Abstract
Metabolic syndrome has a multifactorial origin; however, epidemiological data correspond to populations located at sea level. It has been reported that the altitude can affected the prevalence due to physiological changes. The aim of this study is to show the global prevalence of metabolic syndrome at altitude and its components. We use four databases, all studies published up to November 2023. The prevalences from studies were meta-analyzed using a random-effects model. To assess sources of heterogeneity, subgroup analyses were performed. We included 28 studies. The number of participants was 29 195. The prevalence of metabolic syndrome was 30.3% (95% CI 22.8–38.4%). According to the altitude level, at 1500–2500 was 36.5%, 2500–3500 (21.8%), and > 3500 (30.9%), also it was higher in women (35.5%) that men (26.8%). It was observed that there is an inverse relationship between higher altitude and the prevalence of metabolic syndrome. Among its components, abdominal obesity and low HDL were present in more than 40.0%, while high blood pressure, high triglycerides and impaired glucose were present in less than 30.0%. We recommend that our results be considered for future research in populations living at altitude since they have different characteristics from populations at sea level.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is defined as the concomitant presence of several risk factors such as atherogenic adiposity, dyslipidemia, elevated blood pressure and/or insulin resistance, which in turn correspond to their components1,2. The situation predisposes to the development of cardiovascular disease and type 2 diabetes mellitus3. MetS has received increased attention due to its high prevalence in the general population4. In addition, it has been demonstrated that this pathology has continued to grow over the last two decades5,6, with prevalences ranging from 27.4 to 39.0%7,8,9.
MetS has a multifactorial origin, where lifestyle, geographic locations, and ethnic groups are added1; however, epidemiological data correspond mostly to populations located at sea level, without considering those living at altitudes above 1 500 meters above sea level (m.a.s.l). It has been reported that in this environment, the prevalence of MetS can be affected10 due to physiological changes that occur at altitudes above 1 500 m.a.s.l11, where approximately 500 million people live12. It has been found that people living at altitude tend to maintain adequate body weight and low insulin resistance, but high blood pressure and unfavorable serum lipid levels conditions may affect the prevalence of MetS12. However, reports in the literature diverge in explaining these findings. Among the studies reporting MetS prevalences, one study showed a higher prevalence of MetS at altitude compared to sea level (59.0% vs. 21.0%)13. Similarly, each of the MetS components reports variations in reported prevalences, such as high blood pressure, which is higher in Tibetan adults compared to global estimates (31.4% vs. 22.0%)14. As is the prevalence of obesity, which is higher in people living at altitudes (19.7% vs. 9.7%)15. Results contrary to those reported in large Latin America studies such as ENSANUT—ECU where the components of MetS, except hypertriglyceridemia, were less frequent in high altitude areas (29.5% vs. 34.1%)16. Similarly, the CRONICAS cohort study found that the frequency of obesity is lower at higher altitudes (21.6% vs. 88.4%)17. These studies show the controversy regarding the prevalence of MetS at different altitude levels and its components.
Systematic reviews (SR) and meta-analyses have identified a pooled prevalence of MetS of 12.0% in rural areas vs. 22.5% in urban areas18. Similarly, one SR estimated an overall prevalence of MetS between 12.5% and 31.4%19. However, in both studies, the prevalence was not differentiated by altitude level, and these different reported prevalences may be explained by the physiological changes that occur at different altitude levels (intermediate, high, very high, and extreme)11 and by altitude residence regions20 that diverge from each other, even at the genetic level21. In addition, research on metabolic diseases in altitude regions is scarce22,23. The prevalence of MetS is modified by smoking, alcohol consumption, red meat intake24, and hiking activities25, demonstrating that the prevalence of MetS may differ according to geographical factors, nutritional patterns, and physical activity of the population. Therefore, the present study aims to demonstrate through a systematic review and meta-analysis the global prevalence of MetS, each of its diagnostic criteria, the differences between age group, type of resident, level of altitude, continent, type of definition and type of population since they represent variables not evaluated in other studies.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines Supplementary Material 126. The protocol was registered with PROSPERO, number CRD42021291083.
Eligibility criteria
We included cross-sectional and cohort studies that presented information on the prevalence of MetS in people living at high altitudes (resident or native people) worldwide. Among the inclusion criteria, we considered (1) studies with a inhabitants population at an altitude higher than 1 500 m.a.s.l., considering that, according to the literature, physiological and systemic changes occur after this limit11; (2) that each of the studies reports the use of MetS criteria as the International Diabetes Federation’s (IDF)27, National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III)28 and others29; (3) the definition of settlers as resident (defined as a person who leaves his or her city of birth to settle in another place and has a period of stay of more than 12 months in the altitude) and native (defined as a person who has been born and resides in the altitude).
Exclusion criteria included manuscripts that were not available in full text, incorrect study design (referring to editorials, comments, opinions, and narrative reviews that did not present the necessary data for analysis), and studies with incorrect populations (referring to studies with a population that does not belong to altitude regions).
Bibliographic search and selection of studies
A systematic search was performed in four databases: Web of Science (WoS), Scopus, PubMed and Embase. About the search time, all studies published up to November 2023 were considered. No language restrictions were applied. The complete search of each database is available in Supplementary Material 2. The bibliographic references of the included studies were also reviewed for potentially eligible studies.
The studies were exported to the Rayyan Software program30, where duplicates were manually removed. Subsequently, five authors (PGE—FSA—MRCM—JCB—ADCS—JTF) independently reviewed the articles by titles and abstracts to identify potentially relevant articles for inclusion. A third author (JPZV) resolved discrepancies at this stage. The chosen studies proceeded to full-text review where they participated (PGE—FSA—MRCM—JCB—ADCS—JTF). Likewise, this process was performed independently, and discrepancies were resolved by a third author (JPZV). At this point, the excluded studies with their reasons for exclusion can be seen in Supplementary Material 3.
Data extraction and assessment of methodological quality
Four authors (PGE—FSA—JCB—JTF) independently extracted the following data of interest using a Microsoft Excel sheet: author, year of publication, study design, country, sample size, age, sex, type of resident, altitude level, the prevalence of MetS globally and the prevalences of each component, and whether the studies reported by sex (male and female). Discrepancies were resolved by two authors (FSA and MRCM).
Likewise, four authors (JPZV—PGE—JCB—ADCS) independently assessed the methodological quality of prevalence studies using the Joanna Briggs Institute Critical Appraisal Tool31. Another author (JPZV) resolved discrepancies in this process. This scale has 9 items with possible answers of “Yes” (1 point), “No” (0 point) and “Unclear” (0 point). The quality score presented in Supplementary Material 4 was considered as one point for “Yes” and zero points for “No” and “Unclear”. In addition, we categorized the risk of bias into high, moderate, and low according to scores of 0 to 3, 4 to 6, and 7 to 9, respectively. Sensitivity analysis was not performed because all included studies were at low risk of bias. Supplementary Material 4.
Statistical analysis
To assess the proportion of metabolic syndrome, we decided to calculate pooled proportions using mixed-effects models for sample differences in each study with 95% confidence intervals. The Freeman-Turkey Double transformation was used to stabilize variances. In relation to studies with two or more groups, to the analyzes we chose the group with more participants. We decided not to use Egger’s test or funnel plots, as their usefulness for assessing publication bias in the meta-analysis of prevalence is unclear and the results may reflect selection bias. Six subgroup analyses were prespecified according to the available data: type of resident (rural and urban), type of population (native and resident), continent (Asia and America), altitude levels (1500 to 2500, 2500 to 3500 and > 3500 m.a.s.l), type of criteria definition used according to the guideline used for the diagnosis of MetS, age group (<55 years and >= 55 years) and sex (men and women), at this point, we believe that at least two studies should have the necessary information. Assessment of heterogeneity included the Cochrane Q p-value (< 0.10 denotes statistical significance) along with the I2 statistics. When the percentages of I2 value s were 0–40, 30–60, 50–90 and 75–100, they represented possibilities of minor, moderate, substantial and significance heterogeneity, respectively. Statistical significance of the summary estimates was determined with a p-value <0.05. To assess heterogeneity and its sources, we used the I2 test and performed subgroup analyses according to population: sex, country, risk of bias, and altitude. Additionally, meta-regression was carried out to establish whether any of the characteristics of the studies were a source of heterogeneity and the residual heterogeneity percentage. The statistical program Stata v.17 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
Results
Selection and inclusion of studies
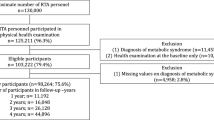
After eliminating duplicates, 780 studies remained to be evaluated by title and abstract. A total of 56 studies were evaluated in full text to assess their eligibility, from which 23 were selected, and another five were selected from other sources. Twenty-eight studies were included in the review (Fig. 1).
Characteristics of studies evaluating the prevalence of metabolic syndrome and its components at altitude (Table 1 )
Of the 28 studies, only one was of cohort design32. The number of participants in all studies was 29,195 and ranged from 18 to 80 years. The altitude level of residence of the study participants ranged from 1500 to 4500 m.a.s.l. The countries where the studies were carried out were: Peru32,33,34,35,36,37,38,39, China12,40,41,42,43, Ecuador16,38,44,45,46,47, Bolivia48, Mexico49,50,51,52,53, and India54,55,56. Some studies evaluated the overall prevalence and components of MetS12,16,32,34,35,36,37,38,40,44,47,55,56,57. On the other hand, only eight studies specified that their populations were natives12,33,34,39,41,42,44,51,55,56,58. About the guideline used for the diagnosis of MetS, nine studies used the IDF16,34,35,38,45,46,47,56,58, nine used the NCEP-ATP III33,34,36,42,43,44,47,50,51,52,56,57,59, and others used other instruments12,37,39,41,46,48,49,54 (Table 1).
Prevalence of metabolic syndrome according each parameter
Twenty-eight studies were synthesized for meta-analysis of metabolic syndrome (MetS) prevalence at altitude (> 1500 m.a.s.l). The pooled prevalence of MetS was 30.3% (95% CI 22.8–38.4%), with significant heterogeneity (I2: 99.5%) among studies. In men, the pooled prevalence of MetS was 26.8% (95% CI 18.5–36.0 %; I2: 98.9%), in women was 35.5% (95% CI 24.8–47.01%; I2: 99.5%), both synthesized from 21 studies (Fig. 2).
Blood pressure had a pooled prevalence of 28.4% (95% CI 17.2–41.1%; I2: 99.6%). Abdominal perimeter with a prevalence of 43.5 (95% CI 34.0–53.2%; I2: 99.3%). Alteration of blood glucose with a prevalence of 13.2% (95% CI 6.7–21.4%; I2: 99.4%). The high level of triglycerides had a prevalence of 22.3 % (95% CI 16.0–29.3%; I2: 97.6%), and the low HDL levels had a prevalence of 43.0% (95% CI 23.1–64.1%; I2: 99.8%). Which had different results according to the sex Table 2.
Prevalence of metabolic syndrome according the subgroup analysis and sex
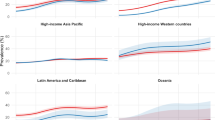
Regarding subgroup analysis by definition, the pooled prevalences were 29.8% (95% CI 18.9–40.7%; I2: 99.4%) for the IDF group, 37.3% (95% CI 26.9–47.7%; I2: 99.2%) for ATP III and 26.8% (95% CI 11.5–42.1%; I2: 99.7%) for the other definitions. In the subgroup analysis by altitude, groups and their pooled prevalences were 1500–2500 m.a.s.l (36.5%; 95% CI 23.1–51.2%; I2: 99.6%), 2500–3500 m.a.s.l (21.8%; 95% CI 10.8–35.4%; I2: 99.3%), and > 3500 m.a.s.l (30.9%; 95% CI 20.0–43.0%; I2: 98.9%). Regarding subgroup analysis by continent, prevalences were 33.2% (95% CI 25.6–41.3%; I2: 99.2%) for America and 23.4% (95% CI 11.0–38.6%; I2: 99.5%) for Asian people Tables 3 and 4.
According to type of population, prevalence was 33.0% (95% CI 25.0–41.6%; I2: 99.3%) for residents and 25.6% (95% CI 12.5–41.5%; I2: 99.6%) for native people. In relation to type of resident, prevalence was 26.4% (95% CI 14.9–40.0%; I2: 97.9%) for rural, 25.0% (95% CI 7.2–48.8%; I2: 99.1%) for urban and 32.9% (95% CI 22.9–43.7%; I2: 99.6%) for both. Finally, people who is < 55 years had the prevalence of 28.3% (95% CI 21.5–35.2%; I2: 99.5%) and >= 55 year was 42.4% (95% CI 26.3–58.4%; I2: 99.4%). The prevalences by sex it can be found in Tables 3 and 4.
Meta-regression analyses
In the meta-regression analyses, no association was found between the type of resident (p=0.188), type of population (p=0.187), continent (p=0.260), level of altitude (p=0.941), type of MetS definition (p=0.214) and male/female sex (p=0.804/0.839) with the prevalence of MetS. Unlike the age group (p=0.002) that did show an association. The residual heterogeneity in the meta-regression was 93.5%.
Quality assessment
More than 80% of the studies met criteria for adequate sample size, detailed description of subjects and setting, appropriate sampling frame and methods, and reliable measurement of conditions in all participants. 76% of the studies met criteria for appropriate statistical analysis. Additionally, most of the studies met criteria for data analysis with sufficient coverage of the identified sample, which could potentially lead to underestimation or overestimation of the prevalence, depending on factors such as the sex and level of altitude of the participants Supplementary Material 4.
Discussion
According our knowledge this systematic review is the first that assesses the prevalence of MetS in altitude regions above 1500 m.a.s.l. worldwide. We found that the prevalence of MetS was 30.3% (> 3500 m.a.s.l: 30.9% vs. < 2500 m.a.s.l: 36.5%) which is inferior to other systematic reviews, which were done at sea level60,61,62, as those carried out in Latin American countries with a prevalence of up to 41.0%53,63, middle eastern countries since 2.2 to 50%64, India 30.0%65, Africa 32.4%66 and people with mental health disorders till 38.7%67,68. These differences according to altitude could be attributed to altitude populations having lower risk factors, lower socioeconomic status, higher mineral intake, lower calorie diets, lower rates of obesity and diabetes10,69. Likewise, the high-altitude environment influences reduced energy intake and increased resting metabolic rate. Coupled with increased physical activity, driven by agricultural and livestock tasks15,70,71, all of which decrease the risk factors that lead to a lower prevalence of MetS.
Abdominal obesity, was present in 4 out of 10 of the participants, we observed that women with MetS have a higher prevalence than men. Similarly, an Asian studied identified that in 5 out of 10 participants showed abdominal or centra obesity72. Different to a systematic review in Latin America where countries of different altitudes were included and reported in 6 out of 10 residents61. It is also lower than another review at sea level (5 out of 10)73. Studies on the components of MetS have reported a high prevalence of abdominal obesity in the Latin American population, similar to or even higher than in developed countries, which can be explained by lifestyle changes61,74. These reports are not entirely comparable with another study in Latin America showing a high prevalence of abdominal obesity but a low prevalence of hypertriglyceridemia, hypertension, and low HDL values33. Overweight, obesity, and related noncommunicable diseases have become major public health problems in the regions, although rates still vary in some countries75.
In relation to Blood pressure, it was present in 3 out of 10 participants, in which the male sex presents a higher value than the female. Similarly, a reviews carried out in Tibetan settler, where found a prevalence between 2 to 5 out of 10 residents76,77. Likewise, it is similar to another study conducted al Altitude in Latin America countries with a prevalence of 1 out of 1078. The result is lower than others studies that were carried out at sea level with prevalences reported between 379 to 5 residents at sea level80. At altitude has been found that living at high altitude is a unique risk factor for hypertension, with a 2.0 % increase in the prevalence of hypertension per 100 m of altitude, which could be due in part to physiological adaptation to hypobaric hypoxia76.
Regarding Hypertriglyceridemia, it was present in almost 2 out of 10 participants with the male sex being the most affected. Different to reported by studies carried out to sea level, where it found a prevalence between of 4 to 5 out of 10 residents81,82. When observing the metabolic components potentially associated with MetS found in the review, it was determined that adiposity, low HDL values, hyperglycemia, and hypertension were mostly reported in the Asian altitude population, a behavior corroborated by another study where hypertension and obesity are strongly associated with a high prevalence of MetS12,83.
Finally, Hyperglycemia was present in 1 out of 10 participants, result that it was the same for both sexes. This result is similar to that found in systematic reviews carried out at sea level (16.0%)73. However, a study carried out in Nepal showed a prevalence of hyperglycemia and MetS was in 3 out of 10 residents84. It is relevant to know that hyperglycemia is not the first sign of MetS and develops as a later complication85. On the other hand, hyperglycemia is the most prevalent component in Latin America, with 25.0%, which is supported by the fact that hyperglycemia is the least prevalent component, as its prevalence is significantly lower in Asian countries compared to other studies61,86. This is supported by the Latin American multicenter CARMELA study, which showed hypertriglyceridemia to be the most prevalent component (85.9%) and glucose the least prevalent (31.2%)36,87.
Subgroups analyses
Our study found that the prevalence of MetS in rural areas is higher than in urban areas. Our study differs from other systematic reviews and meta-analyses conducted in Brazil and Iran where the prevalence of MetS was higher in urban areas compared to rural areas, 34% vs 15% in Brazil63 and 0.39% vs 0.26% in Iran88. It is also different from what was found in a study carried out in Ghana, where it was evident that the prevalence was higher in urban areas than in rural areas and it could also be evidenced according to sex, in men it was 23.6% in urban areas89. While the difference in prevalences is not significant, it probably reflects the multifactorial origin of MetS among populations with their own epidemiological characteristics. However, there is a lack of studies conducted at altitude that show both groups separately.
The finding of the study is that there is a higher prevalence of MetS in the resident population compared to the native population. No similar studies comparing these characteristics were found. This difference could be due to the fact that it has been evidenced that in the native population, due to their own exposure to the hypoxic hypobaric environment, it has been noted that these people present a greater increase of the anorexigenic hormone leptin, which is related to weight loss, especially of the abdominal adipose tissue, which is associated with a lower prevalence of developing MetS90. In contrast, the resident population, as observed in the context of Ladakh, often represents a migrant population moving from a high-altitude rural region to a high-altitude city. These residents tend to adopt the lifestyle of rapidly developing countries, which may lead to an increase in the prevalence of all components of the metabolic syndrome compared to those who continue to live at higher altitudes. This phenomenon reflects the different lifestyles and customs that residents bring with them, which may condition the development of a higher prevalence of MetS54. Our review did not include studies of populations living at altitudes above 4000 m.a.s.l. due to the scarcity of existing information, which represents a significant limitation. This lack of information leaves a gap in knowledge about the metabolic health of high-altitude inhabitants and underscores the need for more research in these extreme areas. Future studies addressing this gap are needed to better understand how altitude affects the development of metabolic syndrome.
In our study, we found that there is a higher prevalence in individuals aged 55 years or older than younger. Our study differs from another systematic review, where an equal prevalence (42%) was found63. Likewise, it is similar to a study carried out in Latin America, where a higher prevalence was reported in subjects over 50 years of age61. Additionally, a study carried out in Chile found that as age increases there is an increase in the prevalence of each of the MetS components91, which is consistent with what was found in our study. Therefore, a person, as they age, is more predisposed to having MetS because they are influenced by the high prevalence of metabolic risk factors developed at these ages92,93.
We found that American was the continent with the highest reported prevalence de MetS, which can be explained by studies reporting that lifestyle (low fruit and vegetable intake, higher meat consumption, and insufficient physical activity) in that continent are highly adaptive risk factors for this MetS94,95, in combination with certain genetic factors and metabolic adaptations during fetal life, may be considered causal factors for this disease74. Likewise, for example, in Ecuador, a Latin America country, more than 30.0% of the adult population aged 18–59 years has MetS and about 85.0% of the population has at least one metabolic abnormality defined as a disorder of MetS16, but reports suggest that the prevalence is higher in coastal cities than in high-altitude cities; furthermore due to intake of sugar and excessively processed foods, high consumption of carbohydrates and salt, and sedentary lifestyle96. Finally, Asian reported prevalence of MetS (2 out of 10), despite being the largest developing continent in the world and studies reporting a high prevalence of MetS97 was the lowest reported in high-altitude populations; concerning three different countries, these prevalences may vary due to the diversity of populations, cultural behaviors, and lifestyle60,98.
It is believed that more than 500 million people worldwide live at altitudes above 1500 m.a.s.l, which causes severe physiological problems for people living in these areas12. In our review, the lowest altitude considered, 1500–2500 m.a.s.l, 4 out of 10 inhabitants has a high prevalence of MetS, while the highest altitude, above 3500 m.a.s.l, the prevalence it was present in 3 out of 10. While is that true, genetic and physiological adaptations to altitude determined by hypoxia affect glucose metabolism, leading to appetite suppression and reduced caloric intake99. The adaptations may explain why prevalence is lower at higher altitudes. Therefore, future research should focus on proposing reference values for each of the five components that determine MetS in populations at high altitudes because of phenotypic and genotypic differences100. The data and studies on MetS components, reference values and cut-off points in different highland regions of the world, particularly in South American countries, are still incipient, and future research should aim to develop new approaches for more accurate assessment, diagnosis, and monitoring of highland populations. However, evidence is lacking as to why at 2500–3500 m.a.s.l. most prevalences are lower, with the exception of low HDL.
The prevalences were 2, 4 and 3 out 10 for IDF, ATP III and other definitions. Unlike, to other systematic review and meta-analysis, the prevalences were 31% (IDF), 31% (ATP III), and 25% (JIS)63. And another study that reported prevalences of 54.0% (IDF), 48.0% (AHA/NHLBI), 36.0% (ATP III) and 31.0% (WHO)53. We emphasize that the studies mentioned above which have been chosen for comparison correspond to studies carried out with resident populations at sea level and they are systematic reviews. Thus, to the best of our knowledge, our results (at altitude) are lower than at sea level. However, it must be recognized that high altitude regions exhibit certain deficiencies, including primary health care services, unequal access to essential medicines and a significant lack of effective programs for the prevention, treatment and management of chronic diseases101,102.
In our study, 4 women and 3 men out of 10 had MetS. Similar to other studies, like the Gulf Cooperation Council countries, where a higher prevalence of MetS is reported in women than in men103. While it is true, the MetS components probably overlap at the physiological level, and evidence shows that males develop this syndrome earlier than females, as reported in studies of Australian adults and high-altitude populations in China12,104,105,106. The higher prevalence of MetS in women reported in the articles reviewed may also be due to the higher prevalence of adiposity in women (46.0%) than in men (24.0%), according to a report on the current outlook for obesity and hypertension in LATAM, it is projected that by 2025, 80–90% of the population will suffer from hypertension and obesity and consequently acquire MetS, and it has been shown that only people with one or two criteria will show greater development of MetS105,107. Globally, statistical trends of physical inactivity reported a higher prevalence in women, explaining women’s greater susceptibility to MetS predisposition, this finding is also complemented by a LATAM study on sedentary lifestyles that focused on gender differences reporting that women were less physically active than men108,109. The intake of energy-dense foods is associated not only with obesity, but also with impaired glucose regulation, hypertension, and dyslipidemia, leading to an increased risk of MetS110,111, in our review, we found no significant differences between men and women in hyperglycemia, but there were significant differences in other indicators such as hypertension and hypertriglyceridemia, which were higher in men than in women.
According to several studies, altitude may affect the incidence of MetS from multiple aspects, for example, there is a lower prevalence of obesity and diabetes at higher altitudes12 also, the prevalence of hypertension in high-altitude residents is lower than in low-altitude residents13,78.
People residing at high altitudes have physiological adaptations to environmental pressure and reduced oxygen availability, such as elevated hemoglobin concentration, enlarged lung volume and decreased hypoxic ventilatory response1. These adaptations have resulted in changes in cardiovascular and respiratory efficiency, lower LDL cholesterol values, higher HDL cholesterol, better-fasting glucose levels compared to sea level residents, and improved blood glucose levels1 and direct catabolic effect; for example, in BMI there was a reduction of −1.31 kg/m2 per kilometer of altitude54.
Some health conditions have also been observed in people living at altitudes, such as acute mountain sickness, pulmonary and cerebral edema at altitude, hypoxia, lack of appetite, fatigue, stomach discomfort, and unwillingness to work. These conditions explain the metabolic risk factors and generate physical restriction and mental depression, consequently promoting the development of cardiometabolic disorders13. Regarding lack of appetite, at higher altitudes there is an increase in basal metabolic rate and an increase in leptin levels, leading to lower calorie intake due to lack of appetite112. Also, hypoxia leads to appetite suppression by impairing glucose metabolism90 and, consequently, there is a reduction in caloric intake1 and a lower prevalence of obesity90. Evidence to support our results in each of the components of the MetS.
Implications of the results and recommendations for future studies
There are few systematic reviews related to altitude22. In several regions of the world, the prevalence of MetS tends to increase, and it occurs more frequently in women; it is also mentioned that it is considered a public health problem and needs to be controlled to mitigate this pathology. MetS is a disease in constant increase worldwide. Populations located at altitude present differences with respect to those located at sea level, such as their lifestyles, customs and hypoxic hypobaric environment. MetS causes high public health expenditure, however, being a pathology with modifiable risk factors, the impact on the economy is substantial113. These risk factors should be reduced through prevention focused on rural and high-altitude areas, where costs, access to health services and knowledge of the disease are lower101. Thus, it is suggested that the prevalence and characteristics of the most frequent components of MetS vary among different ethnic groups and may not be specific to each group or race.
The prevalence of MetS is increasing in parallel with the rising prevalence of obesity and is projected to increase by 33.0% over the next two decades and by 2030 (51.0%) of the population will be obese. If these predictions prove accurate, efforts to reduce healthcare costs will be further hampered, and the incidence of cardiovascular disease could spiral out of control as a consequence114. Curiously, in developed countries, there is a transition in which the higher socioeconomic class reduces metabolic risk while it increases in the lower social classes115,116. This possibly indicates that the assumption of a healthier lifestyle occurs in the wealthier classes than in those who are not and are exposed to a health system that is not 100% covered. Therefore, prevention strategies are needed that identify sociodemographic and environmental factors (which especially affect women) and target risk behaviors that can be modified, such as being underweight, prolonged sedentary behavior, and diet. Our result diverges from that reported in studies performed at sea level, so we assume that there is an inverse relationship between altitude and the prevalence of MetS.
We recommend that further studies assess the prevalence of the components and examine the impact of educational measures and lifestyle modification on the prevalence of MetS Similarly, further studies should be developed to compare the prevalence of MetS between populations living at high altitude and those living at sea level and to identify associated risk factors for reversal. Therefore, epidemiological estimates of MetS risk factors and their frequency are essential to develop specific management programs and plan an optimal allocation of healthcare resources for this patient population.
Limitations and strengths
The study has some strengths. First, this is the first systematic review and meta-analysis on the prevalence of MetS in high-altitude populations worldwide so that it may serve as a baseline for future studies, and the systematized information may be helpful, not only for researchers but also for public health professionals working in high-altitude regions and even for the development of national strategies for the prevention and treatment of MetS in mid- and high-altitude regions. Second, we reviewed peer-reviewed publications without language restriction to gain a comprehensive and culturally informed understanding of the topic. Third, we addressed a clear gap in the literature in considering altitude residents for characteristics unique to the characteristics associated with the hypoxic hypobaric environment. This study also has limitations. The prevalence estimates of MetS were not standardized due to the study sample selection and statistical methods of the included studies. Second, there was a considerable heterogeneity due to unmeasurable variables such as nutritional patterns in each country, physical activity, and healthy lifestyles in each population. These limited their comparability. Nevertheless, we have used the best available data to increase our understanding of this important topic. An additional limitation is that we did not include well-designed peer-reviewed studies that took into account socioeconomic factors, cultural factors, ethnics factors, hypoxemia, and the effects of altitude above 3500 m.a.s.l. on the components of the MetS because there is limited information available, otherwise it would have provided a more complex and culturally informed understanding of the subject, especially in relation to inhabitants residing in regions with higher altitude. Despite our thorough review with no language restrictions, the lack of studies considering socioeconomic, cultural, and hypoxemic factors, along with variability in MetS definitions, may have impacted the accuracy and generalizability of the results. Additionally, studies focused on migrant or urban populations may not fully capture the effects of altitude on native residents. Therefore, we recommend that future research include well-designed studies at altitudes above 4000 m.a.s.l. and consider socioeconomic and cultural effects, as well as differences in definitions of MetS. It is also crucial to explore the interaction between age, altitude, and prevalence of hypertension, as well as variations in waist circumference among different ethnic groups.
Conclusion
The prevalence of MetS at altitude (> 1500 m.a.s.l.) was 36.5%, being higher in women (35.5%). It was observed that there is an inverse relationship between higher altitude and the prevalence of MetS. Among its components, abdominal obesity and low HDL were present in more than 40.0%, while high blood pressure, high triglycerides and impaired glucose were present in less than 30.0%. We recommend that our results be considered for future research in populations living at altitude since they have different characteristics from populations at sea level.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Villegas-Abrill, C. B. et al. Diagnostic criteria for metabolic syndrome in high-altitude regions: A systematic review. Medicina (Kaunas). 58(3), 451. https://doi.org/10.3390/medicina58030451 (2022).
Zibaeenezhad, M. J. et al. Potential of four definitions of metabolic syndrome to discriminate individuals with different 10-year cardiovascular disease risk scores: A cross-sectional analysis of an Iranian cohort. BMJ Open. 12(2), e058333. https://doi.org/10.1136/bmjopen-2021-058333 (2022).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 120(16), 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 (2009).
Guembe, M. J. et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 19(1), 195. https://doi.org/10.1186/s12933-020-01166-6 (2020).
Shin, D., Kongpakpaisarn, K. & Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 259, 216–219. https://doi.org/10.1016/j.ijcard.2018.01.139 (2018).
Beltrán-Sánchez, H., Harhay, M. O., Harhay, M. M. & McElligott, S. Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. J. Am. Coll. Cardiol. 62(8), 697–703 (2013).
Athyros, V. G., Ganotakis, E. S., Elisaf, M. & Mikhailidis, D. P. The prevalence of the metabolic syndrome using the national cholesterol educational program and international diabetes federation definitions. Curr. Med. Res. Opin. 21(8), 1157–1159. https://doi.org/10.1185/030079905x53333 (2005).
Song, Q. B. et al. Sex difference in the prevalence of metabolic syndrome and cardiovascular-related risk factors in urban adults from 33 communities of China: The CHPSNE study. Diab. Vasc. Dis. Res. 12(3), 189–198. https://doi.org/10.1177/1479164114562410 (2015).
Mazidi, M., Toth, P. P. & Banach, M. C-reactive protein is associated with prevalence of the metabolic syndrome, hypertension, and diabetes mellitus in US adults. Angiology. 69(5), 438–442. https://doi.org/10.1177/0003319717729288 (2018).
Woolcott, O. O. et al. Inverse association between diabetes and altitude: A cross-sectional study in the adult population of the United States. Obesity. 22(9), 2080–2090. https://doi.org/10.1002/oby.20800 (2014).
Imray, C., Booth, A., Wright, A. & Bradwell, A. Acute altitude illnesses. BMJ. 343, d4943. https://doi.org/10.1136/bmj.d4943 (2011).
Huang, X. et al. Metabolic syndrome in native populations living at high altitude: a cross-sectional survey in Derong, China. BMJ Open. 10(1), e032840. https://doi.org/10.1136/bmjopen-2019-032840 (2020).
Tyrovolas, S. et al. Health care access and prevalence of the metabolic syndrome among elders living in high-altitude areas of the Mediterranean Islands: The MEDIS study. Rev Diabet Stud. 8(4), 468–476. https://doi.org/10.1900/RDS.2011.8.468 (2011).
Wang, H. et al. Association between dietary patterns and metabolic syndrome and modification effect of altitude: A cohort study of Tibetan adults in China. Nutrients. 15(9), 2226. https://doi.org/10.3390/nu15092226 (2023).
Merrill, R. M. Explaining the inverse association between altitude and obesity. J. Obes. https://doi.org/10.1155/2020/1946723 (2020).
Pérez-Galarza, J. et al. Prevalence of overweight and metabolic syndrome, and associated sociodemographic factors among adult Ecuadorian populations: the ENSANUT-ECU study. J. Endocrinol. Invest. 44(1), 63–74. https://doi.org/10.1007/s40618-020-01267-9 (2021).
Benziger, C. P. et al. Metabolic abnormalities are common among South American Hispanics subjects with normal weight or excess body weight: The CRONICAS cohort study. PLOS ONE. 10(11), e0138968. https://doi.org/10.1371/journal.pone.0138968 (2015).
Jaspers Faijer-Westerink, H., Kengne, A. P., Meeks, K. A. C. & Agyemang, C. Prevalence of metabolic syndrome in sub-Saharan Africa: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 30(4), 547–565. https://doi.org/10.1016/j.numecd.2019.12.012 (2020).
Noubiap, J. J. et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc. Health https://doi.org/10.1016/S2352-4642(21)00374-610.1016/S2352-4642(21)00374-6 (2022).
Tremblay, J. C. & Ainslie, P. N. Global and country-level estimates of human population at high altitude. Proc. Natl. Acad. Sci. 118(18), e2102463118. https://doi.org/10.1073/pnas.2102463118 (2021).
Heinrich, E. C. et al. Genetic variants at the EGLN1 locus associated with high-altitude adaptation in Tibetans are absent or found at low frequency in highland Andeans. Ann. Hum. Genet. 83(3), 171–176. https://doi.org/10.1111/ahg.12299 (2019).
Zila-Velasque, J. P. et al. Adaptation and altitude sickness: A 40-year bibliometric analysis and collaborative networks. Front. Public Health. https://doi.org/10.3389/fpubh.2023.1069212 (2023).
Zila-Velasque, J. P. et al. Mountain sickness in altitude inhabitants of Latin America: A systematic review and meta-analysis. PLOS ONE. 19(9), e0305651. https://doi.org/10.1371/journal.pone.0305651 (2024).
Yao, F. et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients. 13(12), 4475. https://doi.org/10.3390/nu13124475 (2021).
Neumayr, G. et al. Effects of hiking at moderate and low altitude on cardiovascular parameters in male patients with metabolic syndrome: Austrian moderate altitude study. Wilderness Environ. Med. 25(3), 329–334. https://doi.org/10.1016/j.wem.2014.01.003 (2014).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Zhu, L., Spence, C., Yang, W. J. & Ma, G. X. The IDF definition is better suited for screening metabolic syndrome and estimating risks of diabetes in Asian American adults: Evidence from NHANES 2011–2016. J. Clin. Med. 9(12), 3871. https://doi.org/10.3390/jcm9123871 (2020).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2(5–6), 231–237. https://doi.org/10.1242/dmm.001180 (2009).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome. Circulation. 112(17), e285–e290. https://doi.org/10.1161/CIRCULATIONAHA.105.169405 (2005).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews. 5(1), 210. https://doi.org/10.1186/s13643-016-0384-4 (2016).
Munn, Z., Moola, S., Lisy, K., Riitano, D. & Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 13(3), 147–153. https://doi.org/10.1097/XEB.0000000000000054 (2015).
Mejia, C. R. et al. Age as a risk factor for developing metabolic syndrome in mine workers at high altitude. Revista Argentina de Endocrinologia y Metabolismo. 53(1), 29–35. https://doi.org/10.1016/j.raem.2016.05.002 (2016).
Baracco, R., Mohanna, S. & Seclén, S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in Peru. Metab. Syndr. Relat. Disord. 5(1), 55–62. https://doi.org/10.1089/met.2006.0019 (2007).
Herrera-Enriquez, K. & Narvaez-Guerra, O. Discordance of metabolic syndrome and abdominal obesity prevalence according to different criteria in Andean highlanders: A community-based study. Diabetes Metab. Syndr. 11(Suppl 1), S359–S364. https://doi.org/10.1016/j.dsx.2017.03.016 (2017).
Miele, C. H. et al. Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Alt. Med. Biol. 17(2), 93–100. https://doi.org/10.1089/ham.2015.0084 (2016).
Ninatanta-Ortiz, J. A., Núñez-Zambrano, L. A., García-Flores, S. A. & Romaní, F. R. Frequency of metabolic syndrome in residents of an andean region in Peru. Rev. Peru Med. Exp. Salud Publica. 33(4), 640–650 (2016).
Medina-Lezama, J. et al. Prevalence of the metabolic syndrome in Peruvian Andean Hispanics: The PREVENCION study. Diabetes Res. Clin. Pract. 78(2), 270–281. https://doi.org/10.1016/j.diabres.2007.04.004 (2007).
Lopez-Pascual, A., Arévalo, J., Martínez, J. A. & González-Muniesa, P. Inverse association between metabolic syndrome and altitude: A cross-sectional study in an adult population of Ecuador. Front. Endocrinol. https://doi.org/10.3389/fendo.2018.00658 (2018).
Sanchez-Samaniego, G. et al. Metabolic syndrome in rural Peruvian adults living at high altitudes using different cookstoves. PLOS ONE. 17(2), e0263415. https://doi.org/10.1371/journal.pone.0263415 (2022).
Sherpa, L. Y., Deji, S. H., Chongsuvivatwong, V., Nafstad, P. & Bjertness, E. Prevalence of metabolic syndrome and common metabolic components in high altitude farmers and herdsmen at 3700 m in Tibet. High Alt. Med. Biol. 14(1), 37–44. https://doi.org/10.1089/ham.2012.1051 (2013).
Matsubayashi, K. et al. Comprehensive geriatric assessment of elderly highlanders in Qinghai, China I: Activities of daily living, quality of life and metabolic syndrome. Geriatr. Gerontol. Int. 9(4), 333–341. https://doi.org/10.1111/j.1447-0594.2009.00548.x (2009).
Peng, W. et al. The prevalence and associated factors of metabolic syndrome among Tibetan pastoralists in transition from nomadic to settled urban environment. Zhonghua Liu Xing Bing Xue Za Zhi. 43(4), 533–540. https://doi.org/10.3760/cma.j.cn112338-20211118-00900 (2022).
Peng, W. et al. Metabolic syndrome and its relation to dietary patterns among a selected urbanised and semi-urbanised Tibetan population in transition from nomadic to settled living environment. Public Health Nutr. 24(5), 984–992. https://doi.org/10.1017/S1368980019004798 (2021).
Baldeón, M. E. et al. Prevalence of metabolic syndrome and diabetes mellitus type-2 and their association with intake of dairy and legume in Andean communities of Ecuador. PLoS One. 16(7), e0254812. https://doi.org/10.1371/journal.pone.0254812 (2021).
Salazar-Lugo, R. et al. Factores bioquímicos y nutricionales asociados a la viscosidad sanguínea en adultos de la sierra urbana (Imbabura). Ecuador. Investigación Clínica. 57(3), 293–304 (2016).
Orces, C. H. & Gavilanez, E. L. The prevalence of metabolic syndrome among older adults in Ecuador: Results of the SABE survey. Diabetes Metab. Syndr. 11(Suppl 2), S555–S560. https://doi.org/10.1016/j.dsx.2017.04.004 (2017).
Chimbo-Yunga, J. M., Chuchuca-Cajamarca, Á. J., Wong, S. & Encalada-Torres, L. E. Metabolic syndrome and physical activity in elderly people from the Ecuadorian highlands. Rev. Salud Publica (Bogota). 19(6), 754–759 (2017).
Hurtado-Arestegui, A. et al. Higher prevalence of unrecognized kidney disease at high altitude. J Nephrol. 31(2), 263–269. https://doi.org/10.1007/s40620-017-0456-0 (2018).
Porchia, L. M., Gonzalez-Mejia, M. E., Torres-Rasgado, E., Ruiz-Vivanco, G. & Pérez-Fuentes, R. Low serum uric acid concentration augments insulin effects on the prevalence of metabolic syndrome. Diabetes Metab. Syndr.: Clin. Res. Rev. 12(3), 325–331. https://doi.org/10.1016/j.dsx.2017.12.012 (2018).
Díaz-Cisneros, F. J., Rodríguez-Guzmán, L., Rodríguez-Guzmán, E. & García-González, M. R. Prevalencia del síndrome metabólico en profesores de Guanajuato, México. Anales de la Facultad de Medicina. 71(2), 75–78 (2010).
Echavarría-Pinto, M., Hernández-Lomelí, A., Alcocer-Gamba, M. A., Morales-Flores, H. & Vázquez-Mellado, A. Síndrome metabólico en adultos de 20 a 40 años en una comunidad rural mexicana. Rev. Med. Inst. Mex. Seguro Soc. 44(4), 329–335 (2006).
Jiménez, A. E. R. et al. Prevalencia del síndrome metabólico en relación con las concentraciones de ácido úrico. Med. Int. Mex. 25(4), 278–284 (2009).
Gutiérrez-Solis, A. L., Datta Banik, S. & Méndez-González, R. M. Prevalence of metabolic syndrome in Mexico: A systematic review and meta-analysis. Metab. Syndr. Relat. Disord. 16(8), 395–405. https://doi.org/10.1089/met.2017.0157 (2018).
Lin, B. Y. et al. The prevalence of obesity and metabolic syndrome in Tibetan immigrants living in high altitude areas in Ladakh, India. Obes. Res. Clin. Pract. 12(4), 365–371. https://doi.org/10.1016/j.orcp.2017.03.002 (2018).
Kapil, U. et al. Prevalence of metabolic syndrome and associated risk factors among geriatric population living in a high altitude region of rural Uttarakhand, India. J. Family Med. Prim. Care. 7(4), 709–716. https://doi.org/10.4103/jfmpc.jfmpc_261_17 (2018).
Thakur, S., Raina, S., Thakur, S., Negi, P. C. & Verma, B. S. Prevalence of metabolic syndrome among newly diagnosed hypertensive patients in the hills of Himachal Pradesh, India. Indian J. Endocrinol. Metab. 17(4), 723–726. https://doi.org/10.4103/2230-8210.113768 (2013).
Juna, C. F., Cho, Y. H. & Joung, H. Low elevation and physical inactivity are associated with a higher prevalence of metabolic syndrome in Ecuadorian adults: A national cross-sectional study. Diabetes, Metab. Syndr. Obes. Targ. Ther. 13, 2217–2226. https://doi.org/10.2147/DMSO.S253099 (2020).
Sherpa, L. Y. et al. Prevalence of metabolic syndrome and common metabolic components in high altitude farmers and herdsmen at 3700 m in Tibet. High Alt. Med. Biol. 14(1), 37–44. https://doi.org/10.1089/ham.2012.1051 (2013).
González-Chávez, A. et al. Prevalencia del síndrome metabólico entre adultos mexicanos no diabéticos, usando las definiciones de la OMS, NCEP-ATPIIIa e IDF. Rev. Med. Hosp. Gen. Mex. 71(1), 11–19 (2008).
Cai, W., Tang, X. & Pang, M. Prevalence of metabolic syndrome in patients with Rheumatoid Arthritis: An updated systematic review and meta-analysis. Front. Med. https://doi.org/10.3389/fmed.2022.855141 (2022).
Márquez-Sandoval, F. et al. The prevalence of metabolic syndrome in Latin America: a systematic review. Public Health Nutr. 14(10), 1702–1713. https://doi.org/10.1017/S1368980010003320 (2011).
Panagiotakos, D. B., Pitsavos, C., Skoumas, Y. & Stefanadis, C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J. Am. Diet. Assoc. 107(6), 979–987. https://doi.org/10.1016/j.jada.2007.03.006 (2007).
de Siqueira Valadares, L. T. et al. Prevalence of metabolic syndrome in Brazilian adults in the last 10 years: A systematic review and meta-analysis. BMC Public Health. 22(1), 327. https://doi.org/10.1186/s12889-022-12753-5 (2022).
Ansarimoghaddam, A. et al. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab. Syndr. 12(2), 195–201. https://doi.org/10.1016/j.dsx.2017.11.004 (2018).
Krishnamoorthy, Y. et al. Prevalence of metabolic syndrome among adult population in India: A systematic review and meta-analysis. PLOS ONE. 15(10), e0240971. https://doi.org/10.1371/journal.pone.0240971 (2020).
Bowo-Ngandji, A. et al. Prevalence of the metabolic syndrome in African populations: A systematic review and meta-analysis. PLoS One. 18(7), e0289155. https://doi.org/10.1371/journal.pone.0289155 (2023).
Rosenbaum, S. et al. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 64(8), 926–933. https://doi.org/10.1016/j.metabol.2015.04.009 (2015).
Tao, H. et al. A systematic review and meta-analysis of metabolic syndrome prevalence in Chinese inpatients with bipolar disorder. Horm. Metab. Res. 54(9), 587–592. https://doi.org/10.1055/a-1882-8423 (2022).
Hirschler, V. Cardiometabolic risk factors in native populations living at high altitudes. Int. J. Clin. Pract. 70(2), 113–118. https://doi.org/10.1111/ijcp.12756 (2016).
Voss, J. D., Masuoka, P., Webber, B. J., Scher, A. I. & Atkinson, R. L. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int. J. Obes. (Lond). 37(10), 1407–1412. https://doi.org/10.1038/ijo.2013.5 (2013).
Kayser, B. & Verges, S. Hypoxia, energy balance, and obesity: An update. Obes. Rev. 22(Suppl 2), e13192. https://doi.org/10.1111/obr.13192 (2021).
Peng, W. et al. Prevalence, management, and associated factors of obesity, hypertension, and diabetes in Tibetan population compared with China overall. Int. J. Environ. Res. Public Health. 19(14), 8787. https://doi.org/10.3390/ijerph19148787 (2022).
de Carvalho, Vidigal F., Bressan, J., Babio, N. & Salas-Salvadó, J. Prevalence of metabolic syndrome in Brazilian adults: a systematic review. BMC Public Health. 13, 1198. https://doi.org/10.1186/1471-2458-13-1198 (2013).
Cuevas, A., Alvarez, V. & Carrasco, F. Epidemic of metabolic syndrome in Latin America. Curr. Opin. Endocrinol. Diabetes Obes. 18(2), 134–138. https://doi.org/10.1097/MED.0b013e3283449167 (2011).
Gill, T. Epidemiology and health impact of obesity: an Asia Pacific perspective. Asia Pac. J. Clin. Nutr. 15(Suppl), 3–14 (2006).
Mingji, C., Onakpoya, I. J., Perera, R., Ward, A. M. & Heneghan, C. J. Relationship between altitude and the prevalence of hypertension in Tibet: a systematic review. Heart. 101(13), 1054–1060. https://doi.org/10.1136/heartjnl-2014-307158 (2015).
Zhang, X., Zhang, Z., Ye, R., Meng, Q. & Chen, X. Prevalence of hypertension and its relationship with altitude in highland areas: a systematic review and meta-analysis. Hypertens Res. 45(8), 1225–1239. https://doi.org/10.1038/s41440-022-00955-8 (2022).
Zila-Velasque, J. P. et al. Prevalence of hypertension in adults living at altitude in Latin America and the Caribbean: A systematic review and meta-analysis. PLOS ONE. 18(10), e0292111. https://doi.org/10.1371/journal.pone.0292111 (2023).
Zhou, B. et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 398(10304), 957–980. https://doi.org/10.1016/S0140-6736(21)01330-1 (2021).
Haile, T. G. et al. Prevalence of hypertension among type 2 diabetes mellitus patients in Ethiopia: a systematic review and meta-analysis. Int. Health. 15(3), 235–241. https://doi.org/10.1093/inthealth/ihac060 (2023).
Ambachew, S. et al. The prevalence of metabolic syndrome in Ethiopian population: A systematic review and meta-analysis. J. Obes. https://doi.org/10.1155/2020/2701309 (2020).
Tabatabaei-Malazy, O. et al. Prevalence of Dyslipidemia in Iran: A systematic review and meta-analysis study. Int. J. Prev. Med. 5(4), 373–393 (2014).
Xu, S. et al. Prevalence and clustering of cardiovascular disease risk factors among Tibetan adults in China: A population-based study. PLOS ONE. 10(6), e0129966. https://doi.org/10.1371/journal.pone.0129966 (2015).
Koirala, S. et al. Current health status and its risk factors of the Tsarang villagers living at high altitude in the Mustang district of Nepal. J. Physiol. Anthropol. 37(1), 1–10. https://doi.org/10.1186/s40101-018-0181-y (2018).
Khasanova, A. K. et al. Blood and urinary biomarkers of antipsychotic-induced metabolic syndrome. Metabolites. 12(8), 726. https://doi.org/10.3390/metabo12080726 (2022).
Chirinos, D. A., Morey-Vargas, O. L., Goldberg, R. B., Chirinos, J. A. & Medina-Lezama, J. Metabolic syndrome in andean populations. Glob. Heart. 8(4), 349-354.e1. https://doi.org/10.1016/j.gheart.2013.10.001 (2013).
Escobedo, J. et al. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: The CARMELA cross sectional study. Cardiovasc. Diabetol. 8, 52. https://doi.org/10.1186/1475-2840-8-52 (2009).
Fatahi, A., Doosti-Irani, A. & Cheraghi, Z. Prevalence and incidence of metabolic syndrome in Iran: A systematic review and meta-analysis. Int. J. Prev. Med. 11, 64. https://doi.org/10.4103/ijpvm.IJPVM_489_18 (2020).
van der Linden, E. L. et al. The prevalence of metabolic syndrome among Ghanaian migrants and their homeland counterparts: The research on obesity and type 2 diabetes among African Migrants (RODAM) study. Eur. J. Public Health. 29(5), 906–913. https://doi.org/10.1093/eurpub/ckz051 (2019).
Castillo-Sayán, O., Woolcott, O. Obesidad en la altura. Anales de la Facultad de Medicina. 78(2), 186-191 (2017). https://doi.org/10.15381/anales.v78i2.13215
Bustos, P., Amigo, H., Vásquez, A. & Vargas, C. Evolution of the metabolic syndrome and its components in a follow up of 10 years in adults from Valparaíso region. Rev. Med. Chil. 142(5), 579–586. https://doi.org/10.4067/S0034-98872014000500005 (2014).
Pucci, G. et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 120, 34–42. https://doi.org/10.1016/j.phrs.2017.03.008 (2017).
Kautzky-Willer, A., Harreiter, J. & Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 Diabetes Mellitus. Endocr. Rev. 37(3), 278–316. https://doi.org/10.1210/er.2015-1137 (2016).
Hegsted, D. M. & Kritchevsky, D. Diet and serum lipid concentrations: where are we?. Am. J. Clin. Nutr. 65(6), 1893–1896. https://doi.org/10.1093/ajcn/65.6.1893 (1997).
Verma, P., Srivastava, R. K. & Jain, D. Association of lifestyle risk factors with metabolic syndrome components: a cross-sectional study in eastern India. Int. J. Prev. Med. 9, 6. https://doi.org/10.4103/ijpvm.IJPVM_236_17 (2018).
Franco, O. H. et al. Trajectories of entering the metabolic syndrome: The Framingham heart study. Circulation. 120(20), 1943–1950. https://doi.org/10.1161/CIRCULATIONAHA.109.855817 (2009).
Li, R. et al. Prevalence of metabolic syndrome in mainland China: A meta-analysis of published studies. BMC Public Health. 16(1), 296. https://doi.org/10.1186/s12889-016-2870-y (2016).
Lao, X. Q. et al. Dramatic escalation in metabolic syndrome and cardiovascular risk in a Chinese population experiencing rapid economic development. BMC Public Health. 14, 983. https://doi.org/10.1186/1471-2458-14-983 (2014).
Leavitt, M.O. Physical activity guidelines for Americans. Published online 76 (2008).
Bigham, A. et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLOS Genet. 6(9), e1001116. https://doi.org/10.1371/journal.pgen.1001116 (2010).
Calderón, M. et al. Health need assessment in an indigenous high-altitude population living on an island in Lake Titicaca, Perú. Int. J. Equity Health. 18(1), 94. https://doi.org/10.1186/s12939-019-0993-3 (2019).
Hu, X. J., Zhang, X. & Chen, X. P. The disparities of hypertension control rate and risk factors among hypertensive residing in high-altitude and plain in Sichuan province. Sichuan Da Xue Xue Bao Yi Xue Ban. 51(3), 376–382 (2020).
Mabry, R. M., Reeves, M. M., Eakin, E. G. & Owen, N. Gender differences in prevalence of the metabolic syndrome in Gulf Cooperation Council Countries: A systematic review. Diabetic Med. 27(5), 593–597. https://doi.org/10.1111/j.1464-5491.2010.02998.x (2010).
Bizuayehu Wube, T., Mohammed Nuru, M. & Tesfaye, Anbese A. A comparative prevalence of metabolic syndrome among type 2 diabetes mellitus patients in Hawassa university comprehensive specialized hospital using four different diagnostic criteria. Diabetes Metab. Syndr. Obes. 12, 1877–1887. https://doi.org/10.2147/DMSO.S221429 (2019).
Hwang, L. C., Bai, C. H., You, S. L., Sun, C. A. & Chen, C. J. Description and prediction of the development of metabolic syndrome: A longitudinal analysis using a Markov model approach. PLOS ONE. 8(6), e67436. https://doi.org/10.1371/journal.pone.0067436 (2013).
Cameron, A. J., Magliano, D. J., Zimmet, P. Z., Welborn, T. & Shaw, J. E. The metabolic syndrome in Australia: Prevalence using four definitions. Diabetes Res. Clin. Pract. 77(3), 471–478. https://doi.org/10.1016/j.diabres.2007.02.002 (2007).
Ruilope, L. M., Nunes Filho, A. C. B., Nadruz, W., Rodríguez Rosales, F. F. & Verdejo-Paris, J. Obesity and hypertension in Latin America: Current perspectives. Hipertens Riesgo Vasc. 35(2), 70–76. https://doi.org/10.1016/j.hipert.2017.12.004 (2018).
Brazo-Sayavera, J., Aubert, S., Barnes, J. D., González, S. A. & Tremblay, M. S. Gender differences in physical activity and sedentary behavior: Results from over 200,000 Latin-American children and adolescents. PLoS One. 16(8), e0255353. https://doi.org/10.1371/journal.pone.0255353 (2021).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health. 6(10), e1077–e1086. https://doi.org/10.1016/S2214-109X(18)30357-7 (2018).
Després, J. P. et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28(6), 1039–1049. https://doi.org/10.1161/ATVBAHA.107.159228 (2008).
Kishida, K., Funahashi, T., Matsuzawa, Y. & Shimomura, I. Visceral adiposity as a target for the management of the metabolic syndrome. Ann. Med. 44(3), 233–241. https://doi.org/10.3109/07853890.2011.564202 (2012).
Díaz-Gutiérrez, J. et al. Living at higher altitude and incidence of overweight/obesity: Prospective analysis of the SUN cohort. PLoS One. 11(11), e0164483. https://doi.org/10.1371/journal.pone.0164483 (2016).
Bolnick, H. J. et al. Health-care spending attributable to modifiable risk factors in the USA: An economic attribution analysis. Lancet Public Health. 5(10), e525–e535. https://doi.org/10.1016/S2468-2667(20)30203-6 (2020).
Finkelstein, E. A. et al. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 42(6), 563–570. https://doi.org/10.1016/j.amepre.2011.10.026 (2012).
Blanquet, M. et al. Metabolic syndrome and social deprivation: Results of a French observational multicentre survey. Fam. Pract. 33(1), 17–22. https://doi.org/10.1093/fampra/cmv086 (2016).
Wells, J. C. K., Cole, T. J., Bruner, D. & Treleaven, P. Body shape in American and British adults: between-country and inter-ethnic comparisons. Int. J. Obes. (Lond). 32(1), 152–159. https://doi.org/10.1038/sj.ijo.0803685 (2008).
Ninatanta-Ortiz, J. A., Núñez-Zambrano, L. A., García-Flores, S. A. & Romaní, Romaní F. Frecuencia de síndrome metabólico en residentes de una región andina del Perú. Revista Peruana de Medicina Experimental y Salud Publica. 33(4), 640–650 (2016).
Funding
The study was self-financed.
Author information
Authors and Affiliations
Contributions
J.P Z-V and P G-E were responsible for the conceptualization, methodology, investigation, data curation, writing—original draft preparation, and writing—review and editing. MR C-M, A-D CS, EA. H-B, J T-F, A P F, J P-M and VA B-Z were responsible for the methodology, investigation, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zila-Velasque, J.P., Grados-Espinoza, P., Challapa-Mamani, M.R. et al. Prevalence of metabolic syndrome and its components according to altitude levels: a systematic review and meta-analysis. Sci Rep 14, 27581 (2024). https://doi.org/10.1038/s41598-024-77928-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77928-z