Abstract
Weekly Steroids in Muscular Dystrophy (WSiMD) was a pilot study to evaluate once weekly prednisone in patients with Limb Girdle and Becker muscular dystrophy (LGMD and BMD, respectively). At study endpoint, there were trends towards increased lean mass, reduced fat mass, reduced creatine kinase and improved motor function. The investigation was motivated by studies in mouse muscular dystrophy models in which once weekly glucocorticoid exposure enhanced muscle strength and reduced fibrosis. WSiMD participants provided blood samples for aptamer serum profiling at baseline and after 6 months of weekly steroids. A subset completed magnetic resonance (MR) evaluation of muscle at study onset and endpoint. At baseline compared to age and sex-matched healthy controls, the aggregate serum protein profile in the WSiMD cohort was dominated by muscle proteins, reflecting leak of muscle proteins into serum. Disease status produced more proteins differentially present in serum compared to steroid-treatment effect. Nonetheless, a response to prednisone was discernable in the WSiMD cohort, even at this low dose. Glucocorticoids decreased muscle proteins and increased certain immune process- and matrix-associated proteins. Muscle MR fat fraction showed trends with functional status. The prednisone-responsive markers could be used in larger trial of prednisone efficacy.
Similar content being viewed by others
Introduction
Glucocorticoid steroids are used to treat Duchenne Muscular Dystrophy, where their use has been shown to prolong ambulatory duration and to correlate with improved cardiac and respiratory muscle function1,2. The most commonly used glucocorticoid agents in DMD are prednisone and deflazacort, typically across a range of dosing regimens including once daily, high dose weekend, and “ten day on/off” strategies3. With DMD patients living longer, in part due to steroid use, non-invasive respiratory support, and supportive cardiac care, many DMD patients are now receiving glucocorticoids for two or more decades. Prolonged use of glucocorticoids in DMD associates with adverse outcomes like delayed puberty with altered testosterone levels, adrenal suppression, obesity, osteoporosis, cataracts and metabolic syndrome4,5. Among the more troubling side effects of steroid use in DMD are obesity and behavioral issues, which prompted studies of alternative dosing strategies or led to cessation of steroid use in some patients. High-dose steroid given twice weekly on weekends has been found to have benefit in DMD6.
In an effort to identify glucocorticoid dosing that provided benefit with fewer side effects, we evaluated the use of once weekly steroids in mouse models of DMD and LGMD7,8. We identified that once weekly steroids improved muscle strength and reduced fibrosis in the mdx model of DMD and in mouse models of LGMD due to sarcoglycan or dysferlin mutations, Sgcg and Dysf, respectively7,8. In the mdx model, where the molecular consequences of this intermittent steroid dosing were detailed, once weekly steroids “reprogrammed” muscle, seen as altered gene expression and epigenetic marks9. Notably, the KLF15 and MEF2C pathways were specifically increased by once weekly steroids, and the gene expression cascades driven by the transcriptional regulators KLF15 and MEF2C shift nutrient uptake into muscle7,9. Once weekly steroids also reduced inflammatory pathway genes and resulted in an ability to recover better from muscle injury. We found that the effect of glucocorticoid dosing was dependent on time of day dosing10. Mice with genetically disrupted circadian rhythm no longer benefitted from once weekly dosing10.
To evaluate whether this same steroid dosing strategy in human muscular dystrophy (MD), we conducted a pilot, open label study in 19 LGMD patients and one BMD patient. The Weekly Steroids in Muscular Dystrophy (WSiMD) study recruited participants for once weekly glucocorticoid at 0.75–1.0 mg/kg/week, depending on body mass, taken once weekly in the evening for 24 weeks11. Participants included a range of patients with LGMD subtypes and disease progression, including approximately one-third ambulatory, one-third nonambulatory and one-third transitioning to nonambulatory status. All patients were adults, age 18–60, and males and females were included. Overall, in this pilot study, we observed that once weekly steroid dosing was well tolerated with few side effects over six months. Participants showed trends towards improved lean body mass, reduced CK, and greater distance walked in 6 min11.
Serum profiling and magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) in DMD patients provide important biomarker correlates of disease trajectories and treatment effects12,13,14,15,16. The goal of this study was to identify clinically relevant biomarkers associated with the WSiMD cohort as an aggregate group, and to evaluate whether we could discern a biomarker signature of intermittent prednisone treatment at this comparatively low dose of glucocorticoid exposure. Past aptamer profiling studies in MD have focused on DMD, including steroid responsiveness, and because these were DMD studies, they included children and males12,17. We evaluated the serum aptamer profiles in the WSiMD cohort and compared to these prior studies to assess MD markers as a whole and to identify potentially useful steroid responsive biomarkers.
Methods
Ethical declaration
As previously noted, the WSiMD study was (NCT04054375, Date of Registration 13/08/2019 (Aug 13, 2019)) was approved by the Institutional Review Board at Northwestern University Feinberg School of Medicine (STU00208443)11. All participants provided written informed consent (n = 20) following a discussion of study goals, design, risks, and benefits preceding enrollment in compliance with the ethical standards of the Helsinki declaration of 1975.
Inclusion and exclusion criteria
All WSiMD participants (n = 20) were recruited from the Northwestern University Muscular Dystrophy Association clinic between June 2019 and January 2020. WSiMD participants were between 18 and 60 years (mean age of 35), electrocardiogram within 2 months prior to enrollment with no evidence of atrial fibrillation or recent myocardial infarction, genetic testing confirming BMD or LGMD status, echocardiogram showing left ventricular ejection fraction greater than 25%, and no changes to medication within three months of enrollment. Exclusion criteria were a history of oral steroid use in the preceding three years for longer than one month, history of diabetes mellitus, BMI greater than 35 kg/m2, uncontrolled hypertension, congestive heart failure, chronic kidney disease, full time ventilator dependency, orthopedic surgery within past 6 months, current pregnancy, and any history of tuberculosis.
Study design
Participants completed a 24-week, open-label trial. At week 0, baseline measurements included heart rate, blood pressure, weight, height, forced vital capacity, and muscle imaging. These same measures were repeated at study endpoint at 24 weeks. Whole blood samples were collected at week 0 and week 24. The Vignos and Brooke scales were used for lower and upper extremity measurements, and the NorthStar Ambulatory assessment for Dysferlinopathy (NSAD) was used. Each participant was tested for grip strength. Six-minute walk test and 10-m run test data were collected on ambulatory patients. All data was collected on site by study coordinators and therapists. Study participants received monthly phone calls at weeks 4, 8, 12, 16, and 20 from study coordinators confirming medication adherence, dosage, and any adverse events. DEXA studies were completed at week 0 and study endpoint for most participants. Completion of all study data points was limited for some participants due to the COVID-19 pandemic in March 2020.
Magnetic resonance imaging
Participants (n = 13) completed upper and lower extremity imaging according to previously established methods at study onset and endpoint. Quadriceps and Triceps surae muscles were analyzed for fat fraction18,19,20,21. Muscle fat fraction (FF) was assessed using both an imaging method (chemical shift encoded or Dixon MRI) and the gold standard of volume-localized magnetic resonance spectroscopy (MRS). Fat fraction images were calculated using a muscle-based multi-peak analysis22 from a 12-point Dixon dataset, which combined three multi-slice, multi-echo scans (TR = 430 ms, 18 four mm slices, 20 degree flip angle), each obtained with four unique echo times [TEs] with overall TEs of 2.4, 3.6, 4.8, 5.9, 8.4, 9.4, 9.6, 13.0, 13.2, 14.3, 17. 19.1 ms. Single voxel 1H-MRS muscle FF was derived from Vastus lateralis (VL) and Soleus (Sol) muscles respectively in both the lower leg and thigh using Stimulated Echo Acquisition Mode (STEAM;TR = 9,000 ms, TM = 16 ms, BW = 2 kHz, complex data points = 2048) without water suppression and with multiple echo times (TE = 11, 27, 54, and 243 ms) to measure water T2. 1H MRS spectra were corrected for T2 relaxation as previously described, and the water and lipid regions were integrated to determine muscle FF23. In cases where disease progression eliminated any visible VL and Sol muscle, an alternative upper and/or lower limb muscle was selected. Cross-validation of the imaging sequence and system quality control were conducted at each imaging session using a co-axial phantom19 and by co-registering MRI and MRS-determined FF22.
To quantify FF based on Dixon imaging, custom-written IDL software was used to generate pixel by pixel FF maps from water and fat images of both LL and thigh. Regions of interest (ROIs) were manually drawn on the FF maps for the four muscles combining to form quadriceps (VL, Vastus intermedius (VI), Vastus medius (VM), Rectus femoris (RF)) and three lower leg muscles combining to form Triceps surae (Sol, medial gastrocnemius (MG), lateral gastrocnemius (LG)). Each muscle was analyzed on the same three consecutive slices based on pre-determined landmarks. The mean FF for each slice was determined for all pixels within ROI using the following formulae:
Muscle FF for the four thigh and the three lower limb muscles were averaged to get average fat fraction for quadriceps and Triceps surae muscle groups.
Serum aptamer profiling and analysis
Sera was collected from WSiMD participants at study onset and after 24 weeks of once weekly prednisone. Age/sex matched healthy individuals without steroid exposure were used for controls. Sera was analyzed using SomaScan aptamer profiling. The SomaScan platform utilizes modified DNA aptamers to detect 6411 unique proteins from small volumes of serum. A subset of proteins are detected by more than one aptamers resulting in 7322 aptamers in total. Analysis was conducted by importing data into R using the SomaDataIO R package, and subsequent analysis was performed in R Studio. Serum biomarkers associating with disease and prednisone treatment were evaluated using a linear mixed model from the variancePartition R package24, with a model of y ~ Disease + Prednisone + (1|Subject), with a random effect accounting for basal-level differences across participants. Disease and prednisone-responsive terms were extracted using the TopTable function, and adjusted p value and logFC thresholds were used as described in the results. Pathway analysis was performed using the ClusterProfiler package on significantly increased or decreased terms. Lists of significantly different terms between disease and treatment were compared to determine the set of disease-associated treatment-responsive biomarkers.
Spearman correlation was used to assess the relationships between age, muscle functional test outcomes, fat fraction and water T2 MRI/MRs measures, and DEXA-determined body composition. Linear regression analysis was used to assess the relationship between individual biomarkers and outcomes. To reduce multiple hypothesis burden, we used the group biomarkers associated with MD (p adj ≤ 0.05) to assess biomarker level against functional assessments and MRI outcomes. Similarly, we used the paired change in GC responsive markers (p adj ≤ 0.05) against change in outcomes to assess the relationship of pharmacodynamic markers with individual patient outcomes. Patients with missing test values were omitted from the analysis. For each analysis, coefficients and p-values were extracted and FDR hypothesis correction was applied.
Results
Proteins differentially present in serum from adults with MD.
The WSiMD cohort was previously described11, and included 19 individuals with LGMD and one adult with BMD. Of participants with LGMD, 9 participants had subtype 2A (CAPN3), 3 participants had subtype 2B (DYSF), 2 participants had subtype 2I (FKRP), 1 participant had type 2J (TTN), and 2 participants had type 2L (ANO5). The participant with BMD had an exon 45–47 deletion in DMD. The ages of participants ranged between 18 and 60, with a mean age of 35. Thirteen participants were male, and 7 were female. Six participants were non-ambulatory, 9 were ambulatory, and 5 were transitioning from ambulatory to non-ambulatory status. The control group was age- and sex-matched (Table 1), and was not exposed to steroid use, and therefore enabled baseline comparisons between the WSiMD cohort and individuals without muscular dystrophy.
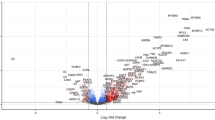
We used an aptamer-based method to measure 6411 proteins in the serum of WSiMD participants. We analyzed MD-responsive biomarkers using a linear mixed effects model by comparing aptamer profiles from baseline WSiMD participants with age- and sex-matched healthy controls. This comparison identified 507 proteins with an adjusted p value < 0.05 and an absolute log fold change > 0.5. Of these, 109 (22%) were increased and 398 (78%) were decreased in WSiMD participants at baseline compared to healthy controls (Fig. 1A, Supplemental Table 1). These proteins increased in the serum were mainly structural sarcomeric proteins, markers involved in skeletal muscle function and differentiation, and metabolic proteins, consistent with leak of muscle proteins into the serum and reflecting ongoing muscle breakdown that is common across many MD subtypes (Fig. 1B). The sarcomeric proteins ACTN2, MYL3, TNNI2, MYOM2, MYOM3, TNNT3, TTN, TPM3, PDLIM3, PDLIM5, ANKRD2, and TNNT2 were the most increased family of biomarkers, by representation and degree of upregulation. The clinically measured protein CKM, along with CA3, were also in the top 20 proteins most highly associated with disease. The measurement of CK correlated well with what was measured clinically (Supplemental Fig. 1). Constant muscle turnover and regeneration is a hallmark of muscular dystrophies, and consistent with this, many proteins were increased that are implicated in muscle differentiation and repair including KLHL41, CACNB4, CFL2, and TRIM72. Additional increased serum proteins included long chain fatty acid binding proteins FABP3 and FABP4 and these markers have been previously associated with muscular dystrophy12,17,25,26,27.
MD Associated Biomarkers. (A) Volcano plot of aptamer profiling from WSiMD participants compared to age/sex matched controls compared using a linear mixed effects model. Benjamini–Hochberg corrected p-value < 0.05 and abs(log2FC) > 0.5 were used as thresholds for significance. (B) Gene Ontology terms were used to cluster the increased (upper panel) and decreased (lower panel) groupings of protein biomarkers. Increased proteins were largely proteins important for muscle structure, function, and development. Decreased proteins included those implicated in cellular adhesion, membrane fusion, actin organization, growth factor signaling and coagulation.
Proteins decreased in the serum of the WSiMD cohort were those implicated in cellular adhesion, membrane fusion, actin organization, growth factor signaling and coagulation (Fig. 1B). The membrane fusion proteins STX4, and STX8 and the vesicle trafficking/fusion protein VTI1B were significantly decreased. RHOQ and EZR, which are both involved in the organization and stabilization of the actin cytoskeleton, were decreased, as were the Src family kinases downstream of Fc-receptor signaling like LYN, FGR, and FYN. VAV1 and VAV3, both involved in regulation of intracellular calcium levels were reduced in serum from WSiMD participants compared to healthy controls. The platform lacks an aptamer for VAV2, whose catalytic efficiency has been positively associated with muscle mass28. ANXA1, a membrane stabilizing and immunosuppressive biomarker, was also reduced in WSiMD participants compared to healthy controls.
Prednisone-responsive biomarkers in the WSiMD cohort.
Figure 2 shows a hierarchical clustered heatmap assessing both disease and treatment status across both the WSiMD and control cohorts. When compared in this manner, differences associated with overall MD status were more evident than the effect of steroid treatment. No clear patterns were evident based on LGMD subtype (Supplemental Fig. 2), likely limited by small sample size, and genotype and functional status heterogeneity. To assess the effect of 24 weeks of intermittent prednisone treatment, we evaluated the baseline and study endpoint serum aptamer profiles in the WSiMD participants. We found that glucocorticoid dosing significantly increased 24 proteins and decreased 132 proteins with an adjusted p-value < 0.05, and an absolute value logFC > 0.2 (Fig. 3A, Supplemental Table 2). Twenty-four proteins had adjusted p-values < 0.01. Consistent with the known effect of glucocorticoids on the immune system, most of decreased ontology terms associated with immune system function (Fig. 3B). Additionally, we observed muscle structural constituents decreased (Fig. 3B). The Tumor Necrosis Factor α (TNFα) and interferon gamma (IFN-γ) pathways are inflammatory pathways activated in response to muscle damage and implicated in the progression of muscular dystrophies29,30. Intermittent prednisone reduced the IFN-γ-responsive proteins WNT5A, IL12B, and the chemoattractant cytokines CCL21 and CCL22. WNT5A and IL12B are known to be responsive to TNFα31,32. C1QTNF4, CD33, and LILRA4 were also decreased in this group. Overall, these findings indicate that this relatively low, once-weekly dose of prednisone modulated inflammatory pathways in the WSiMD cohort.
Protein biomarkers in WSiMD participants responsive to 6 months of intermittent glucocorticoid (GC) treatment. (A) Volcano plot depicting results from linear mixed-effects model for prednisone treatment status. Benjamini–Hochberg corrected p-value < 0.05 and abs(log2FC) > 0.2 were used as thresholds for significance. (B) Gene Ontology groups of GC increased and GC decreased serum proteins. Increased serum proteins are implicated in extracellular matrix disassembly and immune/inflammatory function. Decreased serum proteins were largely extracellular matrix proteins, proteins involved in muscle structure and function, growth factor signaling, and immune/inflammatory cell adhesion and chemotaxis.
When evaluating proteins that were increased by glucocorticoid treatment, multiple proteases were seen, including ELANE, MMP3, and MMP8 (Fig. 3A). These components of the “extracellular matrix disassembly” ontology grouping may participate in matrix turnover during regeneration, growth and repair (Fig. 3B). Prednisone treatment was associated with an increase in transcriptional regulators like ID2, MNDA, SPI1, and BPI. Other proteins of interest that were increased included ADIPOQ, ANXA1 and MUL1; these proteins are implicated in inflammatory responses and protein turnover and may be specifically important for muscle growth.
The effect of prednisone on muscular dystrophy-associated proteins
Since more proteins were differentially present based on disease status, rather than responsive to prednisone, we assessed whether any disease-associated ontology terms were shifted towards normalization by exposure to prednisone (Fig. 4). This analysis included terms increased in muscular dystrophy that were decreased by prednisone treatment and vice versa (Table 2). Figure 4A shows the individual responses to MMP3, MMP8, CCL21 and CCL22. MMP3 and MMP8 were both significantly increased after GC treatment while CCL21 and CCL22 were significantly decreased after GC treatment. Although not significantly different between the controls and the WSiMD cohort at baseline, there were mild trends suggestive of differences at baseline and consistent towards the direction of normalization for these biomarkers. Figure 4B shows examples of muscle proteins that were significantly increased in muscular dystrophy baseline and then significantly decreased after prednisone. This group includes clinically monitored markers like CKM, as well as CKB plus CKM heterodimer, TNNI2 and TNNI3. Similar trends were evident for MYL3, MYOM2, and MYL6B (Table 2). There were several proteins involved in receptor ligand activity that also showed this same pattern including the innate immune and TNF-α related proteins C1QTNF4, PTN, THBS4, and PENK (Table 2).
Individual shifts in prednisone- and disease-associated Biomarkers. Boxplots with paired results depicting representative behavior of biomarkers which had a significant response in both LGMD and the paired prednisone comparison. P-values are derived from the linear mixed model, thresholds are (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). (A) Glucocorticoid-only responsive marker. (B) Clinically monitored creatine kinase and troponin markers. (C) Proteins displaying both dystrophy association and glucocorticoid responsiveness. GC treatment had disease normalizing effects in this group.
Proteins decreased in MD that were increased by prednisone included mainly proteins implicated in immune process. Notably, PADI4, a protein deiminase that can reduce the affinity of chemoattractant ligands was the most decreased biomarker by logFC in the WSiMD cohort at baseline, and PADI4 was the most increased after prednisone treatment (Figs. 3 and 4C). Similarly, the membrane stabilizing and immunosuppressive proteins ANXA1, was also increased by prednisone treatment (Figs. 3 and 4C). SPI1, a master regulator of myeloid cell differentiation was also strongly increased by prednisone. Other proteins in this category were ITGB7, ELANE, NCF1, S100A8, BPI, and VAV3 (Fig. 5). When viewed according to LGMD subtype, no clear patterns were evident (Supplemental Fig. 3). Interestingly, prednisone treatment also increased ID2, a transcription factor that is in an inhibitor of differentiation. ID2 has been shown to also inhibit MyoD, a myogenic regulatory factor, and this observation coupled with stabilized sarcolemma from an increase in ANXA1 could indicate a lessened need for regeneration with prednisone exposure.
Comparisons with previous studies
Five prior MD studies used the similar aptamer platform12,17,25,33,34. These studies included a DMD cohort of young and slightly older patients with DMD, and a study evaluating the response of DMD patients to steroid treatment12,33. It also included a study of FSHD patients34 (Table 3). Across all studies comparing results to healthy controls, CKM, CA3, TNNI2 and FABP3 were increased. Overlapping proteins between DMD and LGMD are included in Supplemental Fig. 4. MB and ANP32B were increased in the DMD studies relative to healthy controls12,17,25. There was substantial overlap between the list of decreased proteins in the WSiMD cohort and those increased in younger DMD patients12. These proteins included 14–3-3 proteins YWHAB, YWHAH, and YWHAZ, as well as ENO1, XRCC6, EIF4G2, EIF4H, and HNRNPA2B1. Also of note, in the WSiMD cohort, compared to the younger DMD cohort, there was less immune downregulation and fewer increased proteins at baseline. This distinction may reflect the more intense nature of DMD, reflected by its younger age of onset and faster progression to loss of ambulation in DMD compared to the adult MD patients in WSiMD.
The treatment responsive serum proteins in the WSiMD cohort shared some overlap with the glucocorticoid treated DMD group described by12. Notably, 24 weeks of once weekly prednisone in WSiMD yielded a set of biomarkers that had overlap with the Hathout 2019 study, displaying increased MMP3 and the membrane-stabilizing and immune-suppressive ANXA1. CCL22, CCL21, CSF1R, CKM, PTN, BGN, FCER2, THBS4, MMP12, and ILL22RA2 were all decreased in both studies. This “normalizing” effect of once weekly prednisone was similar to higher doses used in the DMD patients and may indicate these biomarkers are especially relevant to mitigating the muscular dystrophy process.
Integrating MRI, serum profiles and functional status.
Despite the small size and genetic and functional heterogeneity of the WSiMD cohort, we assessed for correlations between MR imaging, functional status and serum aptamer profiles.
We evaluated fat fraction (FF) in the lower extremity studies with focus on the quadriceps muscle group and Triceps surae (Fig. 6A). We included data for those participants who completed the MRI at study endpoint and for whom there was a sufficient quality image for analysis (n = 10 for quadriceps, n = 13 for Triceps surae). Overall, most participants showed a small trend towards increasing fat fraction during the open label study. We correlated functional study and body composition with body composition determined by DEXA scanning (Fig. 6B). We noted a negative correlation between muscle FF and grip strength, and with NSAD. NSAD also showed a high degree of correlation with the other functional assessments. As NSAD includes many different measures, we used it to assess association of disease biomarkers with disease severity by linear regression. We observed a significant association between CKM and NSAD functional status, which reflects what is seen in DMD. This same pattern was evident for the muscle marker PGAM2 (Fig. 6C). We also evaluated the paired changes in GC responsive biomarkers with changes in NSAD. The change in CKM over the 6 months of prednisone treatment suggested that those with greater reduction in CKM had greater gain in functional status, so that change in CKM over this short time frame may reflect improved muscle repair or membrane stability. It is interesting that while higher CKM is associated with better functional status, likely due to increased activity and muscle mass, larger declines were associated with improved functional outcomes. This observation likely relies on a maintenance or increase in lean mass, as was observed in this cohort. There was also a trend toward improved functional in those participants with a decline in CCL22, a marker previously shown as steroid responsive in DMD12.
MRI and biomarkers related to functional outcomes. (A) Muscle fat fraction at study onset and after 6 months of weekly prednisone (V1, visit 1; V2, visit 2) in the quadriceps (RED) and Triceps surae (BLUE) muscle groups. Participants are listed by gene name along the X axis. Data was available from two muscle groups for 10 participants. For the two FKRP participants and one ANO5 participant, data was only available from the Triceps surae (blue). The majority of participants experienced a small increase in muscle fat fraction in the quadriceps and Triceps surae muscle groups. There was not always agreement between the two muscles. (B) Spearman correlation coefficients between age, functional performance measures, MRI water T2 and muscle fat fraction, and body composition as determined by DEXA scanning. (C) Example biomarkers showing a significant association (FDR ≤ 0.05) by linear regression with NSAD measure. The muscle protein markers CKM and PGAM2 correlated positively with functional performance as assessed by NSAD. D) Broad trends towards CKM and CCL33 reduction with change in NSAD over six months of treatment with once weekly prednisone (p value ≤ 0.05, FDR ≥ 0.05).
Discussion
Steroid use in muscular dystrophy
The WSiMD study was conceived to provide pilot information to guide a larger study of steroid use for MD subtypes not typically treated with glucocorticoids. Although DMD patients receive glucocorticoids with demonstrated functional benefit, this treatment comes with significant adverse outcomes, especially over the lifetime of a DMD patient35. These adverse consequences are not specific to MD since they are seen with long term glucocorticoid use for any condition. Long term adverse effects of obesity, metabolic syndrome/insulin resistance, adrenal insufficiency, cataracts, and osteoporosis are known consequences of years of exogenous glucocorticoid exposure. In DMD, glucocorticoid use typically begins in young children, and prolonged steroid during childhood stunts bone growth, body growth, and puberty. To this end, the novel glucose-sparing vamorolone received approval in the US and EU in 2023 for treating DMD based on its efficacy in boys with DMD36,37. For vamorolone, it is encouraging that bone growth is less adversely affected in DMD compared to prednisone36,37. The degree to which vamorolone protects against the other complications from prednisone or deflazacort use will be determined as more DMD patients transition to vamorolone for lifetime use.
An alternative strategy of using intermittent steroids has been employed in DMD, and one advantage of this intermittent dosing is less adrenal insufficiency, obesity, and insulin resistance6,9. In a study comparing 10 day on/off with daily glucocorticoids, intermittent steroids had less benefit on functional outcomes3. This study did not examine high dose weekend dosing, which is likely to have distinct effects from the 10 day on/off regimen. While some intermittent glucocorticoid treatment strategies may have less benefit functionally over the shorter term in DMD, the long term outcomes with respect to side effects and how these findings translate to adults with LGMD needs more study.
For the LGMD subtypes evaluated in this study, especially CAPN3, DYSF, and ANO5, these forms of muscular dystrophy are usually diagnosed in adulthood, and most patients with these subtypes will report having normal muscle function as children. Each of these MD subtypes has elevated serum CK, which if measured in early life, can be used for early diagnosis. We observed across the LGMD subset of WSiMD that many of the same proteins in DMD were elevated, especially muscle proteins. In DMD, most muscle proteins decline with age because of the reduction of muscle mass in older DMD patients17. Indeed, all the patients participating in the WSiMD trial were adults, although with a wide range of functional status, including younger individuals who were nonambulatory and older participants who retained ambulation. Because of this heterogeneity in LGMD and especially in the WSiMD cohort, it is not possible to draw correlations as to how the biomarkers associate with age. We also noted that some of proteins known to be increased in DMD were actually decreased proteins in WSiMD, like the 14–3-3 proteins and proteins implicated in translation. DMD profiles have greater immune dysregulation compared to WSiMD. We believe some of these differences between the WSiMD cohort and DMD reflect age as well as the more aggressive trajectory of MD in DMD, with earlier loss of ambulation.
Diversity of response to intermittent steroids and using biomarkers to guide steroid dosing
The overall outcomes in this small pilot study were highly variable, which was expected based on inclusion criteria. We noted that some patients appeared to have more benefit in this short duration study, showing improved lean mass11. We did not observe striking shifts in MRS muscle fat fraction, with most participants trending towards a modest increase over the 6 month study. We did observe trending correlations across all measures of muscle function with each other, and to a small degree with upper limb fat fraction and muscle function. A longer duration, placebo controlled glucocorticoid study is needed to determine muscle fat fraction trajectory over time and whether it correlates with muscle function.
We did observe proteins that appeared to shift in response to prednisone treatment, even at this low dose and intermittent exposure. These markers included proteins both increased and decreased by glucocorticoids. ADIPOQ was increased by glucocorticoids, consistent with prior reports in DMD steroid use12. We recently showed that intermittent prednisone mitigated diet-induced obesity in mice, and the effect of intermittent prednisone required the Adipoq gene to achieve this result. In this model, intermittent steroid exposure boosts ADIPOQ production from adipocytes, and correspondingly increases the adiponectin receptor (AdipoR1) in skeletal muscle38. Once activated, pathways downstream of the receptor activate CaMKK2 and AMPK to enhance skeletal muscle uptake of nutrients and ATP production. We previously found that mild exercise in mdx mice also activated ADIPOQ pathways, suggesting activity in combination with prednisone may be even more effective39.
The serum proteins decreased by weekly steroid exposure in WSiMD participants that were observed as being steroid responsive in DMD included several proteins implicated broadly in immune function like CCL22, CCL21, CSF1R, PTN, FCER2, and IL22RA2. CCL22 is a chemokine produced by macrophages, dendritic cells and T cells, and it is a steroid responsive chemokine that can be used to predict steroid resistance in other settings40,41. Moving forward, it may be possible to design serum markers to more carefully tailor steroid regimens the optimize efficacy and minimize adverse consequences.
Study limitations
This study is limited by the small sample size, open label design and heterogeneity of participants, both in terms of genotype and functional status. We also only sampled participants at the beginning and end of the study, with no intermediate analyses. We relied exclusively on aptamer measures of serum proteins, and future studies would require validated measures of these proteins. The purpose of the study was to provide pilot data that could inform future studies of steroid use in LGMD and BMD. WSiMD included a heterogenous cohort with a range of different types of MD and stage of clinical disease status. Despite these limitations, this data can help guide larger and more informative studies to use glucocorticoids to treat muscular dystrophy beyond DMD.
Data availability
Data that support the findings in this study are included in this article or are available upon request from the corresponding author and will be shared in manner to protect the privacy of the participants.
Abbreviations
- LGMD:
-
Limb girdle muscular dystrophy
- MD:
-
Muscular dystrophy
References
Bushby, K. et al. Diagnosis and management of Duchenne muscular dystrophy, Part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 9, 77–93 (2010).
Fenichel, G. M. et al. Long-term benefit from prednisone therapy in duchenne muscular dystrophy. Neurology 41, 1874–1877 (1991).
Guglieri, M. et al. Effect of different corticosteroid dosing regimens on clinical outcomes in boys with Duchenne muscular dystrophy: A randomized clinical trial. Jama 327, 1456–1468 (2022).
Schara, U., Mortier & Mortier, W. Long-term steroid therapy in Duchenne muscular dystrophy-positive results versus side effects. J. Clin. Neuromuscul. Dis. 2, 179–183 (2001).
Weber, D. R., Hadjiyannakis, S., McMillan, H. J., Noritz, G. & Ward, L. M. Obesity and endocrine management of the patient with Duchenne muscular dystrophy. Pediatrics 142, S43-s52 (2018).
Escolar, D. M. et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology 77, 444–452 (2011).
Quattrocelli, M. et al. Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J. Clin. Invest. 127, 2418–2432 (2017).
Quattrocelli, M. et al. Intermittent glucocorticoid dosing improves muscle repair and function in mice with limb-girdle muscular dystrophy. Am. J. Pathol. 187, 2520–2535 (2017).
Quattrocelli, M. et al. Pulsed glucocorticoids enhance dystrophic muscle performance through epigenetic-metabolic reprogramming. JCI Insight 4, (2019).
Wintzinger, M. et al. Impact of circadian time of dosing on cardiomyocyte-autonomous effects of glucocorticoids. Mol. Metab. 62, 101528 (2022).
Zelikovich, A. S., Joslin, B. C., Casey, P., McNally, E. M. & Ajroud-Driss, S. An open label exploratory clinical trial evaluating safety and tolerability of once-weekly prednisone in becker and limb-girdle muscular dystrophy. J. Neuromuscul. Dis. 9, 275–287 (2022).
Hathout, Y. et al. Disease-specific and glucocorticoid-responsive serum biomarkers for Duchenne muscular dystrophy. Sci. Rep. 9, 12167 (2019).
Khattri, R. B. et al. Magnetic resonance quantification of skeletal muscle lipid infiltration in a humanized mouse model of Duchenne muscular dystrophy. NMR Biomed. 36, e4869 (2023).
Kim, S. et al. Multivariate modeling of magnetic resonance biomarkers and clinical outcome measures for Duchenne muscular dystrophy clinical trials. CPT Pharmacometrics Syst. Pharmacol. 12, 1437–1449 (2023).
Barnard, A. M. et al. Mr biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology 94, e897–e909 (2020).
Rooney, W. D. et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology 94, e1622–e1633 (2020).
Hathout, Y. et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 112, 7153–7158 (2015).
Forbes, S. C. et al. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: Validation of multicenter study of evaluation with Mr imaging and Mr spectroscopy. Radiology 269, 198–207 (2013).
Forbes, S. C. et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: A multicenter cross sectional study. PLoS ONE 9, e106435 (2014).
Willcocks, R. J. et al. Longitudinal measurements of mri-T2 in boys with Duchenne muscular dystrophy: Effects of age and disease progression. Neuromuscul. Disord. 24, 393–401 (2014).
Willcocks, R. J. et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann.Neurol. 79, 535–547 (2016).
Triplett, W. T. et al. Chemical shift-based mri to measure fat fractions in dystrophic skeletal muscle. Magn. Reson. Med. 72, 8–19 (2014).
Forbes, S. C. et al. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: Validation of multicenter study of evaluation with Mr imaging and Mr spectroscopy. Radiology, 121948, (2013).
Hoffman, G. E. & Schadt, E. E. Variancepartition: Interpreting drivers of variation in complex gene expression studies. BMC Bioinform. 17, 483 (2016).
Parolo, S. et al. Combined use of protein biomarkers and network analysis unveils deregulated regulatory circuits in Duchenne muscular dystrophy. PLoS ONE 13, e0194225 (2018).
Dowling, P., Gargan, S., Zweyer, M., Swandulla, D. & Ohlendieck, K. Proteomic profiling of fatty acid binding proteins in muscular dystrophy. Expert Rev. Proteom. 17, 137–148 (2020).
Burch, P. M. et al. Muscle-derived proteins as serum biomarkers for monitoring disease progression in three forms of muscular dystrophy. J. Neuromuscul. Dis. 2, 241–255 (2015).
Rodríguez-Fdez, S. et al. Vav2 catalysis-dependent pathways contribute to skeletal muscle growth and metabolic homeostasis. Nat. Commun. 11, 5808 (2020).
Collins, R. A. & Grounds, M. D. The role of tumor necrosis factor-alpha (Tnf-Alpha) in skeletal muscle regeneration. Studies in Tnf-Alpha(-/-) and Tnf-Alpha(-/-)/Lt-Alpha(-/-) mice. J. Histochem. Cytochem. 49, 989–1001 (2001).
Cheng, M., Nguyen, M. H., Fantuzzi, G. & Koh, T. J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 294, C1183-1191 (2008).
Rauner, M. et al. Wnt5a is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 27, 575–585 (2012).
Gatica-Andrades, M. et al. Wnt ligands contribute to the immune response during septic shock and amplify endotoxemia-driven inflammation in mice. Blood Adv. 1, 1274–1286 (2017).
Dang, U. J. et al. Serum biomarkers associated with baseline clinical severity in young Steroid-Naïve Duchenne muscular dystrophy boys. Hum. Mol. Genet. 29, 2481–2495 (2020).
Petek, L. M. et al. A cross sectional study of two independent cohorts identifies serum biomarkers for facioscapulohumeral muscular dystrophy (Fshd). Neuromuscul. Disord. 26, 405–413 (2016).
Biggar, W. D., Skalsky, A. & McDonald, C. M. Comparing deflazacort and prednisone in Duchenne muscular dystrophy. J. Neuromuscul. Dis. 9, 463–476 (2022).
Dang, U. J. et al. Efficacy and safety of vamorolone over 48 weeks in boys with Duchenne muscular dystrophy: A randomized controlled trial. Neurology 102, e208112 (2024).
Guglieri, M. et al. Efficacy and safety of vamorolone vs placebo and prednisone among boys with Duchenne muscular dystrophy: A randomized clinical trial. JAMA Neurol 79, 1005–1014 (2022).
Quattrocelli, M. et al. Intermittent prednisone treatment in mice promotes exercise tolerance in obesity through adiponectin. J. Exp. Med. 219, (2022).
Zelikovich, A. S., Quattrocelli, M., Salamone, I. M., Kuntz, N. L. & McNally, E. M. Moderate exercise improves function and increases adiponectin in the Mdx mouse model of muscular dystrophy. Sci. Rep. 9, 5770 (2019).
Pranzatelli, M. R., Tate, E. D., McGee, N. R., Colliver, J. A. & Ransohoff, R. M. Ccr4 agonists Ccl22 and Ccl17 are elevated in pediatric Oms sera: Rapid and selective down-regulation of Ccl22 by Acth or corticosteroids. J. Clin. Immunol. 33, 817–825 (2013).
Zhaoyang, P. et al. Ccl22 and leptin associated with steroid resistance in childhood idiopathic nephrotic syndrome. Front. Pediatr. 11, 1261034 (2023).
Acknowledgements
Supported by the Kurt+Peter Foundation, National Institutes of Health AR052646, NS047726, NS127383, and HL061322 and Parent Project Muscular Dystrophy. We thank Dr. Todd Parrish and the Center for Translational Imaging at Northwestern.
Author information
Authors and Affiliations
Contributions
A.Z. collected data and samples. A.W. performed the biomarker analysis. A.B. performed the MRI analysis. A.B., G.W., K.V. analyzed the images. A.W., A.Z., A.D., and E.M. conceived the studies, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
EMM has been a consultant to Amgen, AstraZeneca, Cytokinetics, Pfizer, Tenaya Therapeutics, and is a founder of Ikaika Therapeutics. ARD is CSO of Ikaika Therapeutics. The other authors have declared that no conflict of interest exists. There are no nonfinancial conflicts to report.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Willis, A.B., Zelikovich, A.S., Sufit, R. et al. Serum protein and imaging biomarkers after intermittent steroid treatment in muscular dystrophy. Sci Rep 14, 28745 (2024). https://doi.org/10.1038/s41598-024-79024-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79024-8
Keywords
This article is cited by
-
Large-scale serum protein biomarkers discovery associated with function and clinical milestones in Duchenne muscular dystrophy
Nature Communications (2025)
-
Circulating protein biomarkers identified in two independent clinical trial cohorts of glucocorticoid-naive Duchenne muscular dystrophy patients.
Scientific Reports (2025)