Abstract
The aim of this study was to investigate the effect of dental pulp stem cell-derived exosomes (DPSCs-Exos) on the biological behaviour of fibroblasts, particularly on keloid fibroblasts (KFs) and normal skin fibroblasts (NFs), with a view to providing new insights into cellular regenerative medicine. We obtained DPSCs-Exos by ultracentrifugation and co-cultured it with KFs and NFs. We detected its effect on cell proliferation using the CCK-8 assay; cell migration ability by cell scratch and Transwell assays; extracellular matrix synthesis using the hydroxyproline content assay; the expression levels of genes associated with fibrosis by PCR assay; and the expression levels of proteins related to fibrosis in the cells using the Western Blot method. DPSCs-Exos was able to be taken up by fibroblasts after addition to the culture medium and affected the biological behavior of NFs and KFs. DPSCs-Exos promoted the proliferation of NFs, inhibited the migration and extracellular matrix synthesis of KFs. In addition, DPSCs-Exos was able to inhibit the expression of fibrosis-related genes and proteins in KFs. This study highlights the role of DPSCs-Exos in regulating the biological behaviour of fibroblasts, providing new insights for future applications in the field of cell-free regenerative medicine.
Similar content being viewed by others
Introduction
Dental pulp stem cells (DPSCs) were first isolated and characterized by Gronthos et al.1 in 2000 and were found to have the ability to differentiate in multiple directions. With low immunogenicity, low rejection, and a wide range of sources, DPSCs can be obtained from orthodontic teeth or third molars extracted for therapeutic needs, avoiding controversy over ethical and moral issues, and have become a hotspot for tissue engineering research, with a wide range of clinical applications2. However, some uncontrollable risks of stem cell therapies limit their use, such as immune incompatibility, tumorigenicity, and problems associated with the spread of infection3,4.
Exosomes are nanoscale extracellular disc-shaped vesicles (teato-like structure) secreted by cells. Exosomes from different cellular sources contain their specific protein molecules5, e.g., Exosomes secreted by dendritic cells can induce or amplify acquired immunity due to the inclusion of almost all antigen-presenting molecules6; Exosomes secreted by intestinal epithelial cells contain a variety of metabolic enzymes7; Exosomes derived from dental pulp stem cells (DPSCs-Exos) play important roles in immunomodulation, anti-inflammation, angiogenesis, and apoptosis of tumor cells8,9. In comparison to individual drugs or therapeutic approaches, the collective impact of exosomes may offer a more comprehensive and efficacious outcome. Furthermore, exosomes exhibit a diminished immunogenic response and favorable biocompatibility, thereby mitigating the risk of immune rejection and adverse effects during allografting procedures10, and have great development value and broad application prospects in medicine11,12. Furthermore, recent studies indicate that the biological activity of DPSCs-Exos is influenced by donor age. Specifically, exosomes from younger donors demonstrate enhanced potential for promoting cell proliferation and differentiation compared to those from older donors, suggesting that DPSCs-Exos from younger individuals may be more effective in regenerative applications13.
Fibroblasts have long been considered as key cells in maintaining organ structure and extracellular matrix stability, and with increasing scientific research, it is now recognised that fibroblasts play an even more important role in maintaining tissue integrity14. And during the process of skin wound healing, fibroblasts are able to proliferate in large numbers at the wound site, synthesize collagen fibres and matrix, form granulation tissue, and participate in later tissue reconstruction, and secrete specific enzymes to adjust the repair of skin tissues, whereas the recovery of wounds can be significantly facilitated through the regulation of the biological behaviours of fibroblasts15. Keloid is a benign tumour-like proliferation due to abnormal fibroblast proliferation and collagen overdeposition during post-traumatic skin repair16, which is invasive and has the potential for recurrence. As a skin disease characterised by fibrous tissue overgrowth, the activity of fibroblasts plays a key role in its formation, and it has been suggested that fibroblasts are the core effector cells in the development of keloids17,18. In this study, we focus on connective tissue growth factor (CTGF) due to its critical role in fibrosis. CTGF promotes fibroblast proliferation, migration, and extracellular matrix synthesis19, and its expression is significantly elevated in keloid formation. Additionally, we also assess transforming growth factor-β1 (TGF-β1) and transforming growth factor-β2 (TGF-β2), which are important in fibrosis regulation.
In a previous stem cell therapy study20, we co-cultured DPSCs with various types of keloid fibroblasts (including hypertrophic scars, atrophic scars, and keloids) and normal skin fibroblasts (NFs), and found that DPSCs inhibited biological behaviours such as migration of keloid fibroblasts (KFs). In this study, we further explored the effects of DPSCs-Exos on the biological behaviours of fibroblasts, especially in KFs and NFs. By analysing the regulatory role of DPSCs-Exos in key biological processes such as cell proliferation, migration and fibrosis, the present study aims to provide new insights into the field of cell-free regenerative medicine (Fig. 1).
Materials and methods
Isolation and culture of DPSCs
Sampling was performed at the Oral and Maxillofacial Surgery Department of Guiyang Hospital of Stomatology where intact healthy permanent teeth were collected from patients aged 18 to 25 years old that had been extracted due to impacted or orthodontic reasons, and DPSCs were isolated by tissue block culture method. In brief, The pulp was isolated under aseptic conditions and washed with PBS 3–5 times, then the pulp tissue was sheared into tissue fragments of about 1–2 mm3, and then the tissue fragments were slowly picked up with a probe, and affixed to the bottom wall of the culture flask or Petri dishes, maintaining a growth range of about 5 mm between each tissue fragment. And they were maintained in α-MEM medium with 20% fetal bovine serum (FBS) and 1% penicillin–streptomycin, only DPSCs in passages 3–5 were used in the experiments.
Identification of DPSCs
Oil Red O staining
DPSCs were inoculated on a 6-well plate, and when the cells were cultured to cover about 70–80% of the bottom of the plate, change to adipogenesis induction medium for adipogenesis induction. After 2 weeks Use Oil Red O staining to observe the formation of lipid droplets under a microscope and take pictures.
Alizarin red S (ARS) and Alkaline phosphatase (ALP) staining
DPSCs were inoculated in a 6-well plate, and when the cells were cultured until they covered about 90%-100% of the plate bottom, change to an osteogenic induction medium for osteogenic induction. After 7 days, ALP staining was performed, and the staining results were recorded by taking photographs under a microscope. In the same way, after 21 days, ARS staining was performed, and the formation of mineralized nodules was observed under a microscope and photographed.
Flow cytometry
Flow cytometry was used to detect surface markers of MSCs to identify the phenotype of DPSCs, and DPSCs were incubated with anti-human CD29, CD34, CD45, CD90, and CD105 fluorescent antibodies according to the instructions.
Isolation and identification of DPSCs-Exos
Isolation of DPSCs-Exos by ultracentrifugation
When the fusion rate of DPSCs reached 70%-80%, the original medium was discarded and replaced with serum-free α-MEM medium, cultured for 48–72 h. The cell culture supernatant was collected and centrifuged at 2,000 × g and 10,000 × g to remove cells and debris. Exosomes were then isolated by ultracentrifugation at 100,000 × g for 70 min at 4 °C, resuspended in PBS, and stored at -80℃. The concentration of exosomes was determined using the Bicinchoninic Acid (BCA) Assay.
Identification of DPSCs-Exos
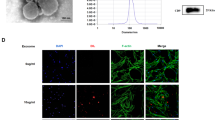
The morphology and size of DPSCs-Exos were observed by transmission electron microscopy (TEM), and the appropriate field of view and magnification were selected for photographing; protein expression of TSG101, CD9, CD63 on the surface of DPSCs-Exos was determined by western blot (WB) technique; and particle size distribution of DPSCs-Exos was determined by nanoparticle tracking analysis technique.
Isolation and culture of KFs and NFs
Sampling was performed at the Guiyang Hospital of Stomatology to collect keloids and normal skin tissues that needed to be excised for treatment, and KFs and NFs were isolated by enzymatic digestion. Briefly, keloid and normal skin tissues were washed 3–5 times with PBS, then cut into tissue fragments and digested with 0.25% collagenase type I for 2 h at 37 °C until there were no visible tissue fragments to the naked eye. After centrifugation at 1000 rpm, resuspend the cell precipitate in culture flasks or petri dishes. Only passages 3–5 KFs or NFs were used. In addition, we used reverse-transcription polymerase chain reaction (RT-PCR) to detect fibroblast specific genes, and primer sequences are listed in Table 1.
CTGF, Connective tissue growth factor; TGF-β1, transforming growth factor-β1; TGF-β2, transforming growth factor-β2; FSP1, Fibroblast-specific protein 1.
DPSCs-Exos uptake assay
DPSCs-Exos were stained according to the instructions of PKH26 dye kit, and PKH26-labeled DPSCs-Exos were collected by ultracentrifugation at 100000xg for 1 h, co-cultured with KFs or NFs for 2 h, and the cells were fixed by adding 4% paraformaldehyde for 20 min, and stained with DAPI, and observed and photo-documented by the help of fluorescence microscope for observation and photo recording.
Cell proliferation assay
KFs or NFs were seeded in 96-well plates at a density of 1 × 103/well, and after 24 h, the medium was changed and DPSCs-Exos (0 μg/ml, 25 μg/ml, 50 μg/ml, 100 μg/ml) were added to continue the culture. After that, the assay was performed using Cell Counting Kit-8 (CCK8) at 1, 2, 3, 4, and 5 days respectively, according to the instructions.
Cell migration assay
Scratch assay
KFs or NFs were inoculated in 6-well plates at a density of 1 × 106/well for 24 h. To minimize the effect of cell proliferation on the assay, cell division was inhibited by treatment with mitomycin-C for 2 h, and the medium was replaced with a serum-free medium. We used a 200ul pipette tip to make a scratch and then PBS rinsing 3 times, the medium was replaced with serum-free medium containing DPSCs-Exos and photographed and recorded at 0 h, 24 h, and 48 h, respectively, and finally, the migratory ability of cells was assessed according to the change of cell migration area.
Transwell assay
To test the three-dimensional migratory capacity of KFs or NFs, we also performed a transwell assay using 8 μm pore transwell chambers. KFs or NFs were inoculated in the upper chamber of Transwell at 1 × 104/well, and add 600 μl of medium containing 10% fetal bovine serum to the Transwell lower chamber, and both the upper and lower chambers contained DPSCs-Exos. After 48 h, the Transwell chambers were removed from the 24-well plates, transferred to methanol for fixation for 20 min, and then stained in rows with 0.1% crystalline aqueous solution for 15–20 min. The non-migrated cells in the upper chamber were wiped off with cotton swabs, washed in PBS 2–3 times, and finally photographed and documented under the microscope.
Hydroxyproline content determination
KFs or NFs were seeded in 96-well plates at a density of 1 × 103/well, and after 48 h, the medium was changed and DPSCs-Exos (0 μg/ml, 25 μg/ml, 50 μg/ml, 100 μg/ml) were added to continue the culture. After 72 h, the test was performed according to the instructions of the hydroxyproline kit.
Real-time quantitative polymerase chain reaction (RT-qPCR)
KFs or NFs were inoculated in 6-well plates at a density of 1 × 106/well, and after 24 h, DPSCs-Exos were added to continue the culture for 48 h. RNA was extracted from each group of cells by the kit method, and the mRNA was reverse transcribed into cDNA according to the instructions of the cDNA Reverse Transcription Kit. RT-qPCR method was used to detect the expression of CTGF, TGF-β1, and TGF-β2. Primer sequences are listed in Table 1.
Western blotting
The pre-treatment of cells was the same as RT-qPCR. After that, the lysate of RIPA protein was added, and the supernatant was taken as total protein by centrifugation 20 min at 4 ℃ at 12000r. The quantitative protein was denatured by heating in a metal bath, then electrophoretic, the sample separated by electrophoresis was transferred to the ECL film for fixation and closed for 1 h (5% skimmed milk). After antibody incubation, the exposure photos were taken and the exposure results were quantitatively analyzed by ImageJ software.
Result
Identification results of DPSCs
Human dental pulp tissue fragments were inoculated and cultured for about 3–5 days (Fig. 2A). The expression of the main marker molecules on the cell surface was detected by flow cytometry, and it was found that the isolated DPSCs expressed the marker molecules of mesenchymal stem cells: the positive expression rate of CD105 was 99.9%, the positive expression rate of CD90 was 99.7%, the positive expression rate of CD29 was 99.9%, but the marker molecules of hematopoietic stem cells were not expressed: the positive expression rate of CD34 was 0.06%, and the positive expression rate of CD45 was 0.004% (Fig. 2E). In addition, DPSCs induced and cultured by osteogenic induction medium showed a blue-purple color by ALP staining (Fig. 2C), and calcium nodules were visible by alizarin red staining (Fig. 2B), which confirmed the osteogenic differentiation of DPSCs; for DPSCs induced and cultured by lipogenic induction medium, lipid droplets formed in the cells were stained orange by oil red O staining, which confirmed the lipogenic differentiation of DPSCs (Fig. 2D). Therefore, the cell identification results indicated that the DPSCs cultured by the tissue block method were stem cells of mesenchymal origin.
Isolation and identification of DPSCs. (A) DPSCs crawling out of the pulp tissue mass were pike-shaped. (B) Results of alizarin red staining with visible calcium nodules. (C) The result of ALP staining shows that the cells are bluish-purple. (D) Oil red O staining results in visible orange lipid droplets (The lipid droplets are indicated by red arrows) and nuclei stained blue by hematoxylin. (E) The phenotype of DPSCs was analyzed by flow cytometry.
Identification of DPSCs-Exos
TEM was used to observe the structure of exosomes, and DPSCs-Exos isolated by ultracentrifugation had a double-layered membrane and a “cup-and-tray”-like structure (Fig. 3B). The average value of particle size of DPSCs-Exos detected by the particle size analyzer was 132.17 nm, which was consistent with the structure of the exosome (Fig. 3A). In addition, WB analysis revealed exosome markers TSG101, CD9, and CD63 (Fig. 3C), consistent with exosomes.
Isolation and identification of fibroblasts
Under the inverted microscope, it can be seen that the primary cells obtained by enzyme digestion started to stick to the wall about 1 d after seeding (Fig. 4A). After adhering to the wall, the morphology transitioned from ovoid to long spindle, and then finally showed typical fibrous and long spindle morphology, which was in line with the morphology of fibroblasts. And there was no significant difference in the morphology of KFs and NFs.
In addition, we used RT-PCR to identify isolated and cultured fibroblasts expressing Vimentin, Collagen-I, and Fibroblast-specific protein 1(FSP1), which proved to be consistent with the characteristics of fibroblasts (Fig. 4B).
Fibroblast uptake of DPSCs-Exos assay
In this study, after co-incubating DPSCs-Exos labelled with PKH26 with NFs, we detected red fluorescence in NFs by fluorescence microscopy (Fig. 5A). Similarly, after co-incubating DPSCs-Exos labelled with PKH26 with KFs, red fluorescence was also detected in KFs by fluorescence microscopy observation (Fig. 5B). This result suggests that DPSCs-Exos can be taken up by KFs and NFs.
DPSCs-Exos promoted the proliferation of NFs
The proliferation profile of fibroblasts was examined using the CCK-8 method. The results were found: For NFs, 25 μg/ml of DPSCs-Exos had no significant effect on NFs during 5 days of culture, while 50 μg/ml and 100 μg/ml of DPSCs-Exos significantly promoted the proliferation of NFs on days 3, 4, and 5 (P < 0.05) (Fig. 6A). For KFs, 25 μg/ml, DPSCs-Exos had no significant effect on KFs during 5 days of culture (Fig. 6B).
DPSCs-Exos reduces the migration rate of KFs
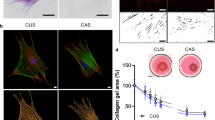
The cell scratch assay can simulate the ability of cells to migrate toward the wound during wound healing under in vitro conditions. The speed of migration of KFs (Fig. 7A) and NFs (Fig. 7B) toward the center from 0 to 24 h and 48 h can be seen in the figure. Furthermore, statistical analysis of the area of KFs and NFs migrating toward the center by image J software revealed that 25 μg/ml, 50 μg/ml and 100 μg/ml of DPSCs-Exos reduced the migration rate of KFs toward the inner delineation line (Fig. 7C); while 25 μg/ml, 50 μg/ml and 100 μg/ml of DPSCs-Exos did not affect the migration rate of NFs were all not significantly affected (Fig. 7D). These data suggest that DPSCs-Exos can reduce the migration of KFs under in vitro conditions and has no significant effect on the migration of NFs.
In addition, we performed a Transwell assay to examine the migration rate of the cells in a three-dimensional manner, and the results showed similar results to those of the cell scratch assay (Fig. 8A), DPSCs-Exos were all able to reduce the migratory effects of KFs in a three-dimensional manner (Fig. 8B), whereas there was no significant effect on the migration effects of NFs (Fig. 8C).
DPSCs-Exos inhibits synthesis of extracellular matrix by KFs
We used a hydroxyproline kit to detect the concentration of hydroxyproline in the cell culture medium of two types of cells, KFs and NFs, after treatment with DPSCs-Exos for 72 h. The final test results showed that 25 μg/ml of DPSCs-Exos did not affect the ability of KFs to synthesize hydroxyproline. In comparison, 50 μg/ml and 100 μg/ml of DPSCs-Exos significantly inhibited the ability of KFs to synthesize hydroxyproline (Fig. 9A). In addition, DPSCs-Exos did not affect the ability of NFs to synthesize hydroxyproline (Fig. 9B).
DPSCs-Exos inhibits the expression of genes related to the pro-fibrotic phenotype of KFs
We employed RT-qPCR to examine the expression of CTGF, TGF-β1, and TGF-β2 in KFs and NFs after culture treatment with DPSCs-Exos for 48 h. We found that DPSCs-Exos significantly inhibited the expression of CTGF, TGF-β1, and TGF-β2 in KFs (Fig. 10A–C). In addition, DPSCs-Exos had no significant effect on the expression of CTGF, TGF-β1, and TGF-β2 in NFs (Fig. 10D–F).
DPSCs-Exos inhibits the expression of proteins related to the pro-fibrotic phenotype of KFs
Western blotting results showed that DPSCs-Exos inhibited the expression of CTGF, TGF-β1 and TGF-β2 in KFs with statistical significance (Fig. 11A–D). In addition, DPSCs-Exos had no significant effect on the expression of CTGF, TGF-β1, and TGF-β2 in NFs (Fig. 11E–H).
(A) Western blotting protein electrophoresis results for each group of KFs. (B–D) Relative expression of proteins associated with the pro-fibrotic phenotype of KFs. (E) Western blotting protein electrophoresis results for each group of NFs. (F–H) Relative expression of proteins associated with the pro-fibrotic phenotype of NFs.
Discussion
Fibroblasts play a key role in tissue repair and their biological behaviour is essential for maintaining a normal healing process. During skin wound repair, fibroblast activity may lead to excessive collagen deposition, which may manifest clinically as scarring after wound healing21. Hydroxyproline is used as a marker of collagen synthesis and changes in its content reflect the level of collagen synthesis. In the experiments measuring hydroxyproline content in this study, we observed that the ability of KFs to secrete hydroxyproline was much higher than that of NFs, and the application of DPSCs-Exos reduced the secretion of hydroxyproline from KFs, implying its potential effect in reducing collagen synthesis, which in turn could be effective in suppressing the growth of the keloid and the possibility of recurrence. In another study, Zhu et al. found that miR-204-5p in exosomes derived from bronchoalveolar lavage fluid was able to reduce hydroxyproline as well as collagen content in rat lung tissues, and miR-204-5p could down-regulate the expression of AP1S2 in primary lung fibroblasts, thereby promoting fibroblast proliferation, a finding that provides a new potential targets22. In addition, abnormal fibroblast activity may lead to scar formation accompanied by discomfort and possible dysfunction, such as joint ankylosis and muscle contractures, which may significantly affect an individual’s quality of life23,24. Therefore, exploring methods that can modulate the biological behaviour of fibroblasts, especially when their activity is abnormal, is important to improve the associated symptoms and prognosis25.
In this study, we chose DPSCs as the source of exosomes, and we found that DPSCs-Exos had a significant effect on biological behaviours such as proliferation and migration of fibroblasts. This may be related to the fact that DPSCs-Exos contain a variety of bioactive components, which include proteins, nucleic acids, lipids, and sugars26,27, and they can affect fibroblast functions through multiple pathways and mechanisms28. As exosomes are important mediators of intercellular communication29, they can facilitate the transfer of information between cells and regulate the biological activities of fibroblasts, including their proliferation, differentiation, and migration, by releasing bioactive substances30. In addition, the study of exosomes is not limited to DPSCs-Exos, and exosomes from other sources have also shown potential applications in the functional regulation of KFs31. For example, Shen et al.'s study pointed out that melanocyte-derived exosomes, which activate the TGF-β/Smads pathway in fibroblasts, promote keloid formation32; another study found that that miR-7846-3p in adipose-derived MSC exosomes was able to inhibit the NRP2 and Hedgehog signalling pathways, thereby reducing the proliferation and angiogenic capacity of fibroblasts33. These findings provide new clues for understanding the potential mechanisms of exosomes in regulating fibroblast function. Thus, exosomes have significant research and application prospects as an emerging cell-free therapy. Future research needs to further explore the bioactive components of exosomes and their specific mechanisms in regulating the function of fibroblasts, in order to develop more effective therapeutic strategies and promote the development of acellular therapy.
The aim of our study was to examine the effect of DPSCs-Exos on the biological behaviour of KFs, due to the fact that keloid is a skin disease characterised by fibroplasia, and the viability of fibroblasts is an important factor in its formation34. In addition, our study focused on the key role of fibroblasts in keloid formation, which is considered by some scholars to be a benign skin tumour and referred to fibroblasts as the key effector cells of keloid formation17, due to the fact that fibroblasts exhibit abnormal proliferative and migratory behaviours during keloid progression, which are typical features of tumour cells34,35, which is similar to the results of the present study, in which we observed that KFs had a stronger proliferative capacity compared to NFs by CCK-8 assay. This finding not only supports the central role of fibroblasts in keloid formation, but also provides a new direction for future therapeutic research. Whereas the present experiments confirmed that DPSCs-Exos could promote the proliferation of NFs, this result may help to accelerate wound healing and improve the repair efficiency in regenerative medicine and tissue engineering. By promoting the proliferation of NFs, DPSCs-Exos may help to maintain the structural and functional integrity of the skin, which is essential for maintaining the normal physiological state of the skin and preventing chronic wound formation. This role of DPSCs-Exos is not only valuable in basic science research, but also provides new perspectives for clinical treatment.
Our findings emphasise the importance of studying KFs under in vitro conditions, as this helps us to better understand the pathomechanisms of keloids and lays the foundation for the development of new therapeutic approaches. Since fibroblasts play a crucial role in the healing process after skin injury36, their migration is part of the normal healing process, but in keloid formation, this migration may be overactive. Whereas, the results of our scratch experiments as well as Transwell experiments showed that DPSCs-Exos could inhibit the migration of KFs, which may help to balance the healing process and prevent excessive keloid formation. In addition, this study confirmed that DPSCs-Exos was able to reduce the expression level of pro-fibrotic genes under in vitro conditions, while TGF-β is a key regulator in the fibrotic process, which promotes the expression of fibrotic genes through the Smad and non-Smad signalling pathways37, and DPSCs-Exos may contain molecules capable of interfering with this signalling pathway, thereby inhibiting fibrotic gene expression. Therefore, the regulatory effect of DPSCs-Exos on pro-fibrotic gene expression in fibroblasts shows its potential application in the treatment of fibrotic diseases, such as scarring, hepatic fibrosis and pulmonary fibrosis.
Our exosome uptake experiments further confirmed the potential of DPSCs-Exos in regulating fibroblast function, and by using PKH26 labelling we observed that fibroblasts were able to uptake DPSCs-Exos, a finding that not only revealed the interaction between DPSCs-Exos and fibroblasts, but also demonstrated DPSCs-Exos’ application value in the treatment of keloids. In addition, the study by Ji et al. further highlighted the advantages of DPSCs-Exos in immunomodulation, which demonstrated more significant immunomodulatory properties compared with bone marrow MSC-derived exosomes, which may be related to the unique biological properties of dental pulp stem cells38. Moreover, dental pulp tissues are more convenient to obtain compared with bone marrow tissues, which makes DPSCs-Exos a more desirable target for research and application, and DPSCs-Exos can be prioritised in future studies on MSC-derived exosomes, which also provides a promising pathway for future scholars to investigate the potential application of DPSCs-Exos in fibroblasts of different tissue types.
These findings provide ideas for our next studies to further explore the molecular mechanisms by which DPSCs-Exos affect the biological behaviour of KFs. In addition, in order to improve the aesthetic and functional outcomes of keloid treatment, new therapeutic strategies are being developed in the research of treatment methods to improve the therapeutic efficacy and reduce the incidence and recurrence of keloids39,40,41. For example, Xiao et al. showed an improvement in keloid atrophy rate by using a modified drug injection technique, which may be due to the improved distribution of the drug within the keloid tissue, thus enhancing the therapeutic efficacy42; meanwhile, Song et al.'s study showed that hyperbaric oxygen therapy was performed on postoperative patients, and it was found that this method was able to significantly reduce keloid recurrence rates43; In addition, advances in laser technology have made laser therapy a viable and effective treatment for keloids44. Nonetheless, the treatment of keloids still faces challenges. For example, Rafael et al. conducted a comprehensive statistical analysis of 15 randomised controlled trials to assess the efficacy of laser treatment of keloids; unfortunately, their findings showed that substantial evidence supporting the efficacy of laser treatment of keloids was significantly insufficient45, suggesting that, despite the fact that laser treatment has been widely used in clinical practice, its efficacy still requires further scientific validation. Since the mechanism of keloid formation is a complex biological process, scholars are committed to understanding the intrinsic mechanisms of keloid formation, which requires the study of the molecular and cellular processes involved in keloid tissue structure, cellular composition and molecular signalling pathways. Recent findings have revealed a range of potential molecular targets that may be critical for the development of new therapeutic strategies. For example, a study by Song et al. identified a set of genes associated with keloid formation, such as ARSA, GBA2, and HEXB, through single-cell sequencing technology and microarray data analysis; changes in the expression of these genes may affect keloid development and may be a new target for future therapy46. In terms of molecular signalling pathways, Chao et al. conducted a study showing that down-regulation of IL-13RA2 in fibroblasts was able to promote the fibrotic process of keloid scars by activating the JAK/STAT6 pathway47. Similarly, Chen et al. showed that keloid formation could be effectively inhibited by targeting the Akt/PI3K/mTOR pathway48. In another study, Satoko et al. observed that the expression of HtrA1 in KFs was significantly higher than that in NFs, and silencing HtrA1 expression significantly inhibited the proliferative capacity of fibroblasts, suggesting that HtrA1 may play a key role in keloid formation49.
In our study, we investigated the effects of DPSCs-Exos on the biological behavior of KFs and NFs. Although we are currently unable to conduct additional experiments to further elucidate specific mechanisms, we hypothesize that DPSCs-Exos may exert their anti-fibrotic effects through multiple pathways. The peroxisome proliferator-activated receptor-γ (PPAR-γ) pathway has been well-documented in the context of fibrosis reversal. For instance, Zhu et al. demonstrated that PPAR-γ agonists inhibit collagen synthesis in keloid fibroblasts by upregulating miR-543, which in turn suppresses early growth response gene 1 (Egr1) expression50. Additionally, Teng et al. highlighted the pivotal role of the PPAR-γ pathway in the transdifferentiation of keloid fibroblasts/myofibroblasts into adipocytes, a process that may contribute to fibrosis reversal51. Recent studies have provided preliminary insights into the potential mechanisms by which DPSCs might regulate fibrosis through PPAR-γ-mediated pathways. For example, Pisciotta et al. conducted co-culture studies under pro-fibrotic conditions, suggesting that DPSCs may modulate fibrosis via PPAR-γ52. Our findings indicate that DPSCs-Exos significantly inhibit the expression of CTGF, TGF-β1, and TGF-β2 in KFs, while having no significant effect on NFs. These effects may be mediated through the PPAR-γ pathway. Although this hypothesis has not been directly tested in our study, future research could explore the specific mechanisms by which DPSCs-Exos influence fibrosis through PPAR-γ signaling.
The main shortcoming of this study is that we only explored the effects of DPSCs-Exos on some biological behaviors of KFs and NFs in vitro, but did not carry out in vivo research and further explore its mechanism. Although the advantages of using animal models for in vivo experiments are obvious, the pathological manifestations of keloids are only seen in human subjects53,54 and, due to the vast physiological and immunological differences between humans and animals55,56, this calls into question the reliability of animal models in clinical studies simulating human keloids57,58,59. In addition, the exact mechanism of keloid formation is still unclear, and its pathological process involves a complex immune response. Existing animal models mostly rely on immunodeficient hosts, which limits the comprehensive study of the role of the immune system. Although new animal models containing human keloid cells and humanized mouse models reconstructed with human immune cells are currently being developed, these models have not yet been able to fully simulate the characteristics of human keloids. Therefore, we chose to focus on in vitro studies in order to lay the foundation for future studies that are closer to human conditions. Another limitation of this study is the exclusion of transforming growth factor-β3 (TGF-β3), which has anti-scar characteristics and could provide significant insights into the effects of DPSCs-Exos on fibroblasts. Future research should investigate the role of TGF-β3 in the context of DPSCs-Exos treatment to provide a more comprehensive understanding of its therapeutic potential. Additionally, investigating the expression levels of TGF-β1, TGF-β2, and TGF-β3 using ELISA before and after the effect on fibroblast cells would offer valuable insights into the mechanisms of DPSCs-Exos. This analysis is proposed as a valuable direction for future studies.
In summary, we can infer that DPSCs-Exos have a significant impact on the biological behavior of KFs. This may be due to the ability of DPSCs-Exos to regulate gene expression, signaling pathways, and immune responses within cells, thereby modulating the proliferation, differentiation, and inflammatory responses of KFs. However, despite the encouraging current research results, we still need to conduct more studies and gain a deeper understanding of the mechanisms by which DPSCs-Exos affect fibroblasts. Further research may contribute to advancing exosome therapy technology and provide more effective treatment options for patients with hypertrophic scars. The innovation of this article lies in the systematic study of the effects of DPSCs-Exos on KFs for the first time and the exploration of its possible molecular mechanisms, offering new insights for the treatment of hypertrophic scars. Nevertheless, this study also has certain limitations, such as a limited sample size and a primary focus on in vitro experiments. Future studies are needed to further verify the efficacy and safety of DPSCs-Exos in animal models and clinical trials. In addition, the optimal dosage, administration method, and treatment duration of DPSCs-Exos in clinical applications also require further study.
Conclusion
In this study, we investigated the role of DPSCs-Exos in regulating the biological behaviours of fibroblasts, and our results showed that DPSCs-Exos could be taken up by both NFs and KFs, and significantly affected the biological behaviours of both types of cells. The results showed that DPSCs-Exos displayed some potential in promoting the proliferation of NFs and exhibited some effects in inhibiting the migration of KFs, extracellular matrix synthesis, and the expression of pro-fibrotic genes and proteins. These results not only provide scientific support for applications in the field of cell-free regenerative medicine, but also offer possible new avenues for the treatment of keloids. Although the exact mechanism of action of DPSCs-Exos in these cellular processes still needs to be further investigated and validated, our study provides a starting point for future work and will hopefully stimulate more research in order to more comprehensively understand the role of DPSCs-Exos in tissue repair and regeneration, and to develop new therapeutic strategies in the future.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G. & Shi, S. Postnatal human dental pulp stem cells (Dpscs) in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 97(25), 13625–13630. https://doi.org/10.1073/pnas.240309797 (2000).
Zayed, M. et al. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 12(1), 302. https://doi.org/10.1186/s13287-021-02240-w (2021).
Liu, C. et al. Dental Pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J. Nanobiotechnol. 20(1), 65. https://doi.org/10.1186/s12951-022-01273-4 (2022).
Huang, C. C. et al. 3D encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 126, 199–210. https://doi.org/10.1016/j.actbio.2021.03.030 (2021).
Zhou, C. et al. Exosomally targeting MicroRNA23a ameliorates microvascular endothelial barrier dysfunction following rickettsial infection. Front. Immunol. 13, 904679. https://doi.org/10.3389/fimmu.2022.904679 (2022).
Besse, B. et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5(4), e1071008. https://doi.org/10.1080/2162402x.2015.1071008 (2016).
Kourembanas, S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Ann. Rev. Physiol. 77, 13–27. https://doi.org/10.1146/annurev-physiol-021014-071641 (2015).
Mai, Z. et al. Translational and clinical applications of dental stem cell-derived exosomes. Front. Genet. 12, 750990. https://doi.org/10.3389/fgene.2021.750990 (2021).
Li, B. et al. Hypoxia alters the proteome profile and enhances the angiogenic potential of dental pulp stem cell-derived exosomes. Biomolecules 12(4), 575. https://doi.org/10.3390/biom12040575 (2022).
He, F. et al. Exosome-mediated delivery of RBP-J decoy oligodeoxynucleotides ameliorates hepatic fibrosis in mice. Theranostics 12(4), 1816–1828. https://doi.org/10.7150/thno.69885 (2022).
Li, Y., Duan, X., Chen, Y., Liu, B. & Chen, G. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. Int. J. Oral Sci. 14(1), 2. https://doi.org/10.1038/s41368-021-00152-2 (2022).
He, C., Zheng, S., Luo, Y. & Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 8(1), 237–255. https://doi.org/10.7150/thno.21945 (2018).
Brunello, G. et al. Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics 14(5), 908. https://doi.org/10.3390/pharmaceutics14050908 (2022).
Di, X. et al. Crosstalk between fibroblasts and immunocytes in fibrosis: From molecular mechanisms to clinical trials. Clin. Transl. Med. 14(1), e1545. https://doi.org/10.1002/ctm2.1545 (2024).
Knoedler, S. et al. Fibroblasts—the cellular choreographers of wound healing. Front. Immunol. 14, 1233800. https://doi.org/10.3389/fimmu.2023.1233800 (2023).
Feng, C. et al. Single-cell RNA sequencing reveals distinct immunology profiles in human keloid. Front. Immunol. 13, 940645. https://doi.org/10.3389/fimmu.2022.940645 (2022).
Xia, Y. et al. Identification of a diagnostic signature and immune cell infiltration characteristics in keloids. Front. Mol. Biosci. 9, 879461. https://doi.org/10.3389/fmolb.2022.879461 (2022).
Su, X., Ma, Y., Wang, Q. & Gao, Y. LncRNA HOXA11-AS aggravates keloid progression by the regulation of HOXA11-AS-miR-205-5p-FOXM1 pathway. J. Surg. Res. 259, 284–295. https://doi.org/10.1016/j.jss.2020.09.035 (2021).
Liu, W. et al. Deciphering key foreign body reaction-related transcription factors and genes through transcriptome analysis. Front. Mol. Biosci. 9, 843391. https://doi.org/10.3389/fmolb.2022.843391 (2022).
Yan, M. et al. Evaluation of the effects of human dental pulp stem cells on the biological phenotype of hypertrophic keloid fibroblasts. Cells 10(7), 1803. https://doi.org/10.3390/cells10071803 (2021).
Lee, S. et al. Contribution of autophagy-Notch1-mediated NLRP3 inflammasome activation to chronic inflammation and fibrosis in keloid fibroblasts. Int. J. Mol. Sci. 21(21), 8050. https://doi.org/10.3390/ijms21218050 (2020).
Zhu, L., Chen, Y., Chen, M. & Wang, W. Mechanism of miR-204-5p in exosomes derived from bronchoalveolar lavage fluid on the progression of pulmonary fibrosis via AP1S2. Ann. Transl. Med. 9(13), 1068. https://doi.org/10.21037/atm-20-8033 (2021).
Delaleu, J. et al. Suppurative keloids: A complication of severe keloid disease. Int. J. Dermatol. 60(11), 1392–1396. https://doi.org/10.1111/ijd.15641 (2021).
Ung, C. Y. et al. Comorbidities of keloid and hypertrophic scars among participants in Uk biobank. JAMA Dermatol. 159(2), 172–181. https://doi.org/10.1001/jamadermatol.2022.5607 (2023).
Xie, J. et al. Single-cell sequencing analysis and weighted co-expression network analysis based on public databases identified that TNC is a novel biomarker for keloid. Front. Immunol. 12, 783907. https://doi.org/10.3389/fimmu.2021.783907 (2021).
Yao, T. et al. Ubiquitinated hepatitis D antigen-loaded microvesicles induce a potent specific cellular immune response to inhibit HDV replication in vivo. Microbiol. Spectrum 9(3), e0102421. https://doi.org/10.1128/Spectrum.01024-21 (2021).
Zhou, X. et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics 10(18), 8197–8210. https://doi.org/10.7150/thno.43968 (2020).
Zhao, W. et al. Exosome derived from mesenchymal stem cells alleviates pathological scars by inhibiting the proliferation, migration and protein expression of fibroblasts via delivering miR-138-5p to target SIRT1. Int. J. Nanomed. 17, 4023–4038. https://doi.org/10.2147/ijn.S377317 (2022).
Chen, S. W. et al. Cancer cell-derived exosomal CircUSP7 Induces CD8(+) T cell dysfunction and Anti-PD1 resistance by regulating the miR-934/SHP2 Axis in NSCLC. Mol. Cancer 20(1), 144. https://doi.org/10.1186/s12943-021-01448-x (2021).
Camões, S. P. et al. 3D-Mscs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J. Adv. Res. 41, 113–128. https://doi.org/10.1016/j.jare.2022.01.013 (2022).
Zhong, Y. et al. Therapeutic role of exosomes and conditioned medium in keloid and hypertrophic scar and possible mechanisms. Front. Physiol. 14, 1247734. https://doi.org/10.3389/fphys.2023.1247734 (2023).
Shen, Z., Shao, J., Sun, J. & Xu, J. Exosomes released by melanocytes modulate fibroblasts to promote keloid formation: A pilot study. J. Zhejiang Univ. Sci. B 23(8), 699–704. https://doi.org/10.1631/jzus.B2200036 (2022).
Wu, D., Liu, X. & Jin, Z. Adipose-derived mesenchymal stem cells-sourced exosomal microRNA-7846-3p suppresses proliferation and pro-angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J. Cosmet. Dermatol. 22(8), 2333–2342. https://doi.org/10.1111/jocd.15721 (2023).
Shan, M. et al. The role of CD28 and CD8(+) T cells in keloid development. Int. J. Mol. Sci. 23(16), 8862. https://doi.org/10.3390/ijms23168862 (2022).
Wang, X., Ma, Y., Gao, Z. & Yang, J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res. Ther. 9(1), 40. https://doi.org/10.1186/s13287-018-0786-4 (2018).
Park, Y. R. et al. NF-ΚB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 67, 183–195. https://doi.org/10.1016/j.actbio.2017.12.006 (2018).
Bertolio, M. S. et al. A novel splice variant of human TGF-Β type II receptor encodes a soluble protein and its Fc-tagged version prevents liver fibrosis in vivo. Front. Cell Dev. Biol. 9, 690397. https://doi.org/10.3389/fcell.2021.690397 (2021).
Ji, L. et al. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 67(4–5), 432–442. https://doi.org/10.1007/s12026-019-09088-6 (2019).
Anggawirya, B. Y., Wardhani, P. H., Indramaya, D. M. & Listiawan, M. Y. Combination of fractional Er: YAG laser, pulsed dye laser, and intralesional triamcinolone with 5-fluorouracil for keloid treatment. J Lasers Med. Sci. 14, e30. https://doi.org/10.34172/jlms.2023.30 (2023).
Ma, Q. Y. et al. Laser combined with radiotherapy for keloid treatment: A novel and efficient comprehensive therapy with a lower recurrence rate. Plast. Reconstr. Surg. 152(6), 1022e-e1029. https://doi.org/10.1097/prs.0000000000010376 (2023).
Lawera, N. G. et al. Keloid intralesional excision reduces recurrence: A meta-analytic study of the available literature on 608 keloids. Plast. Reconstr. Surg. Glob. Open 12(3), e5652. https://doi.org/10.1097/gox.0000000000005652 (2024).
Xiao, H. T., Deng, K., Liu, X. X., Xu, X. W. & Zhang, Y. G. Modified injection technique for improving the treatment of keloids. Chin. Med. J. 133(11), 1378–1379. https://doi.org/10.1097/cm9.0000000000000804 (2020).
Song, K. X. et al. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J. Zhejiang Univ. Sci. B 19(11), 853–862. https://doi.org/10.1631/jzus.B1800132 (2018).
Grove, G. L. et al. Sustained improvement of surgical scar appearance 1 year after early intervention with nonablative fractional laser treatment: A randomized controlled split-wound trial. Br. J. Dermatol. 183(6), 1138–1140. https://doi.org/10.1111/bjd.19400 (2020).
Leszczynski, R., da Silva, C. A., Pinto, A., Kuczynski, U. & da Silva, E. M. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database of Syst. Rev. 9(9), CD011642. https://doi.org/10.1002/14651858.CD011642.pub2 (2022).
Song, B. et al. Revealing the roles of glycosphingolipid metabolism pathway in the development of keloid: A conjoint analysis of single-cell and machine learning. Front. Immunol. 14, 1139775. https://doi.org/10.3389/fimmu.2023.1139775 (2023).
Chao, H., Zheng, L., Hsu, P., He, J., Wu, R., Xu, S. et al. IL-13RA2 downregulation in fibroblasts promotes keloid fibrosis via JAK/STAT6 activation. JCI Insight 8(6). https://doi.org/10.1172/jci.insight.157091 (2023).
Chen, Y. et al. Targeting the Akt/Pi3k/Mtor signaling pathway for complete eradication of keloid disease by sunitinib. Apoptosis Int. J. Progr. Cell Death 27(11–12), 812–824. https://doi.org/10.1007/s10495-022-01744-x (2022).
Yamawaki, S. et al. Htra1 is specifically up-regulated in active keloid lesions and stimulates keloid development. Int. J. Mol. Sci. 19(5), 1275. https://doi.org/10.3390/ijms19051275 (2018).
Zhu, H. Y. et al. Peroxisome proliferator-activated receptor-Γ agonist inhibits collagen synthesis in human keloid fibroblasts by suppression of early growth response-1 expression through upregulation of miR-543 expression. Am. J. Cancer Res. 6(6), 1358–1370 (2016).
Teng, Y. Y. et al. Novel prospects for scarless wound healing: The roles of myofibroblasts and adipocytes. J. Cell. Mol. Med. 26(20), 5113–5121. https://doi.org/10.1111/jcmm.17535 (2022).
Pisciotta, A. et al. Human dental pulp stem cells (Hdpscs) promote the lipofibroblast transition in the early stage of a fibro-inflammatory process. Front. Cell Dev. Biol. 11, 1196023. https://doi.org/10.3389/fcell.2023.1196023 (2023).
Lv, W. et al. Circular RNA circCOL5A1 sponges the MiR-7-5p/Epac1 axis to promote the progression of keloids through regulating Pi3K/Akt signaling pathway. Front. Cell Dev. Biol. 9, 626027. https://doi.org/10.3389/fcell.2021.626027 (2021).
Tuan, T. L. & Nichter, L. S. The molecular basis of keloid and hypertrophic scar formation. Mol. Med. Today 4(1), 19–24. https://doi.org/10.1016/s1357-4310(97)80541-2 (1998).
Perez, R. & Davis, S. C. Relevance of animal models for wound healing. Wounds Compend. Clin. Res. Pract. 20(1), 3–8 (2008).
Ramos, M. L., Gragnani, A. & Ferreira, L. M. Is there an ideal animal model to study hypertrophic scarring?. J. Burn Care Res. 29(2), 363–368. https://doi.org/10.1097/BCR.0b013e3181667557 (2008).
Limandjaja, G. C., Niessen, F. B., Scheper, R. J. & Gibbs, S. The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front. Cell Dev. Biol. 8, 360. https://doi.org/10.3389/fcell.2020.00360 (2020).
Seo, B. F., Lee, J. Y. & Jung, S. N. Models of abnormal scarring. BioMed Res. Int. 2013, 423147. https://doi.org/10.1155/2013/423147 (2013).
Huang, J. et al. Combined analyses of RNA-sequence and Hi-C along with GWAS loci-a novel approach to dissect keloid disorder genetic mechanism. PLoS Genet. 18(6), e1010168. https://doi.org/10.1371/journal.pgen.1010168 (2022).
Acknowledgements
We thank Figdraw for the assistance in creating Figure 1.
Funding
This study was supported by the Guiyang Bureau of Health under the contract number Guiyang Health Technology Contract ([2022] No. 002), and Guizhou Provincial Health Commission provided support through its Science and Technology Fund (gzwkj2022-431). MY was supported by the Merit Scholarship of Hamburg University for International Students (No. 7238065).
Author information
Authors and Affiliations
Contributions
G.Y.C.: collected the tissue specimens required for the experiments and performed the experiments, analyzed the results and wrote the manuscript. L.L.F.: assisted in data analysis and evaluation of the results and edited the manuscript. H.P.Y.: provide specimens and guide experiments. P.C.: guide experiments. M.Y. and H.C.F. designed the study, revised the manuscript, and supervised it throughout the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of Guiyang Stomatological Hospital (Ethics approval number: GYSKLL-KY-20221229-01). All the operational methods in this study were performed in accordance with the relevant guidelines and regulations, and all donors gave their informed consent in writing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, GY., Fu, Ll., Ye, Hp. et al. Effects of exosomes from human dental pulp stem cells on the biological behavior of human fibroblasts. Sci Rep 15, 1134 (2025). https://doi.org/10.1038/s41598-024-78388-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78388-1