Abstract
The Cardiometabolic Index (CMI) represents an innovative metric that encompasses abdominal obesity and lipid levels, offering a comprehensive assessment of cardiometabolic health. It is derived through the multiplication of the waist-to-height ratio by the triglyceride-to-high-density lipoprotein cholesterol ratio. Although obesity and blood lipid levels are known factors affecting sleep quality, the direct correlation between CMI and sleep quality has yet to be elucidated. This study uses NHANES data to explore the potential correlation between CMI and sleep quality. Our research employed a cross-sectional design, utilizing data from the National Health and Nutrition Examination Survey (NHANES) spanning 2011 to 2014. CMI was used as the exposure variable, with sleep quality assessed through three self-reported measures: trouble sleeping, sleep disorders, and sleep duration. We employed multivariate logistic regression models and linear regression model as well as subgroup analyses to explore the independent relationship between CMI and sleep quality. Additionally, interaction tests were conducted to examine differences across various subgroups. The study included 4298 participants, comprising 2134 males and 2164 females. There was a positive correlation between CMI and trouble sleeping (OR = 1.63, 95% CI 1.28–2.08) and sleep disorders (OR = 1.96, 95% CI 1.43–2.67), while there was a negative correlation between CMI and sleep duration (β = − 0.17, 95% CI − 0.31 to − 0.03), indicating that a higher CMI was associated with shorter sleep duration. Subgroup analyses and interaction tests revealed a consistently positive association between CMI and sleep difficulty across various populations, including gender, age, hypertension, diabetes, and cardiovascular disease history. However, the relationship between CMI and sleep disorders was more prominent among participants aged 50 and above. In American adults, a higher CMI is linked to an increased prevalence of sleep disturbances. In clinical practice, CMI can be considered as a supplementary factor in the assessment and management of sleep problems. Our study also provided new insights for improving sleep quality.
Similar content being viewed by others
Introduction
Sleep is the fundamental physiological process essential for human life activities. It is an important guarantee for maintaining physical health and operating body functions. Good sleep quality is not only crucial for cognitive function, emotions, mental health, and cardiovascular, cerebrovascular, and metabolic health, but also reduces the risk of accidents and injuries caused by fatigue. However, with the development of the economy and changes in lifestyle, Nie et al. found that the prevalence of abnormal sleep duration, sleep disorders, and trouble sleeping among adults in the United States is increasing year by year1. A recent study revealed that the estimated global prevalence of sleep disorders during the COVID-19 pandemic stood at 40.49%2. Poor sleep quality is associated with various adverse physiological and mental health outcomes, including cardiovascular disease, diabetes, hypertension, and more3. It may also increase the risk of contracting COVID-19 and worsen its severity4. This is particularly concerning for shift workers, a population already prone to severe sleep disorders5. As we know, sleep disorders pose a burden on individuals’ quality of life and society, so it is very important to identify and address these issues early.
Recently, there has been a surge of interest among researchers in exploring the connection between certain serum biomarkers, like triglycerides (TG), and sleep. In this context, a study conducted within the Baependi Heart Study, a rural-based cohort in Brazil, revealed a statistically significant association between higher TG levels and insufficient sleep quality among adults6. Another study found that the occurrence of sleep disorders is generally associated with cardiometabolic comorbidities, such as lipid metabolism dysfunction and metabolic syndrome7. Prakaschandra et al. indicated that changes in the quantity and quality of sleep are linked to cardiovascular metabolic factors and metabolic syndrome, with insufficient sleep and poor sleep quality being associated with increased waist circumference (WC), blood pressure, glucose, TG, low-density lipoprotein cholesterol (LDL-C), and decreased high-density lipoprotein cholesterol (HDL-C)8. Wang et al. found a significant positive connection between more frequent insomnia symptoms and a high TG to HDL ratio9. These results strongly indicated a tight link between lipid metabolism disorders and sleep disorders and poor sleep quality. Despite numerous studies have shown correlations between blood lipid levels and sleep quality across diverse populations, the findings of these studies remain inconsistent10,11. Such inconsistencies may stem from variations in geographical regions, sample sizes, and a range of confounding factors. Therefore, it is necessary for us to further investigate their relationship.
Cardiometabolic index (CMI) is an indicator reflecting obesity and lipid metabolism disorders, first proposed by Ichiro Wakabayash in 2015. It is calculated by multiplying waist-to-height ratio (WHtR) and the ratio of triglycerides to high-density lipoprotein cholesterol (TG/HDL-C)12. CMI combines blood lipid levels and abdominal obesity, serving as a major component of metabolic syndrome and having significant value in chronic diseases. Previous research has demonstrated a strong correlation between CMI and diabetes, highlighting its simplicity and effectiveness in identifying diabetes13. Recently, multiple studies have established the correlation between CMI and diseases such as liver disease14, lung disease15, cognitive impairment16, and depression17,18. This suggests that CMI is a promising comprehensive indicator for predicting metabolic disorders. However, the relationship between CMI and sleep quality is still unclear. Due to the worsening metabolic status of individuals with lipid metabolism disorders over many years, effective monitoring of fat metabolism disorders in patients with sleep disorders is crucial for developing effective interventions to prevent adverse health outcomes.
Consequently, the primary aim of our research is to explore the correlation between CMI and sleep quality, using the NHANES database. Our findings can provide evidence for the role of lipid metabolism in sleep health, which may be of significant importance for the sleep health of Americans.
Methods
Study participants
This cross-sectional study utilized data gathered from the widely recognized National Health and Nutrition Examination Survey, also referred to as NHANES. This survey is continuous, multi-stage, with a cycle of every two years, focusing on issues related to demographics, socioeconomic factors, dietary habits, and health-related inquiries. The ultimate aim is to evaluate the nutritional well-being of adults and children in the United States, exploring its correlation with the promotion of health and the prevention of diseases.
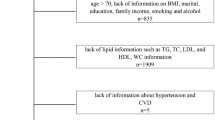
Figure 1 shows the inclusion and exclusion process of our study. (1) First, our study obtained data from 2011 to 2014 for analysis and recruited a total of 19,931 participants. (2) Next, we excluded 14,053 participants who unable to calculate CMI due to missing data on height, waist circumference, TG, and HDL-C. (3) Then, we excluded 591 participants who had missing sleep data. (4) We also excluded individuals younger than 20 years old, pregnant women, and those with a history of cancer. Finally, our study included 4298 participants.
The National Center for Health Statistics Ethics Review Board has granted approval to NHANES (Ethics approval number: Protocol#2011-17). All participants involved in the study provided their written consent.
Calculation of CMI
CMI = [WC (cm)/height (cm)] × [TG (mmol/L)/HDL-C (mmol/L)]12. CMI was regarded as a continuous variable, and group analysis was based on quartiles. The quartiles were defined as follows: Quartile 1 (CMI < 0.29), Quartile 2 (CMI 0.29–0.50), Quartile 3 (CMI 0.50–0.86), and Quartile 4 (CMI > 0.86).
Sleep quality definitions
As with previous studies, sleep quality is defined by three-dimensional questions, including the presence or absence of trouble sleeping, sleep disorders, and sleep duration19. Participants were inquired whether they had ever informed a doctor or another healthcare professional about experiencing trouble sleeping, and those who answered positively were subsequently classified as having sleep troubles. Similarly, participants were queried about whether they had ever been diagnosed with a sleep disorder by a medical doctor or any other health professional, and those who confirmed this were considered to have a sleep disorder. To ascertain the average sleep duration, participants were prompted to indicate, “How many hours of sleep do you typically obtain on weekdays or workdays?”
Covariates selections
In our study, the selection of covariates was guided by professional expertise and prior research. Demographic variables encompassed age, gender, race, educational level, marital status, and poverty income ratio(PIR). Lifestyle factors took into account smoking, alcohol consumption, and moderate physical activity. Laboratory measurements encompassed systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL-C, hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), history of diabetes, hypertension, and cardiovascular disease.
Regarding smoking status, participants were grouped into smokers and non-smokers, depending on whether they had smoked over 100 cigarettes throughout their lives. As for alcohol consumption, individuals were categorized based on whether they consumed a minimum of 12 alcoholic beverages per annum. PIR was stratified into three categories: <1.30, 1.30–3.49, and ≥ 3.520.
The diagnosis of diabetes was established through the satisfaction of one or several of the following criteria: (1) a clinically verified diagnosis of diabetes rendered by a healthcare professional, (2) HbA1c > 6.5%, or (3) FPG ≥ 7.0 mmol/L20.The diagnosis of hypertension is based on the following methods: (1) whether it has been diagnosed as hypertension by a healthcare professional, (2) The average SBP is ≥ 140mmHg or the average DBP is ≥ 90mmHg. The classification of cardiovascular diseases (CVD) followed previous research21 and includes coronary heart disease, angina, congestive heart failure, myocardial infarction, and stroke. CVD was defined by asking the following question: “Have you ever been told by a doctor or other healthcare professional that you have (disease)?”
Using the online software Dagitty version 3.1, a theoretical causal model based on a directed acyclic graph (DAG) was developed, considering the exposure variable (CMI), the outcome (sleep quality), and covariates. To avoid unnecessary adjustments, spurious associations, and estimation errors, the backdoor criterion was employed to select the minimal set of confounding variables required for the fitting analysis (Supplementary Fig. 1)22.
Statistical analysis
Our study utilized a complex, multistage probability sampling design. Following the recommendations from the NHANES official website, we applied fasting subsample weights in our analysis. Employing chi-square tests or t-tests, our research evaluated the characteristics of participants according to quartile of the CMI. As the variables in this study do not follow a normal distribution, we have represented continuous variables using medians and categorical variables using weighted percentages and 95% CI. The generalized variance inflation factor (VIF < 10) was used to check for multicollinearity among the variables. Multivariable logistic regression models and linear regression models were applied to examine the independent association between CMI and sleep quality. The logistic regression models were used to estimate the odds ratios (OR) for categorical outcomes such as trouble sleeping and sleep disorders. On the other hand, linear regression models were applied to evaluate the association between CMI and sleep duration. To control for potential confounding factors that might influence the results, the following three models were employed: Model 1 did not incorporate any covariate adjustments. In Model 2, adjustments were made for age, sex, and race. Model 3 further adjusted for all non-collinear variables based on Model 2, including educational level, marital status, poverty income ratio, alcohol consumption, smoking, moderate work activity, diabetes, hypertension, CVD, TG, HDL-C, and LDL-C. Following the conversion of the CMI value from a continuous variable into classified quartiles, trend tests were carried out to investigate potential linear associations between the CMI and sleep quality. Additionally, subgroup analyses were performed to explore the relationship between the CMI and sleep quality, stratified by factors such as age, gender, diabetes, hypertension, and CVD history. The statistical analysis was performed utilizing the R software package (version 3.4.3) and Empower software (version 4.2). A two-sided P-value less than 0.05 was deemed statistically significant.
Results
Baseline characteristics
Table 1 describes the baseline characteristics of the participants. The study included 4298 participants, comprising 2134 males and 2164 females. Most of them were non-Hispanic whites (65.79%), married or living with a partner (63.24%), and had an educational level above high school (62.50%). The CMI values ranges classified by quartiles are as follows: quartile 1: <0.29; quartile 2: 0.29–0.50; quartile 3: 0.50–0.86; and quartile 4: >0.86. With the increase of CMI, the prevalence of trouble sleeping and sleep disorders also increases (P < 0.001). Notably, the proportion of individuals who self-reported trouble sleeping (25.95%) is higher than those diagnosed with sleep disorders by medical professionals (9.32%). However, there is no significant trend in sleep duration from high to low sleep quality scores. In the group with the highest quartile of CMI, there is an observed increase in age, SBP, DBP, TG, HbA1c, and FPG. Additionally, a higher proportion of males and smokers is noted, while the proportion of HDL-C is lower. Furthermore, as the CMI increases, a greater proportion of participants report a history of diabetes, hypertension, and cardiovascular disease.
The association between the CMI and sleep quality
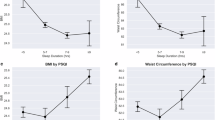
The correlation between CMI and sleep quality is summarized in Figs. 2, 3 and 4. Multiple logistic regression and linear regression analyses were employed to assess the relationship between CMI and sleep quality. In model 3, our findings revealed a positive correlation between CMI and trouble sleeping (OR = 1.63, 95% CI 1.28–2.08) as well as sleep disorders (OR = 1.96, 95% CI 1.43–2.67). It indicates that for every unit increase in CMI, there is a corresponding 63% increase in the likelihood of participants experiencing trouble sleeping and a 96% increase in the likelihood of developing sleep disorders. Additionally, we transformed CMI, which was initially a continuous variable, into a categorical variable (consisting of four groups). When comparing the Q4 group to the Q1 group, a notable elevation in CMI within the Q4 group was linked to a higher occurrence of difficulties in sleeping (Model 3: OR = 1.63, 95% CI 1.15–2.31, P for trend = 0.022) and also to an augmented prevalence of sleep disorders (Model 3: OR = 2.49, 95% CI 1.42–4.37, P for trend < 0.001). Notably, in terms of sleep duration, CMI, as a continuous variable, demonstrated a negative association with it (β = − 0.17, 95% CI − 0.31 to − 0.03), indicating that each unit increase in CMI is associated with a 17% decrease in participants’ sleep duration. However, after treating CMI as a categorical variable and conducting a fully adjusted model, the correlation between CMI and sleep duration was not significant (β = − 0.02, 95% CI − 0.24 to 0.20, P for trend = 0.82).
The relationship between between CMI and sleep duration. Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, education level, marital status, PIR, drinking, smoking, hypertension, diabetes, TG, LDL-C, HDL-C, CVD history, moderate work activity were adjusted. PIR the ratio of income to poverty, Q quartile, TG Triglyceride, LDL-C Low density lipoprotein cholesterol, HDL-C High density lipoprotein cholesterol, CVD Cardiovascular disease.
Subgroup analysis
Table 2 presents the subgroup analysis and interaction tests performed in our research to investigate the robustness of the relationship between CMI and sleep quality, considering variables such as gender, age, hypertension, diabetes, and cardiovascular disease history. This analysis aimed to identify potential population differences. After adjusting for confounding factors, our results indicated a positive correlation between CMI and self-reported trouble sleeping. This association was generally similar across different groups of gender, age, hypertension, diabetes, and CVD history (all P for interaction > 0.05). However, the relationship between CMI and sleep disorders was influenced by age ( P for interaction = 0.01), with individuals aged over 50 years have an increased propensity to develop sleep disorders(OR = 2.30, 95% CI 1.62–3.27). Additionally, a significant association was observed between CMI and sleep duration among participants without a history of CVD (P for interaction < 0.001).
Discussion
A cross-sectional study utilizing the NHANES database was carried out among 4298 participants to elucidate the association between CMI and sleep quality. Our research findings indicate a positive correlation between CMI and trouble sleeping and sleep disorders (OR = 1.63, 95% CI 1.28–2.08; OR = 1.96, 95% CI 1.43–2.67). This suggests that higher levels of CMI were correlated with an increased likelihood of trouble sleeping and sleep disorders. In terms of sleep duration, CMI, as a continuous variable, demonstrated a negative association with it (β= − 0.17, 95% CI − 0.31 to − 0.03), indicating that a higher CMI was associated with shorter sleep duration. Through subgroup analysis and interaction testing, the robustness of the positive correlation between CMI and trouble sleeping has been demonstrated across different population. However, in participants aged 50 and above, the relationship between CMI and sleep disorders was more prominent. Overall, the higher the CMI, the more sleep problems there are. Our research findings highlight the important role of CMI in the development of sleep disorders.
This is the first time evaluating the relationship between CMI and sleep quality to our knowledge. Previous studies have primarily concentrated on examining the correlation between obesity or blood lipid levels and sleep, while lacking direct assessments of the relationship between CMI and sleep quality at present. Hall et al. analyzed data from 1,214 American middle-aged adults aged 30 to 54 years old and discovered that sleep duration exhibits an independent correlation with three key aspects of metabolic syndrome: abdominal obesity, elevated blood glucose concentrations, and heightened triglyceride levels. Abdominal obesity and elevated blood glucose levels are the metabolic syndrome individuals with the strongest correlation with short sleep duration23. A cross-sectional study targeting healthy Saudi adults revealed a significant link between elevated TG levels and both reduced sleep quality and shortened sleep quantity24. Similar to previous research25, we found a positive correlation between CMI (a novel cardiovascular risk marker) and sleep disturbances or sleep problems. This positive correlation underscores the significant role that metabolic factors, particularly abdominal obesity and dyslipidemia, play in sleep health. Several biological mechanisms could explain this observed association. First, there may be a significant cross-sectional link between poor sleep quality and reduced leptin levels coupled with elevated ghrelin levels, which regulate satiety and hunger, respectively. Given the metabolic and endocrine changes, increased appetite and hunger might contribute to poorer sleep quality26. Second, visceral obesity is associated with a heightened risk of obstructive sleep apnea (OSA), a common sleep disorder27. Additionally, we observed that individuals aged over 50 years were more likely to experience sleep disorders with higher CMI levels. This finding suggests that aging may amplify the adverse effects of metabolic disorders on sleep. As people age, the decline in sleep quality could be attributed to changes in circadian rhythms, reduced melatonin production, or alterations in neurotransmitter function28. Interestingly, a stronger inverse correlation was observed between elevated CMI levels and shorter sleep duration. among adults without cardiovascular disease. This could be because in people without cardiovascular disease, high CMI indices and insufficient sleep may be early warning signals of health issues. These signals may reflect poor lifestyle habits, psychological stress, or other underlying health problems that exist before the onset of cardiovascular disease. Sleep is crucial for maintaining cardiovascular health. Insufficient sleep can lead to a series of physiological changes, such as hormonal imbalances and enhanced inflammatory responses, which may affect various components of the cardiac metabolic index. In individuals without cardiovascular disease, these physiological mechanisms may be more sensitive. Therefore, for this population, it is important to focus on and improve sleep quality and cardiac metabolic status to prevent cardiovascular disease. Finally, we should also pay attention to other issues associated with poor sleep quality, which may be risk factors for metabolic disorders. Unhealthy dietary patterns, such as consuming high-sugar diets and ultra-processed foods, can lead to nutrient deficiencies (e.g., vitamin D)29,30. These deficiencies not only contribute to higher levels of inflammation but may also disrupt circadian rhythms and reduce nighttime melatonin secretion, thereby negatively impacting sleep quality31,32. Additionally, sedentary behavior should be considered, as it can lead to metabolic and inflammatory issues closely linked to dyslipidemia, cardiovascular diseases, and poor sleep quality33.
CMI, a novel indicator, offers a comprehensive view of visceral fat distribution and dysfunction. This metric can be swiftly evaluated using blood lipid parameters, waist circumference, and height, providing a convenient and efficient means to assess individual health status. Importantly, CMI holds significant value in predicting the risk of obesity-related metabolic disorders27. CMI is derived by multiplying WHtR with the ratio of TG to HDL-C, where waist-to-height ratio (WHtR) can predict abdominal obesity in individuals, and TG/HDL-C can predict metabolic syndrome and insulin resistance. Some studies have demonstrated a correlation between abdominal obesity and decreased sleep quality34, and the occurrence rate of sleep disorders is often related to metabolic syndrome35. Our research results revealed a noteworthy correlation between elevated CMI and a heightened occurrence of sleep-related issues. Currently, sleep and metabolism are considered to have a bidirectional relationship36,37, Possible explanations for this association can be attributed to several aspects. Firstly, the increase in visceral adipose tissue could cause the secretion of inflammatory cytokines (such as interleukin-6, IL-6), which may contribute to changes in sleep-wake rhythm38. Secondly, sleep disruption may result in metabolic dysregulation due to the overactivation of the hypothalamus-pituitary-adrenal (HPA) axis, modifications in neuroendocrine responses, and alterations in glucose metabolism28,39. At the same time, obesity and obesity-promoting behaviors can lead to sleep disturbances38, creating a bidirectional relationship. Additionally, Chen et al.40proposed that elevated blood lipid levels strongly affect sleep patterns through excessive urination (nocturnal polyuria)41, which in turn may interfere with sleep. Therefore, CMI can reflect the level of metabolic abnormalities in the body. By focusing on early detection and management of elevated CMI, healthcare providers may help patients not only reduce their risk of metabolic diseases but also improve their sleep quality.
The current study boasts several key strengths that significantly enhance the reliability and importance of its findings. Firstly, this is the first time evaluating the relationship between CMI and sleep quality, offering fresh perspectives on the interplay between metabolic characteristics and sleep health. Secondly, the study’s rigorous approach, which took into account potential confounding factors, ensures the accuracy of research results. Finally, the research results have important implications for public health strategies aimed at preventing sleep problems.
Indeed, our study does have some limitations that are worth acknowledging. Firstly, the study’s sample was confined to a specific geographical area and had a relatively small size, which might limit the reliability and generalizability of our findings. This means that our results may not fully reflect the broader population’s characteristics and may not be applicable to other regions or larger populations. Secondly, the assessment of sleep quality was based solely on participants’ self-reported data, which may introduce information bias. Thirdly, the cross-sectional design of our study cannot establish whether the relationship between CMI levels and sleep quality is bidirectional. Future long-term randomized controlled trials are needed to provide clearer evidence for the causal relationship between CMI levels and sleep quality. Additionally, although we used the DAG method to adjust for potential confounders, we cannot entirely rule out the presence of residual confounding factors. Furthermore, while the CMI has been employed in several studies as a marker of cardiometabolic health, we acknowledge that there may be limitations in its validation across different populations. Overall, while our study provides valuable insights into the relationship between CMI levels and sleep quality, these limitations should be carefully considered when interpreting our results. Future studies should aim to address these limitations and further explore the complex relationship between metabolic characteristics and sleep health.
Conclusion
To sum up, the research revealing a strong positive correlation between CMI and sleep disorders as well as trouble sleeping, and a negative association with sleep duration. Our results highlight the potential role of CMI in the early detection and prevention of sleep-related metabolic impairments.
Data availability
The data pertaining to the present study is accessible on the following website: https://www.cdc.gov/nchs/nhanes/index.htm.
Change history
11 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-79200-w
Abbreviations
- CMI:
-
Cardiometabolic index
- NHANES:
-
National Health and Nutrition Examination Survey
- TG:
-
Triglycerides
- WC:
-
Waist circumference
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- PIR:
-
Income to poverty ratio
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycohemoglobin
- CVD:
-
Cardiovascular disease
- OSA:
-
Obstructive sleep apnea
References
Nie, Q. et al. Analysis of sleep for the American population: result from NHANES database. J. Affect. Disord. 347, 134–143. https://doi.org/10.1016/j.jad.2023.11.082 (2024).
Jahrami, H. A. et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 62, 101591. https://doi.org/10.1016/j.smrv.2022.101591 (2022).
Liang, Y. Y. et al. Association between sleep duration and metabolic syndrome: linear and nonlinear Mendelian randomization analyses. J. Transl. Med. 21, 90. https://doi.org/10.1186/s12967-023-03920-2 (2023).
Menezes Júnior, L. A. A. et al. Determinants of poor sleep quality in adults during the coronavirus disease pandemic: COVID-Inconfidentes, a population-based study. Sao Paulo Med. J. 141 (2023).
Menezes-Júnior, L. A. A. et al. O trabalho terceirizado e por turnos está associado ao aumento do risco de COVID-19? Descobertas de um estudo com trabalhadores da mineração. Revista Brasileira de Saúde Ocupacional 48 (2023).
Geovanini, G. R. et al. Poor sleep quality and lipid profile in a rural cohort (The Baependi Heart Study). Sleep Med. 57, 30–35. https://doi.org/10.1016/j.sleep.2018.12.028 (2019).
Dos Santos, A. & Galiè, S. The microbiota-gut-brain axis in metabolic syndrome and sleep disorders: a systematic review. Nutrients 16. https://doi.org/10.3390/nu16030390 (2024).
Prakaschandra, D. R. & Naidoo, D. P. The association of cardiometabolic disorders with sleep duration: a cross-sectional study. Afr. Health Sci. 22, 273–283. https://doi.org/10.4314/ahs.v22i4.32 (2022).
Wang, X. et al. Associations of insomnia with insulin resistance traits: a cross-sectional and Mendelian randomization study. J. Clin. Endocrinol. Metab. 108, e574–e582. https://doi.org/10.1210/clinem/dgad089 (2023).
Lin, P. M. D. et al. Association between self-reported sleep duration and serum lipid profile in a middle-aged and elderly population in Taiwan: a community-based, cross-sectional study. BMJ Open 7, e015964. https://doi.org/10.1136/bmjopen-2017-015964 (2017).
Matsuo, R., Tani, S., Matsumoto, N. & Okumura, Y. Assessment of sex differences in associations between sleep duration and lipid/glucose metabolism in urban Japan: a cross-sectional study. Heart Vessels 37, 1583–1595. https://doi.org/10.1007/s00380-022-02052-9 (2022).
Wakabayashi, I. & Daimon, T. The cardiometabolic index as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta 438, 274–278. https://doi.org/10.1016/j.cca.2014.08.042 (2015).
Song, J. et al. Non-linear associations of cardiometabolic index with insulin resistance, impaired fasting glucose, and type 2 diabetes among US adults: a cross-sectional study. Front. Endocrinol. (Lausanne) 15, 1341828. https://doi.org/10.3389/fendo.2024.1341828 (2024).
Yan, L., Hu, X., Wu, S., Cui, C. & Zhao, S. Association between the cardiometabolic index and NAFLD and fibrosis. Sci. Rep. 14, 13194. https://doi.org/10.1038/s41598-024-64034-3 (2024).
Mo, C. Y., Pu, J. L., Zheng, Y. F. & Li, Y. L. The relationship between cardiometabolic index and pulmonary function among U.S. adults: insights from the National Health and Nutrition Examination Survey (2007–2012). Lipids Health Dis. 23, 246. https://doi.org/10.1186/s12944-024-02235-5 (2024).
Liu, C. et al. Association between cardiometabolic index and cognitive function: a cross-sectional study in a diabetic-based population. Dement. Geriatr. Cogn. Disord 1–11. https://doi.org/10.1159/000539482 (2024).
Cheng, L., Wu, Q. & Wang, S. Cardiometabolic index is associated with increased depression: A population-based study. J. Affect. Disord. 348, 259–264. https://doi.org/10.1016/j.jad.2023.12.073 (2024).
Zhou, X. et al. Association between cardiometabolic index and depression: National Health and Nutrition Examination Survey (NHANES) 2011–2014. J. Affect. Disord. 351, 939–947. https://doi.org/10.1016/j.jad.2024.02.024 (2024).
Lei, X., Xu, Z. & Chen, W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord. 339, 435–442. https://doi.org/10.1016/j.jad.2023.07.040 (2023).
Zhang, L. & Zeng, L. Non-linear association of triglyceride-glucose index with prevalence of prediabetes and diabetes: a cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1295641. https://doi.org/10.3389/fendo.2023.1295641 (2023).
Liu, C. F. & Chien, L. W. Triglyceride glucose index and poor sleep patterns in non-diabetic adults: Evidence from NHANES 2005–2016. Front. Nutr. 10, 1051667. https://doi.org/10.3389/fnut.2023.1051667 (2023).
Menezes-Júnior, L. A. A. et al. How collider bias affects the relationship between skin color and heart attack using directed acyclic graphs, propensity scores, and stepwise approaches. Discov. Public Health 21, 23. https://doi.org/10.1186/s12982-024-00148-3 (2024).
Hall, M. H. et al. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep 31, 635–643. https://doi.org/10.1093/sleep/31.5.635 (2008).
Al-Musharaf, S., Albedair, B., Alfawaz, W., Aldhwayan, M. & Aljuraiban, G. S. The relationships between various factors and sleep status: a cross-sectional study among healthy Saudi adults. Nutrients 15. https://doi.org/10.3390/nu15184090 (2023).
Shimizu, Y. et al. Serum triglyceride levels in relation to high-density lipoprotein cholesterol (TG-HDL) ratios as an efficient tool to estimate the risk of sleep apnea syndrome in non-overweight Japanese men. Environ. Health Prev. Med. 21, 321–326. https://doi.org/10.1007/s12199-016-0532-4 (2016).
Patel, S. R., Malhotra, A., White, D. P., Gottlieb, D. J. & Hu, F. B. Association between reduced sleep and weight gain in women. Am. J. Epidemiol. 164, 947–954. https://doi.org/10.1093/aje/kwj280 (2006).
Wang, D. et al. Application value of cardiometabolic index for the screening of obstructive sleep apnea with or without metabolic syndrome. Nat. Sci. Sleep 16, 177–191. https://doi.org/10.2147/nss.S449862 (2024).
Hirotsu, C., Tufik, S. & Andersen, M. L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 8, 143–152. https://doi.org/10.1016/j.slsci.2015.09.002 (2015).
Menezes-Júnior, L. A. A. et al. Food consumption according to the level of processing and sleep quality during the COVID-19 pandemic. Clin. Nutr. ESPEN 49, 348–356. https://doi.org/10.1016/j.clnesp.2022.03.023 (2022).
Selingardi, S. A. et al. Temporal patterns of food consumption and their association with cardiovascular risk in rotating shift workers. Clin. Nutr. ESPEN 62, 95–101. https://doi.org/10.1016/j.clnesp.2024.04.023 (2024).
Menezes-Júnior, L. A. A. et al. The role of interaction between vitamin D and VDR FokI gene polymorphism (rs2228570) in sleep quality of adults. Sci. Rep. 14, 8141. https://doi.org/10.1038/s41598-024-58561-2 (2024).
de Menezes-Júnior, L. A. A. et al. Influence of sunlight on the association between 25-hydroxyvitamin D levels and sleep quality in Brazilian adults: A population-based study. Nutrition 110, 112008. https://doi.org/10.1016/j.nut.2023.112008 (2023).
Menezes-Júnior, L. A. A. et al. Sedentary behavior is associated with poor sleep quality during the COVID-19 pandemic, and physical activity mitigates its adverse effects. BMC Public Health. 23, 1116. https://doi.org/10.1186/s12889-023-16041-8 (2023).
Mamalaki, E. et al. Associations between sleep and obesity indices in older adults: results from the HELIAD study. Aging Clin. Exp. Res. 31, 1645–1650. https://doi.org/10.1007/s40520-018-01113-2 (2019).
de Souza, L. C. et al. Diet quality, and lipid profile assessment in NCAA Division I acrobatics and tumbling student-athletes: a cross-sectional study. J. Am. Nutr. Assoc. 1–9. https://doi.org/10.1080/27697061.2024.2312843 (2024).
Cooper, C. B., Neufeld, E. V., Dolezal, B. A. & Martin, J. L. Sleep deprivation and obesity in adults: a brief narrative review. BMJ Open Sport Exerc. Med. 4, e000392. https://doi.org/10.1136/bmjsem-2018-000392 (2018).
Nielsen, L. S., Danielsen, K. V. & Sørensen, T. I. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes. Rev. 12, 78–92. https://doi.org/10.1111/j.1467-789X.2010.00724.x (2011).
Muscogiuri, G. et al. Obesity and sleep disturbance: the chicken or the egg? Crit. Rev. Food Sci. Nutr. 59, 2158–2165. https://doi.org/10.1080/10408398.2018.1506979 (2019).
Zhu, B., Shi, C., Park, C. G., Zhao, X. & Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 45, 18–30. https://doi.org/10.1016/j.smrv.2019.02.002 (2019).
Chen, Z. et al. The relationship between sleep duration and blood lipids among Chinese middle-aged and older adults: cross-lagged path analysis from CHARLS. Front. Public Health 10, 868059. https://doi.org/10.3389/fpubh.2022.868059 (2022).
Ohishi, M., Kubozono, T., Higuchi, K. & Akasaki, Y. Hypertension, cardiovascular disease, and nocturia: a systematic review of the pathophysiological mechanisms. Hypertens. Res. 44, 733–739. https://doi.org/10.1038/s41440-021-00634-0 (2021).
Acknowledgements
We would like to extend our heartfelt thanks to everyone involved in this study.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
M. L.: Research Design, Revision of draft. M.X. J.: Data analysis and manuscript drafting and manuscript revision. All authors have reviewed and given their approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The protocols of the NHANES underwent rigorous review and were subsequently approved by the Ethics Review Board. All individuals involved in the study provided written informed consent before participation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors, where the grouped data were not rounded to two decimal numbers. Additionally, in the original version of this Article the variables for TG and HDL-C were inadvertently omitted in Figure 4 legend. Full information regarding the corrections made can be found in the correction for this Article.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, M., Li, M. Association of cardiometabolic index with sleep quality in adults: a population‑based study. Sci Rep 14, 26019 (2024). https://doi.org/10.1038/s41598-024-77855-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77855-z
Keywords
This article is cited by
-
The Cardiometabolic Index (CMI) is associated with an increased risk of obstructive sleep apnea: a population-based study
European Journal of Medical Research (2025)
-
Uncovering the link between cardiometabolic index and depression in diabetes: a large-scale population study
Diabetology & Metabolic Syndrome (2025)
-
Associations of metabolic heterogeneity of obesity, cardiometabolic index with sleep trajectory among middle-aged and older adults: a longitudinal study
BMC Public Health (2025)
-
Associations between cardiometabolic indices and heart disease among elderly and middle-aged Chinese individuals: a retrospective longitudinal study
Scientific Reports (2025)