LV-NC group, whereas it was upregulated in the LV-CCT3 + M-TriDAP group compared to the LV-CCT3 group (all p < 0.001, Fig. 8D). The images from H&E staining showed alleviation of inflammatory infiltration in the tumor tissue of the LV-CCT3 group, which was reversed in the LV-CCT3 + M-TriDAP group (Fig. 8E). Moreover, Ki67 staining demonstrated that the Ki67 positive rate decreased with the down-regulation of CCT3, but increased by M-TriDAP activating the NOD1-NF-κB signaling pathway (p < 0.01 or p < 0.05, Fig. 8F). Western blotting further confirmed the down-regulation of CCT3 protein in the LV-CCT3 group compared to the LV-NC group and its up-regulation in the LV-CCT3 + M-TriDAP group compared to the LV-CCT3 group (all p < 0.001, Fig. 8G). In addition, enhanced expression levels of MDA and Fe2+ and reduced expression levels of GSH, SOD, GPX4, SLC7A11, NOD1, and p-IκBα/IκBα were found in mice after knockdown of CCT3 (all p < 0.001, Fig. 8H-8J), which were all reversed in the LV-CCT3 + M-TriDAP group (p < 0.001 or p < 0.01 or p < 0.05, Fig. 8H-8J).
Silencing CCT3 induced ferroptosis to suppress BCa progression in mice by inhibiting the NOD1-NF-κB pathway. (A) Body weight of mice. (B) Tumor weight. (C) The image of tumors from different groups. (D) The relative mRNA expression level of CCT3 was determined by RT-qPCR. (E) The results of hematoxylin-eosin staining (100×, scale bars = 200 μm). (F) The results of Ki67 staining (100×, scale bars = 200 μm). (G) The relative protein expression level of CCT3 was detected by western blotting. (H) Content detection of MDA, GSH, and SOD by corresponding ELISA kits. (I) The iron assay kits were applied to detect the Fe2+ levels. (J) The protein expression levels of NOD1, IκBα, p-IκBα, SLC7A11, and GPX4. ***p < 0.001 vs. LV-NC group; ###p < 0.001, ##p < 0.01, #p < 0.05 vs. LV-CCT3 group.
Discussion
BCa, especially muscle-invasive BCa, impairs the survival and quality of life of patients seriously21. Thus, it is necessary to develop a novel anti-BCa target. Recently, ferroptosis has emerged as a potential approach to oncotherapy22. In this study, we first obtained DEGs in BCa which were related to ferroptosis, and screened out 5 hub genes by bioinformatics analysis. Then, CCT3 was further selected for verification. The results demonstrated that silencing CCT3 could suppress the progression of BCa by stimulating ferroptosis via inhibiting the NOD-like signaling pathway.
Currently, bioinformatics methods exhibit myriad possibilities for the acquisition of potential therapeutic targets for many diseases, including BCa23, 24. In our study, a total of 5 hub genes were screened out by bioinformatics methods, namely CCT3, CDC25A, PRC1, DIAPH3, and KLF2. Fan et al. have reported that CDC25A overexpression is involved in the process of phospholipase C epsilon promoting tumorigenesis in BCa25. Additionally, PRC1 suppression also can reduce BCa cell viability and induce cell cycle arrest at the G2/M phase by inhibiting the cyclin-dependent kinases1/ Cyclin B1 axis26. DIAPH3 has been reported as an inducer of tumor growth and cisplatin resistance in muscle-invasive BCa, linked to the NF-κB and epithelial-mesenchymal transition pathways27. Small nucleolar RNA host gene 1 improves the progression of BCa by inhibiting KLF2 transcription28, thereby suggesting KLF2 as an antioncogene in BCa. Moreover, down-regulation of CCT3 has exhibited anti-tumor potential in increasing types of tumors, such as liver cancer29 and hepatocellular carcinoma30. However, the role of CCT3 in BCa remains unclear now. Therefore, we selected CCT3 for further research and discovered that silencing CCT3 suppressed BCa progression both in vitro and in vivo, exhibiting the inhibitory effect of CCT3 knockdown in BCa.
Accumulating evidence has suggested that inducing ferroptosis represents a potential approach to oncotherapy, accompanied by a lethal accumulation of lipid peroxides caused by iron22. Downregulation of phosphoglycerate dehydrogenase promotes ferroptosis, leading to the suppression of malignant progression in BCa31. Lung cancer-associated transcript 1 has emerged as a novel oncogenic player due to its ability to suppress ferroptosis in BCa32. Conversely, overexpression of fibronectin leucine-rich transmembrane protein 2 suppresses tumor growth and metastasis by promoting lipid peroxidation and ferroptosis in BCa12. Liu et al. have reported arachidonate 5-lipoxygenase deficiency facilitates BCa resistance to ferroptosis, thereby contributing to BCa progression33. Additionally, Poly C binding protein 1 has been found to facilitate BCa growth by inhibiting ferroptosis34. In this study, we found that silencing CCT3 induced ferroptosis in BCa cells, suggesting that down-regulation of CCT3 inhibited BCa progression via the induction of ferroptosis.
As an important member of the NOD-like receptors, NOD1 is involved in diverse aspects of tumor development, such as tumorigenesis, metastasis, chemoresistance, and stemness. For instance, Bacteroides fragilis enterotoxin-1 directly binds to NOD1, stabilizing its expression and thereby improving breast cancer cell stemness and chemoresistance35. Conversely, membrane-associated ring-CH-type finger 7 reduces NOD1 by promoting its ubiquitin-proteasome degradation, thereby inhibiting the stem-like capacities of bladder tumor cells36. Myeloid-intrinsic NOD1 sustains intra-tumor arginase-1 levels to promote the formation of tumor immunosuppressive microenvironment which contributes to tumorigenesis37. The NF-κB signaling pathway is one of the downstream effectors of NOD1. Activation of the NOD1 signaling facilitates the proliferation and invasion of ovarian cancer cells by promoting the NF-κB signaling pathway38. Suppression of the NOD1-NF-κB signaling pathway inhibits the proliferation and metastasis of triple-negative breast cancer39. Additionally, upregulation of NOD1 triggers the NF-κB signaling pathway, leading to the tumorigenicity and metastasis of human squamous cervical cancer40. In this study, CCT3 knockdown suppressed the expression levels of both NOD1 and p-IκBα/IκBα ratio. Notably, NOD1 agonist M-TriDAP could reverse the effect of CCT3 suppression on tumor growth and ferroptosis in BCa, demonstrating that silencing CCT3 suppressed BCa by inhibiting the NOD1-NF-κB signaling pathway.
In conclusion, CCT3 was identified as an oncogenic gene in BCa by bioinformatics methods, and CCT3 knockdown effectively repressed the progression of BCa by inducing ferroptosis and suppressing the NOD1-NF-κB signaling pathway. This discovery uncovered the anti-BCa potential of CCT3 suppression, suggesting CCT3 as a promising therapeutic target for BCa.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. GSE133624:https://www.ncbi.nlm.nih.gov/geo/browse/GSE42089: https://www.ncbi.nlm.nih.gov/geo/browse/.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49 (2024).
Lopez-Beltran, A. et al. Advances in diagnosis and treatment of bladder cancer. BMJ 384, e076743 (2024).
Dyrskjøt, L. et al. Bladder cancer. Nat. Rev. Dis. Primers. 9 (1), 58 (2023).
Beckabir, W. et al. Spatial relationships in the tumor microenvironment demonstrate association with pathologic response to neoadjuvant chemoimmunotherapy in muscle-invasive bladder cancer. Eur. Urol. 85(3), 242–253 (2024).
Zang, Y. et al. An overview of patients with urothelial bladder cancer over the past two decades: a Surveillance, Epidemiology, and end results (SEER) study. Ann. Transl Med. 8 (23), 1587 (2020).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 22 (7), 381–396 (2022).
Yu, Q. et al. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine. 119, 154999 (2023).
Zhang, C. et al. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol. Cancer. 21 (1), 47 (2022).
Xu, H. et al. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol. Med. 27 (9), 856–867 (2021).
Lee, J. & Roh, J. L. Induction of ferroptosis in head and neck cancer: a novel bridgehead for fighting cancer resilience. Cancer Lett. 546, 215854 (2022).
Zeng, F. et al. Ferroptosis: a new therapeutic target for bladder cancer. Front. Pharmacol. 13, 1043283 (2022).
Jiang, P. et al. FLRT2 suppresses bladder cancer progression through inducing ferroptosis. J. Cell. Mol. Med. 28 (5), e17855 (2023).
Sun, Y. et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell. Death Dis. 12 (11), 1028 (2021).
Liu, W. et al. Current understanding on the role of CCT3 in cancer research. Front. Oncol. 12, 961733 (2022).
Temiz, E., Koyuncu, İ. & Sahin, E. CCT3 suppression prompts apoptotic machinery through oxidative stress and energy deprivation in breast and prostate cancers. Free Radic Biol. Med. 165, 88–99 (2021).
Dou, L. & Zhang, X. Upregulation of CCT3 promotes cervical cancer progression through FN1. Mol. Med. Rep. 24 (6), 12496 (2021).
Chen, S. et al. Suppression of CCT3 inhibits tumor progression by impairing ATP production and cytoplasmic translation in lung adenocarcinoma. Int. J. Mol. Sci. 23 (7), 3983 (2022).
Wang, K. et al. Upregulation of CCT3 predicts poor prognosis and promotes cell proliferation via inhibition of ferroptosis and activation of AKT signaling in lung adenocarcinoma. BMC Mol. Cell. Biol. 23 (1), 25 (2022).
Wasmuth, S. et al. Phenotypic Differences in Primary Murine Microglia Treated with NOD1, NOD2, and NOD1/2 agonists. J. Mol. Neurosci. 70 (4), 600–609 (2020).
Wang, X. et al. Echinatin inhibits tumor growth and synergizes with chemotherapeutic agents against human bladder cancer cells by activating p38 and suppressing Wnt/β-catenin pathways. Genes Dis. 11 (2), 1050–1065 (2024).
Compérat, E. et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 400 (10364), 1712–1721 (2022).
Guan, Q., Zhou, L. L. & Dong, Y. B. Ferroptosis in cancer therapeutics: a materials chemistry perspective. J. Mater. Chem. B. 9 (43), 8906–8936 (2021).
Liu, J. et al. Identifying possible hub genes and biological mechanisms shared between bladder cancer and inflammatory bowel disease using machine learning and integrated bioinformatics. J. Cancer Res. Clin. Oncol. 149 (18), 16885–16904 (2023).
Nedjadi, T. et al. Identification of SPP1 as a prognostic biomarker and immune cells modulator in urothelial bladder cancer: a bioinformatics analysis. Cancers (Basel) 15 (23), 5704 (2023).
Fan, Y. et al. PLCε promotes the Warburg effect and tumorigenesis through AKT/GSK3β/Cdc25a in bladder cancer. Biotechnol. Genet. Eng. Rev. 40 (3), 2155–2169 (2024).
Hao, Y. et al. MicroRNA-30c-5p arrests bladder cancer G2/M phase and suppresses its progression by targeting PRC1-mediated blocking of CDK1/Cyclin B1 axis. Cell. Signal. 110, 110836 (2023).
Wu, Y. et al. Identification of a cisplatin resistant-based prognostic immune related gene signature in MIBC. Transl Oncol. 44, 101942 (2024).
Min, J. et al. Long non-coding RNA SNHG1 promotes bladder cancer progression by upregulating EZH2 and repressing KLF2 transcription. Clin. (Sao Paulo). 77, 100081 (2022).
Liu, Y. et al. CCT3 acts upstream of YAP and TFCP2 as a potential target and tumour biomarker in liver cancer. Cell. Death Dis. 10 (9), 644 (2019).
Zhu, H. et al. CCT3/ACTN4/TFRC axis protects hepatocellular carcinoma cells from ferroptosis by inhibiting iron endocytosis. J. Exp. Clin. Cancer Res. 43 (1), 245 (2024).
Shen, L. et al. PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder Cancer. Int. J. Biol. Sci. 18 (14), 5459–5474 (2022).
Cao, Y. et al. LUCAT1 inhibits ferroptosis in bladder cancer by regulating the mRNA stability of STAT3. Gene. 894, 147974 (2024).
Liu, T. et al. ALOX5 deficiency contributes to bladder cancer progression by mediating ferroptosis escape. Cell. Death Dis. 14 (12), 800 (2023).
Luo, Y. et al. PCBP1 protects bladder cancer cells from mitochondria injury and ferroptosis by inducing LACTB mRNA degradation. Mol. Carcinog. 62 (7), 907–919 (2023).
Ma, W. et al. Microbiota enterotoxigenic Bacteroides fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance through its functional receptor NOD1. Protein Cell. 15 (6), 419–440 (2024).
Zhuang, J. et al. Membrane-associated RING-CH 7 inhibits stem-like capacities of bladder cancer cells by interacting with nucleotide-binding oligomerization domain containing 1. Cell. Biosci. 14 (1), 32 (2024).
Maisonneuve, C. et al. Nod1 promotes colorectal carcinogenesis by regulating the immunosuppressive functions of tumor-infiltrating myeloid cells. Cell. Rep. 34 (4), 108677 (2021).
Zhang, W. & Wang, Y. Activation of RIPK2-mediated NOD1 signaling promotes proliferation and invasion of ovarian cancer cells via NF-κB pathway. Histochem. Cell. Biol. 157 (2), 173–182 (2022).
Shi, X. et al. Eugenol modulates the NOD1-NF-κB signaling pathway via targeting NF-κB protein in triple-negative breast cancer cells. Front. Endocrinol. (Lausanne). 14, 1136067 (2023).
Zhang, Y. et al. Upregulation of NOD1 and NOD2 contribute to cancer progression through the positive regulation of tumorigenicity and metastasis in human squamous cervical cancer. BMC Med. 20 (1), 55 (2022).
Acknowledgements
Not applicable.
Funding
Project supported by the Sichuan Natural Science Foundation, No. 2023NSFSC0331.
Author information
Authors and Affiliations
Contributions
Jianlin Huang and Yizhao Luo: Conceptualization; Formal analysis; Methodology; Writing - original draft; Validation; Resources; Yu Wang , Shize Wang, Runhua Huang and Yu An: Formal analysis; Methodology; Validation; All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experiments conformed to the Guide for the Care and Use of Laboratory Animals. Animal study has been approved by the Animal Ethics Committee of Sichuan Provincial People’s Hospital. All methods are reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
All methods are reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, J., Luo, Y., Wang, Y. et al. Silencing CCT3 induces ferroptosis through the NOD1-NF-κB signaling pathway in bladder cancer. Sci Rep 14, 26188 (2024). https://doi.org/10.1038/s41598-024-76724-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76724-z