Abstract
Colorectal cancer (CRC) is one of the most common and deadly malignancies worldwide, and immune regulation plays a critical role in its development. This study investigates the causal relationships between uveitis, specific immune cell traits, and CRC using Mendelian Randomization (MR) analyses. A total of 21 single nucleotide polymorphisms (SNPs) associated with uveitis were identified, and the analysis revealed that a 1 log-odds increase in uveitis was linked to a statistically significant 3.0% reduction in CRC odds (IVW OR = 0.970, 95% CI: 0.946–0.995, P = 0.021). This protective effect was also observed using the weighted median approach (OR = 0.963, 95% CI: 0.931–0.997, P = 0.034), reinforcing the robustness of the findings. Furthermore, both univariable and multivariable MR analyses highlighted the significant causal influence of specific immune cell traits on CRC odds. Notably, the levels of extracellular monocyte HLA-DR expression emerged as a critical factor, with an associated increase in CRC odds (IVW OR = 1.084, 95% CI: 1.008–1.165, P = 0.030). The proportion of CRC odds mediated by the levels of extracellular monocyte HLA-DR expression, calculated as the ratio of the indirect effect to the total effect using estimates from multivariable MR analyses, was approximately 34.1%(95% CI: 10.23−58.04%). These findings underscore the complex interplay between immune regulation and carcinogenesis, offering insights into potential mechanisms underlying CRC development and suggesting avenues for targeted prevention and therapeutic strategies.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is currently one of the most common types of malignancies affecting the digestive system. According to the latest cancer statistics, it is anticipated that by the year 2024, the United States alone will witness over 150,000 new diagnoses of CRC, culminating in the demise of 53,010 individuals1. This constitutes approximately one-tenth of all cancer cases and fatalities1.The incidence of CRC ascended from the fifth to the second position globally between 2018 and 20202,3. Consequently, enhancing the prevention and screening of CRC is an imperative and prioritized strategy. Currently, the primary therapeutic strategy for CRC involves surgical resection in conjunction with localized pelvic radiotherapy and systemic chemotherapy. Immunotherapy represents one of the novel alternatives in cancer treatment, with vaccines specifically targeting cellular and humoral immune responses holding promise as innovative and effective strategies for intervening in CRC4. However, its application in clinical practice still faces significant challenges.
Previous studies have provided ample evidence indicating that smoking5, alcohol consumption6, Type 2 Diabetes (T2D)7, Body Mass Index (BMI)8, Waist-Hip Ratio (WHR)8, and Total Cholesterol (TC)9 are common risk factors for CRC. However, the precise etiology of this disease remains elusive.In recent years, an increasing body of research has underscored the critical role of the immune system in the onset, progression, and treatment of CRC, thereby amplifying the urgency to decipher the key mechanisms behind tumor development and progression through the lens of immunology.
Immune system dysfunctions can lead to autoimmune diseases and play a crucial role in the prevention, progression, and defense against CRC. The P2 × 7 receptor (P2 × 7R) is important in the pathogenesis of various autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), and inflammatory bowel disease (IBD)10. Research has shown that P2 × 7R also facilitates the proliferation and metastasis of CRC cells, with its expression levels inversely correlated with overall survival rates in CRC patients11. Thus, autoimmune diseases and CRC may share common genetic predispositions.
Extensive research supports the close relationship between autoimmune diseases and CRC. For example, IBDs like Crohn’s disease and ulcerative colitis increase CRC risk due to chronic inflammation in the gastrointestinal tract, which heightens susceptibility to CRC12,13,14. Moreover, Mendelian randomization studies have identified causal links between autoimmune disorders such as Type 1 diabetes, psoriasis, and primary sclerosing cholangitis and an increased risk of CRC15 .
Uveitis is an inflammation of the uvea, the middle layer of the eye. It can affect different parts of the eye and may cause symptoms like vision loss, eye pain, and redness, with severe cases potentially leading to complications such as glaucoma or blindness.The pathogenesis of uveitis is strongly influenced by genetic factors, as demonstrated by various genome-wide association studies (GWAS). Genetic predispositions to uveitis often involve variants in genes related to immune system regulation, particularly those encoding human leukocyte antigen (HLA) molecules. These molecules play a critical role in antigen presentation and T-cell activation, which are central to the immune response in uveitis.For instance, specific alleles of the HLA-DRB1 gene have been associated with an increased risk of developing uveitis. HLA-DRB1 is involved in the presentation of autoantigens to T cells, and its variants may lead to abnormal immune responses, triggering the inflammatory processes seen in uveitis16.
Uveitis is also an autoimmune disease in which Th1 and Th17 cells play pivotal roles in its onset and recurrence17,18. These cells are also integral in CRC development.Th1 cells produce interferon-gamma (IFN-γ), which can suppress tumor growth, while Th17 cells produce interleukin-17 (IL-17), which has pro-inflammatory and pro-tumor effects in CRC19,20. Therefore, immune cells may act as shared regulatory factors in both uveitis and CRC, with uveitis potentially influencing CRC development through immune cell interactions.
MR is an analytical method used to assess causal relationships between diseases, particularly when randomized controlled trials are not feasible to examine causal relationships, and observational studies present biased associations due to confounding or reverse causation21.It utilizes single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to infer causal relationships.Theoretically, this approach can mitigate confounding factors by design. Additionally, by analyzing genetic variations that precede the outcome, it effectively eliminates the possibility of reverse causation21,22,23.
Therefore, in this study, we employed summary data from large-scale GWAS for MR analysis. Our aims are to (i) ascertain the presence of a causal relationship between uveitis and CRC; and (ii) evaluate the extent of the mediating role of immune cells in the impact of uveitis on CRC.
Methods
Study design
The data utilized in our analysis are publicly available and have been approved by the respective research institution’s review boards. Therefore, no further approvals are required. All generated results are presented in the article and its supplementary materials.
We conducted a two-sample MR study to investigate the potential causal relationship between uveitis and CRC, and to examine whether immune cells traits act as a mediating factor. In this study, we utilized SNPs as IVs.To ensure the validity of the data, SNP selection was based on three fundamental assumptions: (1) Relevance assumption: the instrumental variables are strongly correlated with the exposure of interest; (2) Independence assumption: the instrumental variables are independent of confounding factors; (3) Exclusivity assumption: the instrumental variables are unrelated to the outcome and solely influence the outcome through the exposure24.This study was designed with reference to the MR study conducted by Yuan, J. et al.25.
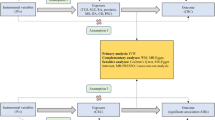
Figure 1 depicts the basic research process of MR with two samples and bidirectional approach26. Figure 2 presents the fundamental design of the two-step MR in this study. The detailed research procedures are outlined below.
Mendelian randomization research process. (A) The total effect between uveitis and colorectal cancer. c represents the overall effect of exposure to colorectal cancer on the outcome of uveitis, while d represents the overall effect of exposure to uveitis on the outcome of colorectal cancer. (B) The overall effect can be divided into two parts .(i) indirect effects (a × b) generated by two-step Mendelian randomization(a represents the effect of uveitis exposure on the outcome of immune cells, b represents the effect of immune cell exposure on the outcome of colorectal cancer). (ii) direct effect (c′=c–a×b). The proportion of the mediated effect is the ratio of the indirect effect to the total effect.
-
(1)
A MR investigation was carried out to explore the potential causal relationship between uveitis and CRC, with the objective of quantifying the total effect, represented by the coefficient c, as shown in Fig. 2A.
-
(2)
Leveraging the acquired GWAS data on 731 immune cell characteristics as the exposure and CRC as the outcome, a MR study was undertaken to identify the immune cell types causally related to CRC and to obtain the effect value b, as depicted in Fig. 2B.
-
(3)
Considering uveitis as the exposure and the previous step’s positive immune cells causally associated with CRC as the outcome, a two-sample MR study was undertaken to determine the effect magnitude, denoted as a, as illustrated in Fig. 2B.
-
(4)
The direct effect produced by immune cells was computed as c’ = c - a × b. Proportion mediated was calculated as (a × b) / c. The 95% confidence interval for the intermediate effect was obtained through the delta method with a 95% confidence level.
GWAS summary data sources
Summary statistics for immune cells traits in the GWAS Catalog (accession numbers GCST0001391 to GCST0002121) are easily accessible. The GWAS study involved 3,757 non-overlapping European individuals27. Genotypes were estimated for around 22 million SNPs using a high-density array based on a Sardinian sequence reference panel. Association testing was conducted controlling for covariates such as age, age squared, and sex. A total of 731 immune phenotypes were analyzed, including 192 relative cell counts (RC), 32 morphological parameters (MP), 118 absolute cell counts (AC), and 389 median fluorescence intensities (MFI) representing surface antigen levels. The MP features comprised CDC and TBNK panels, while MFI, RC, and AC features included B cells, CDC, T cell maturation stage, myeloid cells, monocytes, and TBNK (T cells, B cells, natural killer cells).Further details and download links for these immune cell traits are provided in Supplementary Table S1.
Uveitis-related summary data were retrieved from the GWAS Catalog, accessible at https://www.ebi.ac.uk/gwas/28, under the accession number GCST90018938.The GWAS data comprises 25,844,095 SNPs and includes a total sample size of 480,742 individuals of European descent, consisting of 2,616 cases and 478,126 controls.This data was sourced from a meta-analysis of the UK Biobank (UKB) and FinnGen, with the uveitis data in FinnGen labeled under the disease code H7_IRIDOCYCLITIS. Using FinnGen’s overlap tool, we calculated an overlap sample size of N = 1142 and a Jaccard index of 0.7 between datasets. For more indepth information regarding the GWAS data, refer to the study by Sakaue et al.29.
The GWAS summary data for CRC was obtained from the FinnGen consortium (https://www.finngen.fi/en)30 data version 10 (R10 release version December 18, 2023). This GWAS included 6,847 CRC cases and 314,193 controls. The FinnGen study is a large-scale genomics initiative that has analyzed over 500,000 Finnish biobank samples and correlated genetic variation with health data to understand disease mechanisms and predispositions. The project is a collaboration between research organisations and biobanks within Finland and international industry partners.
Instrumental variables selection
Acquiring SNPs necessitates adherence to three core tenets of MR: (1) Relevance assumption: SNPs ought to exhibit a strong association with the exposure. To fulfill this assumption, we establish the condition as follows: The association between SNP and exposure should have a p-value less than 5 × 10− 6.The clumping process was utilized to assess the linkage disequilibrium (LD) among the SNPs (with r2 < 0.001 and a clumping distance of 10,000 kb). The levels of linkage disequilibrium were estimated from the European samples of the 1000 Genomes Project31. The F value for each SNP is obtained through the following formula: F = (beta/se)2. SNP with weak strength (F-statistic < 10) will be excluded from the analysis32. (2) Independence Assumption: The independence assumption requires that the selected SNPs should not influence the outcome through confounding factors such as population stratification or assortative mating. To satisfy this assumption, all genome-wide association studies (GWAS) were restricted to individuals from a single ancestry group (European ancestry). By doing so, we aim to eliminate potential genetic confounding caused by differences in population structure, ensuring that the selected SNPs are related to the exposure and do not directly influence the outcome through these confounders. (3) Exclusivity Assumption: The exclusivity assumption requires that the selected SNPs influence the outcome only through the exposure, without any direct or indirect effects via other pathways. To validate this assumption, we screened all SNPs using the NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/), which provides phenotype information to ensure that the SNPs are not associated with other diseases related to colorectal cancer, such as inflammatory bowel disease, Crohn’s disease, or ulcerative colitis. A significance threshold of P<1 × 10−5 was applied during this screening process to exclude SNPs associated with these confounders. This step helps to eliminate bias by excluding SNPs linked to other traits. Additionally, to address potential reverse causality, we employed Steiger filtering, which identifies and excludes SNPs that explain more variance in the outcome than in the exposure, further ensuring the validity of the causal inference.
Statistical analysis
We utilized three MR methods to investigate potential causal relationships, namely inverse-variance weighted (IVW), MR-Egger, and weighted median (WM). The IVW method was used for primary analyses, given it is the most precise method for assessing causal relationships when all selected SNPs are valid33,34.Effect estimates derived using the IVW approach were compared to those derived using MR-Egger and WM to evaluate the potential for bias due to horizontal pleiotropy. When the proportion of invalid selected SNPs is less than half, the WM method can provide a more accurate assessment of causal relationships35.When the proportion of invalid selected SNPs exceeds half, MR-Egger can still provide causal assessment results, provided that the Instrument Strength Independent of Direct Effect (InSIDE) assumption holds, and that the pleiotropic effects of the SNPs are uncorrelated with their associations with the exposure36.If the analysis suggests statistical significance, IVW should have a P-value < 0.05, and the directions of beta values from the three methods must be consistent. In addition to these approaches, we conducted a MVMR analysis to account for the causal relationships among the identified immune cell traits in relation to colorectal cancer37. MVMR was performed using both the IVW and MR-Egger methods. The IVW approach in MVMR involves regressing all exposure-associated SNPs on the outcome, weighted by the inverse variance of the outcome. MR-Egger was employed to assess and adjust for potential pleiotropy, ensuring that the causal estimates were not biased by pleiotropic effects in the instrumental variables.
Additionally, we conducted a sensitivity analysis to assess the reliability of the analysis results. Cochran’s Q test was utilized to detect heterogeneity among the studies, with heterogeneity present when P < 0.0534. The MR-Egger intercept test was employed to assess the presence of horizontal and directional pleiotropy in the included SNP studies, with pleiotropy indicated when P < 0.0535. Moreover, the MR-PRESSO method was used to evaluate and correct for horizontal pleiotropy38. This method identifies outlier SNPs that may bias causal estimates and, if detected, adjusts the analysis by removing these outliers. MR-PRESSO helps ensure that the causal relationships observed are not driven by pleiotropic effects. The primary statistical analyses were carried out utilizing TwoSampleMR (version 0.5.7) within the R package (version 4.3.0).
Results
Causal relationship between uveitis and CRC
In the MR analysis investigating the effects of uveitis exposure on the outcomes of CRC, a total of 21 SNPs were selected for inclusion in the study after meeting the criteria of P < 5 × 10−6 and undergoing linkage disequilibrium screening. Following the harmonization of exposure and outcome data to eliminate mismatches and palindromic sequences, and filtering based on F > 10, all 21 SNPs met the necessary standards. Further analysis using the NHGRI-EBI Catalog revealed no associations between the selected SNPs and potential confounding factors. As a result, all 21 SNPs were included in the final analysis (Supplementary Tables S2).
The effect estimate using the IVW method indicated that a 1 log-odds increase in uveitis was associated with a 3.0% decrease in the odds of CRC (IVW OR = 0.970, 95% CI: 0.946–0.995, P = 0.021). Consistent findings were observed with the weighted median (OR = 0.963, 95% CI: 0.931–0.997, P = 0.034), which also indicated a protective effect of uveitis against CRC. Although the MR-Egger method yielded a slightly weaker effect (OR = 0.974, 95% CI: 0.939–1.009, P = 0.161), it did not substantially alter the overall conclusions. (Supplementary table S8, Fig. 3).
Forest plot summarizing MR analysis results for causal relationships across uveitis and colorectal cancer. SNPs, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; CRC, Colorectal cancer; IVW, inverse-variance weighted; WM, weighted median; MVMR, multivariable Mendelian randomization.
To ensure the correct direction of causality between uveitis and CRC in our MR analysis, we performed the Steiger test on the 21 SNPs used as instrumental variables. As shown in Supplementary Table S2, the Steiger test confirmed that for all SNPs, the variance explained was greater for uveitis than for CRC, indicating the correct direction of causality. The overall Steiger test result was highly significant (Supplementary table S8), further supporting the robustness of the identified causal relationships and minimizing the likelihood of reverse causality. These findings affirm that the selected SNPs are appropriate for inferring the causal effect of uveitis on CRC.
Univariable and multivariable MR analysis of immune cell traits and CRC
The Cochran’s Q test indicated that 76 immune cell phenotypes exhibited heterogeneity, while pleiotropy analysis with the MR-Egger intercept test identified 11 phenotypes with potential pleiotropic effects. The MR-PRESSO global test was conducted, identifying 50 immune cell phenotypes with outliers. After excluding these outliers and SNPs related to confounding factors (Supplementary Table S3), as identified by the NHGRI-EBI Catalog, a reanalysis was performed. This reanalysis confirmed that 33 immune cell phenotypes still exhibited a significant causal relationship with CRC, Additionally, Steiger tests indicated that there was no evidence of reverse causality.
The analysis revealed that several immune cell traits exhibited significant causal relationships with CRC. Notably, the proportion of HLA DR + + monocyte %leukocyte was positively associated with CRC odds (IVW OR = 1.100, 95% CI: 1.009–1.199, P = 0.031). Conversely, Unswitched memory B cell Absolute Count showed a protective effect against CRC (IVW OR = 0.911, 95% CI: 0.845–0.983, P = 0.016).The detailed results, including estimates of odds ratios, confidence intervals, and p-values for the IVW, MR-Egger, weighted median methods, and associated tests (Steiger tests, MR-PRESSO, and heterogeneity tests), are presented in Supplementary Table S4.
In addition to the methods mentioned above, to investigate the direct effects of immune cells on CRC, we applied the MVMR approach. After adjusting for immune cell interactions, MVMR estimates based on both IVW and MR-Egger methods (Fig. 4, Supplementary Table S5) demonstrated that genetically predicted levels of several specific immune cell traits, including Resting CD4 regulatory T cells% CD4 regulatory T cell (IVW OR = 1.151, 95% CI: 1.02–1.299, P = 0.023), CD25 + + CD45RA + CD4 not regulatory T cell Absolute Count (IVW OR = 0.86, 95% CI: 0.755–0.979, P = 0.023), the levels of extracellular CD19 on IgD + CD38- B cells (IVW OR = 0.749, 95% CI: 0.584–0.961, P = 0.023), the levels of extracellular CD16-CD56 on natural killer T cells(IVW OR = 1.049, 95% CI: 1.002–1.098, P = 0.039), the levels of extracellular monocyte HLA-DR expression (IVW OR = 1.084, 95% CI: 1.008–1.165, P = 0.030), and the levels of extracellular HLA-DR on dendritic cells (IVW OR = 0.935, 95% CI: 0.895–0.977, P = 0.003), can directly influence colorectal cancer, independently of other immune cells.
Causal impact of uveitis on immune cell trait
In the MR study using uveitis as the exposure and six immune cell traits as outcomes, the Cochran’s Q test indicated no evidence of heterogeneity, and the MR-Egger intercept test suggested no pleiotropy. The MR-PRESSO test identified rs28366149 and rs28892580 as outliers when the levels of extracellular monocyte HLA-DR expression was the outcome, and rs28892580 and rs685031 as outliers when the levels of extracellular HLA-DR on dendritic cells was the outcome. The Steiger test results confirmed that there was no reverse causality. Additionally, the NHGRI-EBI Catalog did not reveal any SNPs associated with confounding factors. The final SNPs included in the analysis are listed in Supplementary Table S6.
After removing the outlier SNPs and reanalyzing the data, the MR analysis revealed a significant protective effect of the levels of extracellular monocyte HLA-DR expression against uveitis. The IVW method identified an OR of 0.880 (95% CI: 0.796–0.973, P = 0.012), with consistent results observed using the WM method (OR = 0.880, 95% CI: 0.792–0.978, P = 0.018). Although the MR-Egger method did not achieve statistical significance (OR = 0.855, 95% CI: 0.715–1.023, P = 0.230), the Steiger test confirmed the correct directionality of the causal relationship, with no evidence of reverse causality. Additionally, the MR-Egger intercept test and MR-PRESSO global test did not detect substantial pleiotropy or outliers. All results are summarized in Supplementary Table S7.
Mediating role of immune cell in uveitis and CRC
We evaluated the role of the levels of extracellular monocyte HLA-DR expression as a mediator in the relationship between uveitis and CRC using both univariable and MVMR analyses. Our analysis found that uveitis was associated with a significant reduction in the levels of extracellular monocyte HLA-DR expression (IVW beta = -0.128, 95% CI: -0.228 to -0.028, P = 0.012; OR = 0.880, 95% CI: 0.796 to 0.973). Conversely, an increase in the levels of extracellular monocyte HLA-DR expression was linked to a higher odds of CRC (IVW beta = 0.052, 95% CI: 0.008 to 0.096, P = 0.022; OR = 1.053, 95% CI: 1.008 to 1.101). The MVMR analysis further confirmed the independent effect of the levels of extracellular monocyte HLA-DR expression on CRC odds, with consistent findings (MVMR IVW beta = 0.080, 95% CI: 0.008 to 0.153, P = 0.030; OR = 1.084, 95% CI: 1.008 to 1.165).
Additionally, the MR analysis indicated that uveitis was associated with a reduced incidence of CRC (IVW beta = -0.031, 95% CI: -0.059 to -0.004, P = 0.025; OR = 0.970, 95% CI: 0.946 to 0.995), suggesting that uveitis may decrease CRC odds to some extent. Detailed results can be found in Supplementary Table S8.
Our study demonstrated that the levels of extracellular monocyte HLA-DR expression accounted for 34.1% of the increased odds of uveitis associated with CRC (proportion mediated: 34.1%; 95% CI = 10.23−58.04%).
Discussion
In this study, we utilized MR analyses to investigate the causal relationships between uveitis, immune cell traits, and CRC. Our results indicate that uveitis may have a protective effect against CRC, with a 1 log-odds increase in uveitis associated with a 3.0% reduction in CRC odds (IVW OR = 0.970, 95% CI: 0.946–0.995, P = 0.021). This protective effect was consistently observed across different MR methods, including the weighted median approach (OR = 0.963, 95% CI: 0.931–0.997, P = 0.034). Additionally, our univariable and multivariable MR analyses identified several immune cell traits as significant factors influencing CRC odds. Notably, the levels of extracellular monocyte HLA-DR expression was associated with an increased odds of CRC (IVW OR = 1.084, 95% CI: 1.008–1.165, P = 0.030), while unswitched memory B cell counts were found to have a protective effect (IVW OR = 0.911, 95% CI: 0.845–0.983, P = 0.016). Importantly, the levels of extracellular monocyte HLA-DR expression mediated 34.1% (95% CI: 10.23−58.04%) of the decreased CRC odds associated with uveitis, underscoring the intricate relationship between immune regulation and cancer development.
Due to the immunological and inflammatory associations of autoimmune diseases and CRC, there may be shared pathological mechanisms between the two conditions. Therefore, ongoing exploration into the relationship between these two disease categories continues. A study conducted an integrated bioinformatics analysis to investigate the shared pathological genes and signaling mechanisms between ulcerative colitis (UC) and CRC. The study identified that inflammation and immune responses play critical roles in the development of both diseases. Through the analysis of differentially expressed genes in UC and CRC tissues, the research revealed the potential roles of these genes in disease progression, offering new insights for the prediction, prevention, and personalized medical care of patients with UC and CRC39.Furthermore, researchers have utilized MR to explore the causal relationship between 7 autoimmune diseases and CRC15. They discovered a negative association between Type 1 diabetes and CRC risk, while psoriasis and primary sclerosing cholangitis were positively associated with CRC risk. This suggests that psoriasis and primary sclerosing cholangitis may be promoting the progression of CRC through certain mechanisms. However, there is currently no research available on the relationship between uveitis and CRC.
Uveitis is an inflammatory disease that affects the uveal tract of the eye, including the iris, ciliary body, and choroid. Immune cells play a crucial role in the pathogenesis of uveitis. HLA-DR is a part of the major histocompatibility complex (MHC) class II molecules, primarily expressed on antigen-presenting cells such as monocytes. It plays a key role in regulating immune responses, particularly in inducing specific T cell responses. During the pathophysiological process of uveitis, the increased expression of HLA-DR molecules may contribute to the activation and maintenance of autoimmune responses, leading to ocular inflammation40.Furthermore, studies have indicated that specific HLA-DR alleles are associated with the susceptibility to uveitis. For instance, the HLA-DRB1*0102 allele is strongly linked to the occurrence of nodular anterior uveitis16.
Immune cells play a crucial role in the development and treatment of CRC. Research has shown that tumor-infiltrating immune cells (TIICs) play a pivotal role in the development and progression of cancer. For example, CD66b + tumor-associated neutrophils (TANs), FoxP3 + regulatory T cells (Tregs), and CD163 + tumor-associated macrophages (TAMs) have been closely associated with the clinical characteristics and prognosis of patients in studies on CRC biomarkers. These cells not only play a role in immune surveillance but may also impact patients’ responses to chemotherapy41.In particular, regulatory T cells (Tregs) in the tumor microenvironment are often considered key factors in promoting tumor evasion of immune surveillance. Studies have found that the number of Tregs significantly increases in tumor tissues, and in high-infiltration intensity tumors, there are more Tregs compared to tumors with low infiltration intensity19.Furthermore, tumor-infiltrating immune cells, such as CD8 + T cells, have been closely associated with patients’ survival rates in the tumor microenvironment, indicating their significant role in anti-tumor immune responses42.
HLA-DR is a crucial part of the major histocompatibility complex (MHC) class II molecules, playing a vital role in the immune system. MHC class II molecules are primarily expressed on antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells. Their main function is to capture exogenous antigens and present them to CD4 + T cells on the cell surface, thereby initiating and regulating immune responses. In the context of CRC, inflammation and immune surveillance are two key factors. Inflammation can promote tumor initiation and progression, while effective immune surveillance aids in identifying and clearing cancer cells. There are several possible explanations for a causal effect of increased levels of extracellular monocyte HLA-DR expression on odds of CRC:
-
(1)
Alterations in immune response: An elevation in HLA-DR levels implies active involvement of monocytes in antigen presentation. In certain cases, this heightened antigen presentation may not lead to effective tumor clearance but could potentially result in overactivation of the immune system and chronic inflammation. Persistent chronic inflammation is a known risk factor for CRC, as it can promote the formation of the tumor microenvironment, providing favorable conditions for cancer cell growth and spread.
-
(2)
Impact of the tumor microenvironment: Immune cells in the tumor microenvironment, including activated monocytes, are crucial for cancer development. An increase in HLA-DR may reflect an immune response imbalance that hinders effective tumor cell clearance and may instead facilitate tumor evasion of immune surveillance mechanisms.
-
(3)
Autoimmune diseases and cancer risk: Increased levels of HLA-DR are sometimes associated with certain autoimmune diseases. These conditions may increase the risk of developing certain types of cancer, including CRC, through chronic inflammation and tissue damage.
In conclusion, through these mechanisms, an elevation in the levels of extracellular monocyte HLA-DR expression may indirectly increase the odds of CRC.
Our study has significant strengths. Firstly, the main advantage lies in the MR design, which avoids confounding factors and reverse causality present in observational studies. Secondly, we conducted various sensitivity analyses and combined previous research to explain the potential mechanisms underlying these associations, enhancing the persuasiveness of our conclusions. Thirdly, in situations where randomized controlled trials are challenging to conduct, we utilized GWAS data from public databases for initial causal inference. Fourthly, we demonstrated the causal relationship between uveitis and CRC through immune cells, highlighting the importance of monitoring immune cell changes in uveitis treatment to prevent the occurrence of other diseases caused by inflammation and immune dysregulation.
However, this study has several limitations. Firstly, the GWAS data primarily originated from European populations, which may limit the generalizability of our conclusions to other ethnic groups and populations. Secondly, the relatively small number of uveitis cases included in the GWAS data underscores the need for additional high-quality GWAS studies on uveitis to confirm our findings. Thirdly, although we have made extensive efforts to identify and eliminate outlier variants, we cannot completely rule out unknown confounding factors, particularly those involving shared inflammatory pathways between uveitis and colorectal cancer. Fourthly, the use of summary-level GWAS data without access to individual-level information, such as age and gender, restricts our ability to conduct more detailed stratified analyses. Fifthly, The inclusion of HLA-DR, a component of MHC class II molecules, may introduce biases due to its genetic complexity, pleiotropic effects, and strong linkage disequilibrium (LD), potentially influencing our findings or introducing confounding pathways unrelated to the exposure. Sixthly, we employed a less stringent P-value threshold than standard MR analyses when identifying genetic instruments for uveitis of 5 × 10−6 which can increase the risk of including horizontally pleiotropic SNPs. Though our results were robust to the many sensitivity analyses and strategies we employed to avoid bias from horizontal pleiotropy, the third assumption of MR (that there is no directional horizontal pleiotropy) is ultimately unverifiable. Therefore, we are unable to conclusively rule out the possibility of horizontal pleiotropy, rather than a true causal effect, in explaining our results suggesting a causal effect of uveitis on CRC odds. Lastly, the use of binary exposure variables in MR analysis, such as uveitis and colorectal cancer, may introduce biases and complicate the interpretation of causal estimates43.
In conclusion, our MR study suggests a potential causal relationship between uveitis and CRC, with the immune cell the levels of extracellular monocyte HLA-DR expression playing a regulatory role. However, the reliability of our results may be affected by unknown confounding factors, and our research is still far from being applicable to clinical practice. Further high-quality studies are needed to elucidate the common pathological mechanisms between uveitis and CRC and to better understand the role of immune cells in this relationship.
Data availability
All original data is derived from publicly available databases. All results are presented within the article or supplementary files. For more detailed data, please contact the corresponding author.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Padma, S. et al. Cell surface fibroblast activation protein-2 (Fap2) of fusobacterium nucleatum as a vaccine candidate for therapeutic intervention of human colorectal cancer: An immunoinformatics approach. Vaccines (Basel). 11,525(2023).
Botteri, E. et al. Smoking and colorectal cancer: A meta-analysis. JAMA. 300, 2765–2778 (2008).
Vieira, A. R. et al. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann. Oncol. 28, 1788–1802 (2017).
Guraya, S. Y. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J. Gastroenterol. 21, 6026–6031 (2015).
Bull, C. J. et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 18, 396 (2020).
Rodriguez-Broadbent, H. et al. Mendelian randomisation implicates hyperlipidaemia as a risk factor for colorectal cancer. Int. J. Cancer. 140, 2701–2708 (2017).
Cao, F. et al. P2X7 receptor: A potential therapeutic target for autoimmune diseases. Autoimmun. Rev. 18, 767–777 (2019).
Bai, X. et al. Progress in the relationship between P2X7R and colorectal cancer. Mol. Biol. Rep. 50, 1687–1699 (2023).
Olén, O. et al. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet. 395, 123–131 (2020).
Olén, O. et al. Colorectal cancer in Crohn’s disease: a Scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 5, 475–484 (2020).
Terzić, J., Grivennikov, S., Karin, E. & Karin, M. Inflammation and colon cancer. Gastroenterology. 138, 2101–2114e5 (2010).
Chen, L., Wang, F., Zhang, H. & Cao, B. Exploring potential causal associations between autoimmune diseases and colorectal cancer using bidirectional Mendelian randomization. Sci. Rep. 14, 1557 (2024).
Levinson, R. D. et al. Strong associations between specific HLA-DQ and HLA-DR alleles and the tubulointerstitial nephritis and uveitis syndrome. Invest. Ophthalmol. Vis. Sci. 44, 653–657 (2003).
Sun, D., Liang, D., Kaplan, H. J. & Shao, H. The role of Th17-associated cytokines in the pathogenesis of experimental autoimmune uveitis (EAU). Cytokine. 74, 76–80 (2015).
Luger, D. et al. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008).
Wu, Y., Yuan, L., Lu, Q., Xu, H. & He, X. Distinctive profiles of tumor-infiltrating immune cells and association with intensity of infiltration in colorectal cancer. Oncol. Lett. 15, 3876–3882 (2018).
Tosolini, M. et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 71, 1263–1271 (2011).
Sekula, P., Del, G. M. F., Pattaro, C. & Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265 (2016).
Bowden, J. & Holmes, M. V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods. 10, 486–496 (2019).
Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res.26, 2333–2355 (2017).
Davey, S. G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: Substantive and nomenclatural issues. Eur. J. Epidemiol. 35, 99–111 (2020).
Yuan, J. et al. Genetically predicted C-reactive protein mediates the association between rheumatoid arthritis and atlantoaxial subluxation. Front. Endocrinol. (Lausanne). 13, 1054206 (2022).
Carter, A. R. & Anderson, E. L. Correct illustration of assumptions in Mendelian randomization. Int. J. Epidemiol. 53,dyae050(2024).
Orrù, V. et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 52, 1036–1045 (2020).
Buniello, A. et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47, D1005–D1012 (2019).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53, 1415–1424 (2021).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 613, 508–518 (2023).
Abecasis, G. R. et al. A map of human genome variation from population-scale sequencing. Nature. 467, 1061–1073 (2010).
Pierce, B. L., Ahsan, H. & Vanderweele, T. J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40, 740–752 (2011).
Burgess, S., Scott, R. A., Timpson, N. J., Davey, S. G. & Thompson, S. G. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30, 543–552 (2015).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Bowden, J., Davey, S. G., Haycock, P. C. & Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 40, 304–314 (2016).
Bowden, J., Davey, S. G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260 (2015).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Shi, X. et al. Screening of the shared pathogenic genes of ulcerative colitis and colorectal cancer by integrated bioinformatics analysis. J. Cell. Mol. Med. 28,e17878 (2023).
Mattapallil, M. J. et al. Uveitis-associated epitopes of retinal antigens are pathogenic in the humanized mouse model of uveitis and identify autoaggressive T cells. J. Immunol. 187, 1977–1985 (2011).
Ye, L. et al. Tumor-infiltrating immune cells act as a marker for prognosis in colorectal cancer. Front. Immunol. 10, 2368 (2019).
Shang, S. et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci. Transl Med. 14, eabf0992 (2022).
Burgess, S. & Labrecque, J. A. Mendelian randomization with a binary exposure variable: Interpretation and presentation of causal estimates. Eur. J. Epidemiol. 33, 947–952 (2018).
Acknowledgements
The authors acknowledge Orrù, Valeria et al. for contributing the immunocyte characterization data utilized in this work. The uveitis data furnished by Sakaue, S. et al. is also greatly appreciated. This study made use of data from the GWAS Catalog, which is publicly available for download. The colorectal cancer GWAS data was obtained from the FinnGen database, which the authors wish to thank.We want to acknowledge the participants and investigators of the FinnGen study.
Funding
This research was supported by the Yongchuan District Natural Science Foundation of Chongqing, China (Project No. 2023yc-jckx20060) in 2023.
Author information
Authors and Affiliations
Contributions
L.Z conceived the study . L.Z and J.W.Z jointly completed the downloading and cleaning of the data, and performed statistical analysis. J.W.Z conducted data analysis and drafted the manuscript. L.Z and J.W.Z revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, L., Zhang, J. Immune cells mediate the causal relationship between uveitis and colorectal cancer via Mendelian randomization analysis. Sci Rep 14, 25964 (2024). https://doi.org/10.1038/s41598-024-77758-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77758-z