Abstract
The management of overactive bladder (OAB) in women encompasses a range of strategies, from behavioral modifications to pharmacotherapy and nerve stimulation techniques. This prospective, randomized, controlled trial evaluates the efficacy of the combination of TTNS and mirabegron in symptom improvement over three months in women diagnosed with OAB. The study was designed as a randomized controlled trial. A total of 40 patients were prospectively randomized into two groups. Twenty patients in the combination group accepted TTNS and mirabegron therapy, and the other 20 patients as control only accepted mirabegron therapy. Primary outcomes were changes from baseline in the lower urinary tract symptoms. The severity of symptoms and quality of life (QoL) were assessed using the validated OAB questionnaire (OAB-q). TTNS reduced the clinical symptoms of OAB, and the difference was statistically significant at all study sites (p < 0.05). Regarding secondary outcomes, the OAB-q symptom bother score was lower in the combination group than in the mirabegron group (p < 0.05). The OAB-q score in the combination group was statistically superior to that in the mirabegron group (p < 0.05). The incidence of complications was not statistically significant between the two groups.The combination of TTNS and mirabegron represents a promising therapeutic strategy for women with overactive bladder, significantly improving symptoms and quality of life with a favorable safety profile. Further research with a larger sample size and long-term follow-up is warranted to confirm these findings and explore the underlying mechanisms of this combination therapy’s efficacy.
Similar content being viewed by others
Introduction
Overactive bladder (OAB) syndrome is defined by the International Continence Society (ICS) as a condition of urinary urgency, usually accompanied by urinary frequency and nocturia, with or without urgency urinary incontinence(UUI), and excluding urinary tract infection1. OAB is a highly prevalent disease, with 12.8% of women in the general population suffering from OAB symptoms, according to the ICS2. The problem becomes increasingly prevalent and severe with age, rising to 15% among those over 40 and 20–30% among women over 653,4. The pathogenesis of OAB is complex, including neurological diseases, increased detrusor excitability, urothelial dysfunction, and unstable urethral sphincter function5. Detrusor overactivity can be detected in some OAB patients by urodynamic examination. When UUI accompanies OAB, it is referred to as OAB-wet, while not accompanied by UUI is referred to as OAB-dry6. It is well known that OAB has a severe negative impact on patients’ quality of life(QOL), daily activity performance, sleep, personal relationships, financial burden, and mental health7,8.
The etiology of OAB is multifactorial, involving complex neural pathways, and its management has been an area of extensive research and clinical interest9. There are many ways to treat OAB; the first-line treatment mainly refers to behavioral therapy. Second-line treatment is recommended mainly with drugs, including antimuscarinic drugs and β3 agonists. Third-line treatments include toxin A, tibial nerve stimulation(TNS), and sacral neuromodulation10. The principle of TNS provides neuromodulation by stimulating the tibial nerves with a surface electrode. It includes both PTNS and TTNS modalities.TTNS is a noninvasive treatment that is recommended by guidelines for some patients who have failed medication and behavioral therapy.
However, searching for more effective and tolerable treatment combinations continues, as monotherapy often only provides satisfactory outcomes for some patients. Among the emerging treatment options, Transcutaneous Tibial Nerve Stimulation (TTNS) and the β3-adrenoceptor agonist Mirabegron have separately shown promise. TTNS, a form of peripheral neuromodulation, involves the non-invasive stimulation of the posterior tibial nerve to modulate bladder activity11. It has been recognized for its efficacy and lack of significant side effects. On the other hand, Mirabegron, a novel therapeutic agent, works by relaxing the detrusor smooth muscle during the storage phase of the bladder fill-void cycle, improving bladder capacity without the antimuscarinic side effects12. Hence, we conducted this prospective, randomized trial to explore new treatment models and assess the effectiveness and safety of the TTNS combined with mirabegron in women with overactive bladder.
Materials and methods
Ethical and institutional approval
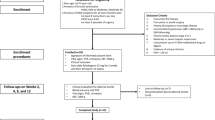
The study is a prospective, controlled, open-label, single-center trial conducted from January to October 2023 at the Department of Urology, the affiliated Jiangning Hospital of Nanjing Medical University. The protocol for this trial has been accepted by the Chinese Clinical Trial Registry (Ethics approval number: ChiCTR2200056752).All patients were informed, and Written informed consent was obtained before the treatment group was allocated.
Randomization
After patients completed informed consent forms, research workers at our center used a computer-generated random number coding table to allocate eligible patients. The sealed envelope held the random assignment.
Inclusion and exclusion criteria
Inclusion criteria: 1. Women 18 years old with a clinical diagnosis of overactive bladder; 2. Able to understand the procedures, advantages, and possible side effects; 3. Able to provide written and informed consent and have the ability to complete urination diaries and quality of life questionnaires.
Exclusion criteria: 1. Active UTI; 2. History of PTNS/TTNS/SNS; 3. History of intravesical botox treatment; 4. Women with stress urinary incontinence; 5. Post-void residual > 100 ml; 6. Pregnancy or intention to become pregnant during the study; 7. Previous urology surgery within three months; 8. Cardiac pacemaker or implanted defibrillator; 9. Peripheral vascular disease or leg ulcer/skin condition affecting lower legs; 10. Absent sensation at the electrode site.
Participants
A total of 40 women met the above criteria and agreed to participate in the study (Fig. 1). They were randomized in a 1:1 ratio to a 3-month maintenance program. Patients allocated to the mirabegron group received 50 mg of mirabegron once daily. Those allocated to the combination group received 50 mg of mirabegron once daily and TTNS twice a week for a total of 3 months. Prior to initiating treatment, patient’s demographic data, medical history, personal habits (smoking, alcohol, tea), and education were collected.
Procedures
In the combination group, patients were treated with 50 mg of mirabegron once daily and TTNS twice weekly for three months. Patients were treated via the neurostimulator device (UroCure, Shanghai).Before treatment, we will provide a detailed description of the procedure. We used two surface round self-adhesive electrodes of approximately 3 cm in diameter for electrical stimulation of the tibial nerve. The correct position was determined by observing the bunion response (plantarflexion of the big toe or flapping of all toes), with one electrode placed 2 cm behind the medial malleolus and the other electrode approximately 10 cm above (Fig. 2). Our stimulation protocol was a frequency of 10 Hz, a pulse duration of 200 µs, and a stimulation duration of 30 min, while the intensity of the stimulation current was determined according to the patient’s tolerance level (range 0–50 mA). The same physician performed the TTNS sessions twice a week over three months. During treatment, participants were asked to complete a 3-day voiding diary once a week, and all women were advised to continue taking medications unrelated to OAB symptoms. Our staff assessed their OABSS and QOL index weekly.
In the mirabegron group, patients received mirabegron with 50 mg once daily for three months. A voiding diary is mandatory, including the amount of fluids ingested and the amount of urine excreted, as well as urges, incontinence, frequency of voiding, and intensity of incontinence.
Evaluation parameters
Three-day bladder diary
Frequency of voiding, incontinence episodes, nocturia, and number of pads used were collected from the 3-day bladder diary.
Health-related quality of life (HRQoL)
This is a 33-item, validated, condition-specific questionnaire showing sound psychometric properties.
Symptom severity: overactive bladder questionnaire (OAB-V8)
The OAB-V8 was used to assess symptom severity in women, and the questionnaire measures the degree of bothersomeness of four symptoms associated with OAB: urinary frequency, urgency, nocturia, and urge incontinence, with a total score between 0 and 4013.
Data collection
The primary outcomes include frequency of urination, frequency of urgency episodes, and frequency of UUI episodes, which participants completed before each study visit. The secondary outcomes were the OAB-q HRQol score and symptom severity, which participants completed during their study visits. Informed consent was obtained from all individuals participating in the study.
Statistical analysis
SPSS v.22.0 for Windows (IBM Corp., Armonk, NY, USA) was used for statistical analysis. A sample size of 11 subjects per group would be required to achieve a 0.05 level of significance and 80% statistical power to detect an increase in the number of urinations by 3 in a 24-hour period, which would constitute a clinically significant worsening of symptoms during maintenance therapy. This significant worsening of OAB symptoms may occur within six weeks of stopping treatment. Therefore, a minimum of 12 participants were recruited in each group to accommodate the 10% dropout rate.
Demographic characteristics, duration of follow-up, and clinical outcomes were compared between the two groups using the independent samples t-test; other clinical characteristics were compared between the two groups using the chi-square test. p < 0.05 was considered a significant difference.
Compliance with ethical standards
All procedures performed in studies involving human participants were by the ethical standards of Affiliated Jiangning Hospital with Nanjing Medical University and with the 1964 Helsinki Declaration.
Results
In our study, 40 patients were randomly assigned to two groups: 20 in the mirabegron group and 20 in the combination group. The patients’ demographics and baseline clinical characteristics of participants are shown in Table 1. There was no significant difference between the two groups in age, BMI, duration of symptoms, hypertension, diabetes history, education, and dietary habits(smoking, drinking, cups of tea)(All P > 0.05). Further studies based on the type of OAB still showed no significant differences between the two groups. In addition, no apparent differences between groups were found for delivery and delivery type.
Tables 2 and 3 showed summary statistics for the bladder diary variables at each of the study points for both treatment groups. At baseline, there were no significant differences between the groups in voiding frequency, incontinence episodes, nocturia, symptom severity, and QoL parameters (P > 0.05). All clinical symptoms, including urgency, frequent urination, and incontinence episodes, were significantly lower in the sixth week and the third month after combination treatment than those in the mirabegron group (P < 0.05).
In addition, the symptom severity and QoL parameters were significantly improved in the combination group as compared to the mirabegron group (P < 0.05). In terms of drug-related adverse events, there was no statistical difference between the two groups (P > 0.05). No other serious complications were found in this study.
Discussion
This study aimed to evaluate the efficacy and safety of combining TTNS with mirabegron therapy in the treatment of women with OAB. Compared to mirabegron monotherapy, our findings indicate that combination therapy significantly enhances urinary symptoms, including urgency, frequency, and nocturia. Additionally, the safety profile of the combination therapy was comparable to that of mirabegron alone, with no significant increase in adverse events.
The tibial nerve is a mixed nerve (L4-S3) from the same spinal cord segment as the nerves innervating the bladder and pelvic floor14. Stimulation of the tibial nerve blocks the transmission of abnormal sensations to the spinal cord and brain, affecting and regulating the behavior of sacral innervated organs such as the bladder, urethral sphincter, and pelvic floor. In the late 1990s, Dr. Marschall Stoller proposed tibial nerve stimulation, initially known as the SANS (Stoller Afferent Nerve Stimulator) protocol, promising to treat non-neurogenic lower urinary tract dysfunction15. The terminology now used is percutaneous or transcutaneous tibial nerve stimulation(TTNS), depending on the electrodes used. The use of self-adhesive surface electrodes during treatment is called transcutaneous tibial nerve stimulation (TTNS).
Bladder smooth muscle activity is primarily influenced by neurotransmitters16, and β3 adrenoceptor agonists17 have appeared as an emerging drug commonly used to treat incontinence, urgency, and frequency of urination caused by OAB. A study found that the effect of mirabegron on OAB symptoms is similar to that of solifenacin monotherapy, and it does not increase the risk of adverse events18.
The superior efficacy of the combination therapy could be attributed to the synergistic effects of TTNS and mirabegron. TTNS, through its neuromodulatory mechanism, potentially enhances the therapeutic effects of mirabegron on the detrusor muscle, leading to improved bladder capacity and control. This synergism aligns with the hypothesis that targeting multiple pathophysiological pathways in OAB can yield better outcomes than monotherapy. Our study showed significant improvement in all bladder diary parameters in the combination group in the sixth week and the third month. These results are consistent with previous studies suggesting the benefits of multimodal treatment approaches in managing complex conditions like OAB. Maida Zonić-Imamović et al.19 demonstrated the effect of a single application of TTNS in patients with an idiopathic overactive bladder. There was a statistically significant improvement in all clinical symptoms, which positively affected QOL.In addition, De Seze et al. conducted a multicenter study in France20, where 83.3% of patients showed statistically significant improvement in clinical symptoms and quality of life after TTNS treatment. Vecchioli et al.21,22 investigated the efficacy and durability of Solifenacin (SS) with PTNS with combination therapy (PTNS + SS) for the treatment of OAB. They found that PTNS + SS was more effective than SS and PTNS separately. Booth et al.23 designed a randomized controlled trial to evaluate the efficacy of transcutaneous tibial nerve stimulation (TTNS) for treating adults with an OAB.There were significant improvements in urgency, frequency of urination, and incontinence episodes. Our study’s findings are reassuring regarding safety, indicating that adding TTNS to mirabegron therapy does not exacerbate the risk of adverse events. The non-invasive nature of TTNS, coupled with the well-established safety profile of mirabegron, supports the clinical feasibility of this combination therapy as a therapeutic option for women with OAB.
The synergistic action of TTNS and mirabegron likely provides a comprehensive approach to modulating bladder activity both directly and indirectly. While TTNS targets the neural control of bladder function, mirabegron’s role in relaxing the detrusor muscle addresses the storage symptoms of OAB, thereby offering a dual mechanism of action. While our study provides valuable insights, it is not without limitations. The short follow-up period may have affected the results. In addition, the study was based on a single center with a small sample size, which may have some sampling error. Moreover, we only counted relevant symptoms by patients’ main complaints, which may have subjective bias. Future research should focus on the long-term outcomes of combination therapy, its cost-effectiveness, and its applicability to broader patient populations, including men with OAB and individuals with different OAB severities.
Conclusion
Combination therapy of TTNS and mirabegron demonstrated significant improvements over mirabegron monotherapy in reducing OAB symptoms and providing a higher quality of life without increasing adverse effects. This method is safe and reproducible in clinical practice. In future studies, patients could be professionally trained and allowed to treat themselves at home.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the nature of this research participants of this study did not agree for their data to be shared publicly, so supporting data is not available,but are available from the corresponding author on reasonable request.
References
Jiang, Y. H. & Kuo, H. C. Current optimal pharmacologic therapies for overactive bladder. Expert Opin. Pharmacother 24(18), 2005–2019 (2023).
Nambiar, A. K. et al. European Association of Urology Guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur. Urol. 82(1), 49–59 (2022).
Carpenter, L. & Campain, N. J. Overactive bladder: Not just a normal part of getting older. Br. J. Nurs. 31(18), S16–S22 (2022).
Dengler, K. L. et al. Overactive Bladder and Cognitive Impairment: The American Urogynecologic Society and Pelvic Floor Disorders Research Foundation State-of-the-Science Conference Summary Report. Urogynecology (Phila) 29(1S Suppl 1):S1–S19 (2023).
Dobberfuhl, A. D. Pathophysiology, assessment, and treatment of overactive bladder symptoms in patients with interstitial cystitis/bladder pain syndrome. Neurourol. Urodyn. 41(8), 1958–1966 (2022).
Staskin, D. et al. Vibegron for the treatment of patients with dry and wet overactive bladder: A subgroup analysis from the EMPOWUR Trial. Int. J. Clin. Pract. 2022, 6475014 (2022).
Tang, D. H. et al. Impact of urinary incontinence on healthcare resource utilization, health-related quality of life and productivity in patients with overactive bladder. BJU Int. 113(3), 484–491 (2014).
Gacci, M. et al. European Association of Urology Guidelines on male urinary incontinence. Eur. Urol. 82(4), 387–398 (2022).
Denisenko, A. A., Clark, C. B., D’Amico, M. & Murphy, A. M. Evaluation and management of female urinary incontinence. Can. J. Urol. 28(S2), 27–32 (2021).
Farag, F. et al. What are the short-term benefits and potential Harms of Therapeutic modalities for the management of overactive bladder syndrome in women? A review of evidence under the auspices of the European Association of Urology, female non-neurogenic lower urinary tract symptoms guidelines panel. Eur. Urol. 84(3), 302–312 (2023).
Sayner, A. M. et al. Transcutaneous Tibial nerve stimulation in the management of overactive bladder: A scoping review. Neuromodulation 25(8), 1086–1096 (2022).
O’Kane, M., Robinson, D., Cardozo, L., Wagg, A. & Abrams, P. Mirabegron in the management of overactive bladder syndrome. Int. J. Womens Health 14, 1337–1350 (2022).
Malde, S., Kelly, S., Saad, S. & Sahai, A. Case-finding tools for the diagnosis of OAB in women: A narrative review. Neurourol. Urodyn. 39(1), 13–24 (2020).
Oliveira, M. C. et al. Evaluation of satisfaction of pelvic floor muscle training isolated and associated with tibial nerve stimulation in women with mixed urinary incontinence: A randomized, single-blinded clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 265, 60–65 (2021).
Schneider, M. P. et al. Tibial nerve stimulation for treating neurogenic lower urinary tract dysfunction: A systematic review. Eur. Urol. 68(5), 859–867 (2015).
Malysz, J. & Petkov, G. V. Urinary bladder smooth muscle ion channels: Expression, function, and regulation in health and disease. Am. J. Physiol. Ren. Physiol. 319(2), F257–F283 (2020).
Funada, S. et al. Bladder training for treating overactive bladder in adults. Cochrane Database Syst. Rev. 10(10), CD013571 (2023).
Chapple, C. R. et al. Safety and Efficacy of Mirabegron: Analysis of a large Integrated Clinical Trial Database of patients with overactive bladder receiving Mirabegron, Antimuscarinics, or Placebo. Eur. Urol. 77(1), 119–128 (2020).
Zonić-Imamović, M. et al. Effects of transcutaneous and percutaneous tibial nerve stimulation in Bosnian female patients with an idiopathic overactive urinary bladder. Acta Med. Acad. 50(2), 235–243 (2021).
de Sèze, M. et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: Results of a multicenter prospective study. Neurourol. Urodyn. 30(3), 306–311 (2011).
Vecchioli-Scaldazza, C., Morosetti, C., Berouz, A., Giannubilo, W. & Ferrara, V. Solifenacin succinate versus percutaneous tibial nerve stimulation in women with overactive bladder syndrome: Results of a randomized controlled crossover study. Gynecol. Obstet. Invest. 75(4), 230–234 (2013).
Vecchioli-Scaldazza, C. & Morosetti, C. Effectiveness and durability of solifenacin versus percutaneous tibial nerve stimulation versus their combination for the treatment of women with overactive bladder syndrome: a randomized controlled study with a follow-up of ten months. Int. Braz J. Urol. 44 (1), 102–108 (2018).
Booth, J., Connelly, L., Dickson, S., Duncan, F. & Lawrence, M. The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: A systematic review. Neurourol. Urodyn. 37 (2), 528–541 (2018).
Funding
This work was supported by the key project of scientific research development fund of Kangda College of Nanjing Medical University in China (no. KD2022KYJJZD077).
Author information
Authors and Affiliations
Contributions
Y.L.: Project development. Y.X. and Y.X.: Data collection. H.T., Y.Y. and R.T.: Data analysis and manuscript writing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The studies involving humans were approved by the clinical research ethics committee of the Affiliated Jiangning Hospital of the Nanjing Medical University.The Chinese Clinical Trial Registry has accepted the protocol (ethics approval number: ChiCTR2200056752). The studies were conducted in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, YX., Xiong, Y., Tian, Hq. et al. Efficacy of the combination of transcutaneous tibial nerve stimulation and mirabegron in women with overactive bladder in a prospective randomized controlled trial. Sci Rep 14, 27248 (2024). https://doi.org/10.1038/s41598-024-78492-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78492-2