Abstract
Myocardial infarction (MI) is a leading cause of heart failure, disability and mortality worldwide. In this study, the effects of intramyocardial injection of human platelet lysate (HPL), bone marrow mesenchymal stem cells pretreated with HPL (PMSCs), and PMSC lysate (lys), alone and in combination were investigated on MI-induced by LAD ligation in male Wistar rats. The experiment was carried out on sham, vehicle (Veh), HPL, PMSCs, PMSC lysate (PMSC lys), HPL + PMSCs, and HPL + PMSC lys groups. SBP, DBP, and ± dp/dt max were monitored by the PowerLab physiograph. The MSC characteristics and CD31, NKX2.5, and cardiac troponin I (cTnI) contents were determined by flow cytometry, immunohistochemistry, and immunofluorescence, respectively. SBP, DBP, and ± dp/dt max that decreased in the MI group were recovered by HPL, PMSC, PMSC lys, HPL + PMSC, and HPL + PMSC lys treatments. CD31 density was higher in all treated groups compared to the Veh group. CD31 density in the HPL + PMSCs and HPL + PMSC lys groups was higher than in the PMSCs group. The number of Dil+/NKX2.5 + and Dil+/cTnI + cells was higher in the HPL + PMSCs group compared to the PMSCs group. The HPL and PMSCs mitigates heart injuries and cardiac dysfunction after MI. HPL provides an appropriate environment for cardiomyocyte differentiation from PMSCs.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) are among the most important causes of disability and mortality worldwide. It is estimated that 17.9 million people pass away from CVDs each year, which accounts for approximately 32% of all deaths. More than four out of five CVD deaths are due to heart attacks and strokes1,2. Heart failure occurs as a result of heart attack, congenital heart disease, coronary artery disease, and extracellular matrix disorders that cause replacement of cardiomyocytes with fibrotic tissue, heart remodeling, and irreversible impairment of cardiomyocytes3.
Despite various pharmacological and other therapeutic interventions, the long-term prognosis of CVDs is poor, with a 30-day mortality rate of approximately 35–50%4. One of the best choices for the treatment of heart failure in the final stages is heart transplantation. Nevertheless, the practicality of this method is low, considering the small number of donors, the side effects of immunosuppression, and the impossibility of transplantation in some cases. Therefore, based on these findings, scientists and clinicians have tried to achieve more efficient ways to stimulate the healing processes of heart injuries and replace lost myocytes5.
After myocardial infarction, approximately one billion cardiomyocytes are lost. Since the intrinsic regenerative capacity of the heart is low (less than 1% per year), using exogenous stem cells and/or stimulating proliferation and differentiation of endogenous cardiac progenitor cells are emerging treatments for producing new functional myocardial tissue6. Furthermore, reprogramming strategies have been suggested for cardiomyocyte production from somatic cells7.
Mesenchymal stem cells (MSCs) of various origins have been used in the treatment of heart disease and cardiomyocyte regeneration. Experimental and clinical findings have suggested MSCs are safe and they can be used for treating cardiovascular disorders8. MSCs induce anti-inflammatory and immunosuppressive effects by interacting with immune cells and releasing paracrine factors such as cytokines, chemokines, and extracellular vesicles9. Therefore, MSCs from young healthy persons can be applied to aged patients undergoing allogenic transplant10.
The beneficial effects of MSCs on myocardial function have been demonstrated in several experimental and clinical studies11. Studies have reported an improvement in cardiac function following bone marrow-derived mesenchymal stem cell (BMSC) injection after six months. Nevertheless, there was no difference in the results of long-term follow-up between treated and untreated patients12,13. Tracing stem cells in the heart has proved that their viability is low. However, MSCs improve heart function by releasing growth factors including cytokines, miRNAs, and angiogenic factors. Consequently, MSCs ameliorate ischemic injuries by increasing angiogenesis and reducing fibrosis, apoptosis, and infarct size14,15,16. The injection of stem cell extract, which contains the above-mentioned factors, into the border zone of the infarct area has shown the same improving effects, suggesting that the effects of stem cells are related to their paracrine properties17,18.
In addition to their conventional functions (coagulation and thrombosis), platelets have multiple physiological and therapeutic functions because they contain growth factors and active molecules, e.g. basal fibroblast-derived growth factor (bFGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), and C-X-X chemokines19. Among different derivatives of platelets, platelet-rich plasma (PRP), and platelet lysate (PL) are more suitable for therapeutic aims owing to the absence of platelet membranes and white blood cells20. There is evidence that PL therapy has positive outcomes in the treatment of osteoarthritis, bone regeneration, ocular disease, infertility, and wound and ulcer healing21,22,23,24. Also PL has been suggested as an alternative inducer of the growth and proliferation of MSCs ex vivo25,26; and an increase in BMSC and adipose-MSC proliferation and growth has been found after treatment with PL14,26. Moreover, adipose-derived MSCs acquire cardiac phenotypes in the presence of PL and 5-azacytidine27,28.
Various studies have demonstrated the therapeutic effects of MSCs mediated by secreting different factors with paracrine mechanisms10,29. The cell lysate has been suggested as a cell-free therapeutic strategy for treating multiple diseases30,31. However, so far, the role of PL, BMSC pretreated with PL (PMSCs), and MSC lysate extracted from PL-pretreated BMSCs have not been tested in the treatment of myocardial infarction. Therefore, to answer the question that which of the above strategies is more appropriate for treatment of MI, this study aimed to investigate the effects of intra-myocardial injection of human platelet lysate (HPL), PMSCs, MSC lysate extracted from HPL-pretreated BMSCs (PMSC lys), and their combination on cardiac function, cardiomyocyte damage and inflammation, fibrosis, angiogenesis, and myogenesis in the hearts of rats with myocardial infarction.
Results
Identification of PMSCs
The flow cytometry results indicated that CD73, CD90, and CD105 (MSC markers) were highly expressed on the surface of the PMSCs in passage 4, but they were negative for expression of CD11b and CD45 (hematopoietic markers) (Fig. 1).
Expression of cardiomyogenic microRNAs and cardiac-related genes in PMSCs
The expression of four cardiomyogenic microRNAs including miR-1, miR-133a, miR-499, and miR-208a32 was assessed in PMSCs prior to injection to investigate whether the treatment of MSCs with HPL affects their differentiation towards cardiomyocytes (Fig. 2). Among the four microRNAs, miR-1 (P < 0.001) and miR-133a (P < 0.001) showed a decrease while miR-499 (P < 0.05) and miR-208a (P < 0.001) exhibited a significant increase in expression. In addition, the expression of two pluripotency factors, Oct-4 (P < 0.05) and Nanog (P < 0.001)33, and two cardiac-related genes, GATA-4 (P < 0.001) and Nkx2.5 (P < 0.001), showed an increase. Furthermore, morphologically the PMSCs appeared elongated and spindle-shaped. Based on these results, it seems that HPL promotes the differentiation of MSCs towards cardiomyocytes.
The effect of HPL on MSC morphology and gene expression. (A): The MSCs exposed to HPL (8%) at passage 4 appear elongated, longer, and spindle-shaped whereas the MSCs not treated with HPL exhibit a broader morphology with more prominent and larger nuclei (light microscopy, Optika, 10X magnification). (B): Among the four cardiomiRs, miR-499, and miR-208a showed increased expression in the HPL + MSCs group. In addition, two stem cell marker genes (Nanog and Oct-4) and two cardiac-related genes (GATA-4 and Nkx2.5) exhibited a significant increase in the HPL + MSCs group compared to the MSCs group. HPL: human platelet lysate, MSCs: mesenchymal stem cells, PMSCs: bone marrow mesenchymal stem cells pretreated with HPL. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. MSCs.
Histopathological findings
Induction of MI and intramyocardial injection of agents caused one-third of the animals to die due to severe arrhythmia in pilot experiments. To reduce the mortality, lidocaine 2% (2 mg/kg) was administered 5–6 min before MI induction34. This reduced the mortality rate to less than 10%. The mortality rate did not differ among the groups. In the case of mortality, new animals were replaced so that the final sample size reached 7 rats in each group. The body weight, left ventricular weight/body weight ratio, and lungs weight/body weight ratio were not different among the groups (Table 1).
According to the TTC staining results, the average infarct size of the heart was 40.1% of the total LV area in the Veh group. Treatment with HPL (P < 0.01), PMSCs (P < 0.001), PMSC lys (P < 0.001), HPL + PMSCs (P < 0.01), and HPL + PMSC lys (P < 0.01) significantly reduced the size of infarct area (Fig. 3). Moreover, the results of H&E and Masson’s trichrome staining showed that treatment with HPL, PMSC lys, HPL + PMSCs, HPL + PMSC lys (P < 0.05 to P < 0.01), but not PMSCs, improved degenerative changes, inflammation and fibrosis in the hearts with MI (Fig. 4).
Effects of different treatments on infarct size in the heart four weeks after LAD occlusion. (A): The heart and its transverse sections (from tip to the base) in one animal from each group, after TTC staining. (B): Quantification of infarct size in study groups. HPL: human platelet lysate, PMSCs: bone marrow-mesenchymal stem cells pretreated with HPL, PMSC lys: PMSC lysate: ##P < 0.01, ### P < 0.001 vs. Veh. n = 7 in each group.
Effects of different treatments on histopathological scores including myocyte damage, inflammation and fibrosis (%) in hearts four weeks after LAD occlusion. (A): The histopathological changes (myocyte damage and inflammation) in one animal of each group with H&E staining. (B, C): Micrograph showing the fibrosis (Masson’s trichrome) in one animal of each group. (D): Quantification of histological scores in study groups. (E): Quantification of fibrosis in study groups. HPL: human platelet lysate, PMSCs: bone marrow-mesenchymal stem cells pretreated with HPL, PMSC lys: PMSC lysate: # P < 0.05, ##P < 0.01, vs. Veh. Magnification = 100x and 400x, n = 7 in each group.
Hemodynamic and cardiac function findings
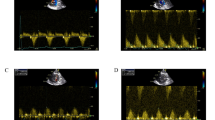
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were lower in MI animals than in the sham group (P < 0.001). There were recoveries in SBP (P < 0.01) and DBP (P < 0.05) in the HPL and HPL + PMSCs groups compared to the Veh group. When HPL was added to PMSCs, the recovery in SBP was more prominent (P < 0.05). The combination of PMSC lysate and HPL also increased SBP and DBP (P < 0.05) relative to the Veh group (Fig. 5A, B). Heart rates did not differ significantly among the studied groups (Fig. 5C).
Also, left ventricular systolic pressure (LVSP) was lower in Veh group than in the sham (P < 0.001). LVSP restored to almost normal value in HPL (P < 0.001), PMSCs (P < 0.01), PMSC lys (P < 0.01), HPL + PMSCs (P < 0.01), and HPL + PMSC lys (P < 0.01) groups. An increase in left ventricular end diastolic pressure (LVEDP) was observed four weeks after MI induction (P < 0.05). Except for PMSC lys, none of the other interventions significantly improved the increase in LVEDP (Fig. 6).
+dp/dt max (maximum rate of increase in left ventricular pressure during systole), which is an index of myocardial contractility, decreased in the Veh group compared to the sham group (P < 0.001). There was a significant recovery of the + dp/dt max in the HPL (P < 0.001), PMSCs (P < 0.001), PMSC lys (P < 0.01), HPL + PMSCs (P < 0.001), and HPL + PMSC lys (P < 0.001) groups (Fig. 7A).
-dp/dt max (maximum rate of decrease in left ventricular pressure during diastole), which is an index of myocardial relaxation, decreased in the Veh group (P < 0.05). HPL (P < 0.05), PMSCs (P < 0.05), HPL + PMSCs (P < 0.01), and HPL + PMSC lys (P < 0.05) were able to improve this lusitropic index (Fig. 7B).
Effects of different treatments on (A): +dp/dt max (maximum rate of increase in left ventricular pressure during systole), (B): -dp/dt max (maximum rate of decrease in left ventricular pressure during diastole) in rats four weeks after LAD occlusion. **P < 0.01, *** P < 0.001 vs. sham; # P < 0.05, ## P < 0.01 ### P < 0.001 vs. Veh. n = 7 in each group.
Angiogenesis
To test this hypothesis that improvement of cardiac function after transplantation of stem cells and other treatments may be caused by angiogenesis. we performed anti-CD31 immunohistochemistry to analyze the extent of neovascularization in the border zone of the infarct area. The findings revealed that in the Veh group, the capillary density significantly decreased at the border zone of the infarct area. CD31 density significantly increased in the HPL (P < 0.01), PMSCs (P < 0.05), PMSC lys (P < 0.01), HPL + PMSCs (P < 0.01), HPL + PMSC lys (P < 0.001) groups compared to the Veh group. Also, the CD31 density was higher in the HPL + PMSCs (P < 0.05) and HPL + PMSC lys (P < 0.01) groups compared to PMSCs alone (Fig. 8).
(A): Sample micrograph of the left ventricle of one animal from each group showing the effect of different treatments on the CD31 + expression area (brown color, marked by the red arrow) assessed by immunohistochemistry (IHC) in hearts four weeks after LAD occlusion. (B): Quantification of the CD31+ expression area (%) in study groups. *** P < 0.001 vs. sham; # P < 0.05 ## P < 0.01 ### P < 0.001 vs. Veh; && P < 0.01 vs. PMSCs; $$ P < 0.01 vs. PMSC lysate. Magnification = 400x. n = 7 in each group.
Myogenesis
Immunofluorescence analysis was performed to observe the maintenance and differentiation of PMSCs and HPL + PMSCs. There were Dil-positive cells in the heart two and four weeks after stem cell injection (Fig. 9). The levels of NKX2.5 and cTnI at the end of week 4 was higher than at the end of week 2 in PMSCs and HPL + PMSCs groups (P < 0.001). In addition, the expression of NKX2.5 and cTnI was higher in the group that received HPL + PMSCs than those received PMSCs (P < 0.001). The number of Dil+/NKX2.5+ and Dil+/ cTnI + cells at the end of week 4 was higher than at the end of week 2 after injection in PMSCS and HPL + PMSCs groups (P < 0.05 to P < 0.01).
Immunofluorescent analysis indicates the expression of NKX2.5 and cTnI (green) in the hearts of rats with MI two and four weeks after PMSCs and HPL + PMSCs injection: (A, B): Sample micrographs of NKX2.5 and cTnI in an animal of each group. Quantification of the levels of NKX2.5 + and cTnI+ (C and E) and the number of Dil+/NKX2.5 + and Dil+/ cTnI+ (D and F) in the study groups. Stem cells stained with Dil dye (red) and nuclei with DAPI (blue). *** P < 0.001 vs. PMSCs 2 weeks; # < P < 0.05, ### P < 0.001 vs. HPL + PMSCs 2 weeks; &&& P < 0.01 vs. PMSCs; $$ P < 0.001, $$$ P < 0.001 vs. HPL + PMSCs. n = 3 in each group.
Discussion
The findings of the present study showed that HPL, PMSCs, PMSC lysate, and the combination of these treatments improved the cardiac function in the MI rats. The hemodynamic parameters, contractility indices, and angiogenesis improved, and infarct size, cardiomyocyte damage, inflammation, and fibrosis decreased in the treatment groups. In the rats treated with HPL + PMSC, improvement in cardiac function and infarct size was associated with the viability and differentiation of PMSCs.
The therapeutic benefits of the different derivatives of platelets including PL and platelet-rich plasma (PRP) have been illustrated in other diseases including osteoarthritis and in the repair of injured tissues, such as spinal cord injuries, and on maintenance of the integrity of blood vessels20,35. The observed positive outcomes of intramyocardial injection of HPL on cardiac function and left ventricular contractility in this study can be attributed to angiogenic growth factors of PL, such as VEGF, TGF-ß, and neuropeptide Y19,36. The increase in CD31 density verified angiogenesis in HPL treated rats with MI. This is probably due to the effect of angiogenic factors existing in the HPL. Other studies have also reported the angiogenic properties of PL37,38,39.
After MI, the inflammatory responses initiate and last 3–4 days (in mice) to remove damaged cells. The platelets also participate in the inflammatory responses due to their membranes’ adhesion factors. However, the released microparticles and exosomes from platelets decrease inflammatory responses by suppressing the leukocytes, neutrophils, monocytes, and macrophages and by potentiation of M2-macrophage activity necessary for repair in the myocardium19. In line with other reports, our findings revealed that fibrosis, cardiomyocyte damage and inflammation decreased after treatment with PL. The cardioprotective effects of released factors from platelet α-granules, including SDF-1α and TGF-β1, have been demonstrated in ischemia-reperfusion lesions19. Cardiomyocytes are responsive to platelet contents, and possibly platelet factors increase the inotropic activity of the heart19.
Studies have suggested that the pretreatment of stem cells with various agents, such as growth factors, chemical substances, microRNAs, and cytokines, improves their quality and differentiation capability40,41. Current evidence suggests that using HPL instead of FBS in the culture medium possess the positive features of FBS, which include the induction of mesenchymal proliferation and differentiation, in addition to the elimination of their undesirable effects of FBS14. Based on the results of this study, it seems that HPL enhances partial cardiomyocyte reprogramming of BMSCs through induction of Oct4 expression as it has been suggested that Oct4 expression mediates partial cardiomyocyte reprogramming of MSCs33. Oct-4 promotes the pluripotency process by inducing the expression of other factors, such as Nanog42. Our finding showed that HPL increased the expression of two cardiomiRs, miR-499 and miR-208a. MicroRNAs − 499, -208a, -1, and − 133a are cardiomiRs involved in cardiac development32, which have been referred to as combo microRNAs that enhance differentiation of fibroblasts to cardiomyocytes43. Furthermore, the expression of cardiac marker genes Nkx2.5 and GATA4, confirm the partial acquisition of cardiomyocyte characteristics in PMSCs. These genes improve the engraftment of PMSCs in cardiac tissue and strengthen their improving effects on cardiac function following myocardial infarction42. This study indicated that PMSCs have beneficial effects on cardiac function, as the ± dp/dt max (indicators of heart contractility and lusitropy), and LVEDP, as an index of heart diastolic function, improved in the MI rats. The IHC results revealed PMSCs also improved angiogenesis in the infarct area, evidenced by increase in CD31, a transmembrane glycoprotein that is highly expressed in endothelium44.
One of the mechanisms by which stem cell therapy induces cardiac repair is the differentiation of stem cells into cardiomyocytes6. The expression of cardiomyocyte indicators, NKX2.5 and cTnI, confirmed differentiating of cardiomyocytes from stem cells. It is noticeable that concomitant injection of HPL and PMSCs increased the capability of angiogenesis and cardiomyogenesis, probably due to providing a rich environment containing growth factors, such as VEGF, miRNAs, and stem cell-derived factor 1 alpha (SDF-1α), which are stored in platelets6,19,20. These factors promote the differentiation of cardiac progenitor cells (CPCs) into cardiomyocytes45.
It has been suggested that the primary improving mechanism of MSCs is paracrine action through the release of bioactive factors, including exosomes, growth factors, cytokines, and interleukins. Therefore, PMSC extract or lysate is proposed as an alternative cell-free strategy for the treatment of many disorders46. In the present study, the effect of cellular lysate prepared from HPL-pretreated MSCs (PMSC lys) was less prominent than the improving effect of PMSCs itself. It appears that the settling of MSCs in target tissue by releasing paracrine growth factors, differentiation of PMSCs to cardiomyocytes, or connecting to host cells are additional ways to cure tissue injuries. Based on the results, HPL potentiates the improving effect of PMSCs and PMSCs lys on cardiac contractility, and on angiogenesis, probably directly due to the adaptive effects of HPL or indirectly through providing an appropriate milieu condition for PMSCs. It has been demonstrated that the enriched milieu condition stimulates the production and release of bioactive materials by stem cells47. The combination of HPL with PMSCs showed no additional effects on left ventricular performance compared to the effect of HPL alone, probably owing to the maximum effects induced by HPL. Given that HPL promotes the differentiation of PMSCs into cardiomyocytes, the beneficial effects of the combination of HPL and PMSCs are expected to appear over the long term.
Conclusion
The findings indicated the improving effects of HPL, PMSCs, and PMSC lysate on the cardiac dysfunction and heart injuries induced by MI. The combination of HPL with PMSCs and/or with PMSC lysate potentiated the ameliorative effects of PMSCs and PMSC lysate, particularly on angiogenesis. HPL also increased cardiomyocyte differentiation from PMSCs. It appears that the common pathways for the beneficial effect of adding HPL and PMSC lysate are angiogenesis and reduction of fibrosis and inflammation in the injured myocardium.
Materials and methods
The animals were purchased from the animal breeding center of Kerman University of Medical Sciences (Pars Research Biocenter) and kept in the Physiology Research Centre’s animal house with free access to water and food and under a 12-hour dark/light cycle. All methods were carried out in accordance with relevant guidelines and regulations. The study was approved by the Ethics Committee of Kerman University of Medical Sciences and Rafsanjan University of Medical Sciences (approval codes: IR.KMU.REC. 1399.259. and IR.RUMS.REC.1399.037 respectively). All methods are reported in accordance with ARRIVE guidelines.
Materials
The following materials were used in this study: TTC from Sigma, UK; Dulbecco’s Modified Eagle Medium (DMEM) and Fetal Bovine Serum (FBS) from Gibco, CD31 polyclonal antibody from Elabscience Biotechnology Inc., ab64261 - rabbit specific HRP/DAB (ABC) detection IHC kit from Abcam. Dil dye was obtained from Invitrogen. Rabbit polyclonal primary antibody for NKX2 (No: orb540657), rabbit polyclonal antibody for cTnI (No: orb304638), and goat anti-rabbit IgG (H + L) antibody (FITC) (No orb688925) were purchased from Biorbyt, United Kingdom.
Methods
The hemodynamic parameters, cardiac contractility indices, and angiogenesis were assessed in seven experimental groups (7 rats in each group) included sham, myocardial infarction receiving phosphate-buffered saline (PBS) (Veh), HPL, MSCs pretreated by HPL (PMSCs), PMSC lysate, HPL + PMSCs, and HPL + PMSC lys. The size of the infarcted area was measured in the above-mentioned groups (extra 5 rats in each group). The survival and myogenesis of stem cells were traced two and four weeks after PMSCs and HPL + PMSCs injection. For this, PMSCs were stained with Dil dye before injection into the border of the infarct zone in two groups of PMSCs and HPL + PMSCs groups (extra three rats in each group).
Isolation, culture, and pretreatment of rat bone marrow mesenchymal cells with HPL
Wistar rats 6–8 weeks old (n = 10) were deeply anesthetized by ketamine and xylazine (80/10 mg, IP). The femur and tibia were isolated from the skin and muscles and washed with 70% ethanol for 10 s and then transferred to a 50 ml conical tube containing sterile DMEM. The end of each bone was cut by sterile scissors under a laminar hood. The bone marrow was aspirated using a 22 G syringe, diluted with 5 ml DMEM, and centrifuged at 1800 rpm for 5 min. The supernatant was removed and the cell pellets were cultured in DMEM containing 8% human platelet lysate (HPL) 48 and 2% pen-strep, and incubated at 37 °C in a 5% CO2 incubator. The culture medium was refreshed every 3 days. When the cells reached 70 to 80% confluency, they underwent passages. Cells in passage 4 were used for characterization and injection procedures48.
PMSCs characterization by flow cytometry
From the cultured medium 106 cells were harvested and washed with PBS before fixation with 4% paraformaldehyde. In order to inactivate the nonspecific antibodies, the cells were incubated with goat serum diluted with PBS (1:10) for 15 min at 4 °C. Then the cells were incubated and labeled with CD105, CD90, CD73, CD11b, and CD45 antibodies for one hour. The expression of all indicators of MSCs was assessed by flow cytometry and analyzed with WinMDI software49.
Preparation of lysates from PMSCs
The lysate from 106 MSCs cells pretreated with HPL was prepared for each injection. For this, after harvesting and washing the cells with PBS, 200 µl of PBS was added to the pellet. For rupture of the cell membrane, the solution was kept at −80 °C overnight and then thawed for 5 min. The freeze-thaw cycle was repeated three times, after that the solution was centrifuged for 5 min at 4 °C and 300 g to remove cell debris50.
Preparation of platelet lysate
To prepare human platelet lysate (HPL), 20 ml of human venous blood was mixed with 2.5 ml of dextrose citrate solution as an anticoagulant (0.8% citric acid, 0.22% sodium citrate, 0.233% dextrose). After centrifugation at 300 g for 15 min, PRP was separated and frozen at -80 °C for 60 min to produce PL and was then thawed at 37 °C for 15 min. This operation was repeated twice. In order to remove platelet fragments and white blood cells, the solution was centrifuged at 4000 g for 20 min and filtered with a 0.22 μm filter. Then, 2 ml of platelet lysate was sent to the laboratory to check for microbial contamination25,51.
Induction of myocardial infarction
The left anterior descending (LAD) coronary artery was occluded to induce MI, as previously explained52. Concisely, after anesthesia with ketamine and xylazine (80/10 mg/kg), the heart was exposed by an incision in the fourth left intercostal space under mechanical ventilation. The pericardium was opened, and the LAD was permanently ligated 2 mm below its origin with a 6/0 silk suture. MI was verified by the pale appearance of the at-risk area and ST-segment elevation in the ECG (Fig. 10). In the sham group, the complete surgical procedure was performed without ligation of the LAD52.
Injection of platelet lysates or stem cells
Thirty minutes after coronary artery ligation, 100 µl of either HPL, PMSC lysate, 106 PMSCs (dissolved in PBS), HPL + PMSCs, or HPL + PMSC lysate was injected using a 30-gauge needle in five points (20 µl in each point) of the border zone of the infarcted area53. After injection, the chest muscles and skin were closed using 4 − 0 vicryl and silk sutures.
Recording of hemodynamic parameters and cardiac indices
Four weeks after injection, the rats were anesthetized with sodium thiopental (50 mg/kg) and blood pressure and heart rate were recorded by a physiograph connected to a catheter filled with heparin saline (10 units/ml) inserted in the right femoral artery. Another catheter was pushed into the left ventricle (LV) via the right carotid artery to measure cardiac function indices52. The animal was ventilated through a tracheal cannula if necessary.
The arterial and ventricular cannulas were connected to pressure transducers and then to an 8-channel PowerLab system (ADInstruments, Australia). The hemodynamic parameters recorded were systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). The LV function indices were left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and cardiac contractility indicators including + dp/dt max (maximum rate of rise in LV pressure i.e. contraction velocity), and -dp/dt max (maximum rate of decline in LV pressure i.e. relaxation velocity)52,54.
Measurement of infarct size
After recording hemodynamic and cardiac function parameters, the hearts were removed and rinsed in cold saline. The samples were kept at -20 °C for 1 to 2 h. Then, 3-mm slices were made and stained with triphenyl tetrazolium chloride 1% (TTC) in PBS for 20 min at 37 °C. Finally, the slices were embedded in formaldehyde 10% for 10 min. The proportion of the infarct area (pale) to the total LV area was measured by ImageJ software52,55.
Preparation of heart samples
Four weeks after treatment, the animals were euthanized under deep anesthesia (thiopental sodium, 50 mg/kg, IP) and the hearts were removed and rinsed with cold saline. The left ventricle (LV) plus the septum were gently separated, weighed, and kept in formaldehyde 10% to assess myocyte damage and inflammation, fibrosis, angiogenesis, and myogenesis.
Morphological examination and measurement of fibrosis, myocyte damage and inflammation in heart tissue
Four weeks after intramyocardial injection of different treatments, the heart was removed under deep anesthesia (thiopental sodium (50 mg/kg, ip) and fixed in 10% formaldehyde for 24 h. After that from the paraffin blocks of the left ventricle, 5-micrometer slices were made and stained with hematoxylin-eosin to examine for myocyte damage and degree of inflammation (histopathology score), or with Masson’s trichrome to detect fibrosis. The histopathology findings were scored as 0, no changes; 1, mild (focal myocyte damage or small multifocal degeneration with a slight degree of inflammatory process); 2, moderate (extensive myofibrillary degeneration and/or diffuse inflammatory process); 3, marked (necrosis with a diffuse inflammatory process)56. A pathologist who was blinded to the animal groups scored the samples with a light microscope (Olympus CX33, Japan) and reported the results. The percentage of fibrosis was measured by ImageJ software in five fields for each slice57.
Immunohistochemistry to evaluate angiogenesis
CD31, as an endothelial and microvasculature (angiogenesis) biomarker, was measured by immunohistochemical staining58. After fixing and dehydrating, 5 μm sections were prepared from the paraffin-embedded sample. Then the deparaffinized samples were incubated with primary monoclonal antibodies overnight at 4 °C. The samples were then washed with PBS three times (each for 5 min) and were incubated with peroxidase-coupled secondary antibody for 30 min at room temperature. The cell nuclei were stained with hematoxylin (Abcam) for 1 min and the slides were imaged with a light microscope (AXIOM Germany-upright microscope). The CD31-positive area was evaluated in five fields using ImageJ software59.
Immunofluorescence staining to evaluate myogenesis
For assessing cardiomyogenesis, the expression of specific myocyte proteins, NKX2.5 and cardiac troponin I (cTnI) were examined two and four weeks after treatments. The heart tissues were placed in formaldehyde 10% for 24 h. Then 5-µm-thick slices were made from the paraffin-embedded hearts dewaxed in xylene, hydrated in a graded series of ethanol, and subjected to Triton 0.3% for 30 min in order to permeabilize the cell membrane. Then 10% goat serum was added to the samples for 45 min to prevent secondary antibody reactions. The samples were incubated overnight in diluted primary antibody (1:100 with PBS) at 2 to 8 °C followed by goat anti-rabbit IgG (H + L) secondary antibody (FITC, green) (1/150) at 37 °C in the incubator (AriaTeb, Iran) for 90 min in dark conditions. Then the samples were stained with DAPI (D9542, Sigma), and their fluorescent pictures were taken with an Olympus fluorescent microscope.
RNA extraction and real-time PCR
For RNA extraction, cultured cells were first washed with PBS and harvested after trypsinization, resulting in a cell pellet obtained through centrifugation. Subsequently, the cells were washed twice with PBS and centrifuged again, after which Trizol (Total RNA extraction kit, Pars Tous, Iran) solution was added to the cell pellet for extraction. The extraction was performed according to the kit’s protocol. For cDNA synthesis, a Pars Tous kit (Easy cDNA synthesis kit) was utilized, and the temperatures used were in accordance with the kit’s instructions, with the addition of RT primers. Gene expression analysis was conducted using the Master Mix from Amplicon (REALQ PLUS 2X Master Mix Green, Denmark) and the Applied Biosystems instrument (StepOnePlus). 18SRNA and RNU6 served as internal controls for the genes and miRNAs, respectively. A list of the primers used is provided in the Table 2.
Statistical analysis
The data were presented as mean ± SEM and analyzed by SPSS software, version 20. After verifying the normal distribution of data using the Shapiro-Wilk test, data were analyzed using Unpaired student t-test or one-way ANOVA, and in case of significance, pairwise comparisons were conducted by Tukey’s post hoc test. The categorical data (histopathology scores) were analyzed with corresponding nonparametric tests. 2^-∆∆ct method was used for analysis of real-time PCR. P-values < 0.05 were considered as statistically significant.
Data availability
Data will be available on reasonable request from the corresponding author.
References
Şahin, B. & İlgün, G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc. Care Community 30, 73–80 (2022).
Ghiroldi, A. et al. Cell-based therapies for cardiac regeneration: A comprehensive review of past and ongoing strategies. Int. J. Mol. Sci. 19 (2018).
Menasché, P. Cell therapy trials for heart regeneration—Lessons learned and future directions. Nat. Rev. Cardiol. 15, 659–671 (2018).
Tyler, J. M. et al. Variability in reporting of key outcome predictors in acute myocardial infarction cardiogenic shock trials. Catheter Cardiovasc. Interv. 99, 19–26 (2022).
Muller, P., Lemcke, H. & David, R. Stem cell therapy in heart diseases-cell types, mechanisms and improvement strategies. Cell. Physiol. Biochem. 48, 2607–2655 (2018).
Beliën, H., Evens, L., Hendrikx, M., Bito, V. & Bronckaers, A. Combining stem cells in myocardial infarction: The road to superior repair? Med. Res. Rev. 42, 343–373 (2022).
Werner, J. H., Rosenberg, J. H., Um, J. Y., Moulton, M. J. & Agrawal, D. K. Molecular discoveries and treatment strategies by direct reprogramming in cardiac regeneration. Transl Res. 203, 73–87 (2019).
Kobayashi, K. & Suzuki, K. Mesenchymal stem/stromal cell-based therapy for heart failure—What is the best source?. Circ. J. 82, 2222–2232 (2018).
Song, N., Scholtemeijer, M. & Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 41, 653–664 (2020).
Gill, J. K., Rehsia, S. K., Verma, E., Sareen, N. & Dhingra, S. Stem cell therapy for cardiac regeneration: Past, present, and future. Can. J. Physiol. Pharmacol. 102, 161–179 (2024).
Seow, K. S. & Ling, A. P. K. Mesenchymal stem cells as future treatment for cardiovascular regeneration and its challenges. Ann. Transl Med. 12, (2024).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Fisher, S. A., Doree, C., Mathur, A. & Taggart, D. P. & Martin-Rendon, E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev. (2016).
Guiotto, M., Raffoul, W., Hart, A. M., Riehle, M. O. & Di Summa, P. G. Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: A systematic review. J. Transl Med. 18, 1–14 (2020).
Heldman, A. W. et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. Jama 311, 62–73 (2014).
Tano, N. et al. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther. 22, 1864–1871 (2014).
Zheng, Y. L. et al. Stem cell-derived exosomes in the treatment of acute myocardial infarction in preclinical animal models: A meta-analysis of randomized controlled trials. Stem Cell. Res. Ther. 13, 151 (2022).
Sahoo, S. & Losordo, D. W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 114, 333–344 (2014).
Walsh, T. G. & Poole, A. W. Do platelets promote cardiac recovery after myocardial infarction: Roles beyond occlusive ischemic damage. Am. J. Physiol. Circ. Physiol. 314, H1043–H1048 (2018).
Zamani, M. et al. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 234, 17172–17186 (2019).
Cui, Y. et al. Research trends of platelet-rich plasma therapy on knee osteoarthritis from 2011 to 2021: A review. Med. (Baltim) 102, e32434 (2023).
Badran, Z., Abdallah, M. N., Torres, J. & Tamimi, F. Platelet concentrates for bone regeneration: Current evidence and future challenges. Platelets. 29, 105–112 (2018).
Nadelmann, J. B., Bunya, V. Y., Ying, G. S., Hua, P. & Massaro-Giordano, M. Effect of autologous platelet-rich plasma drops in the treatment of ocular surface disease. Clin. Ophthalmol. 16, 4207–4213 (2022).
Etulain, J. Platelets in wound healing and regenerative medicine. Platelets. 29, 556–568 (2018).
Becherucci, V. et al. Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol: A cell factory experience. Stem Cell. Res. Ther. 9, 124 (2018).
Siciliano, C. et al. The potential of GMP-compliant platelet lysate to induce a permissive state for cardiovascular transdifferentiation in human mediastinal adipose tissue-derived mesenchymal stem cells. Biomed Res. Int. (2015).
Naaijkens, B. A. et al. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell. Tissue Res. 348, 119–130 (2012).
Yabanoglu, S. et al. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J. Mol. Cell. Cardiol. 46, 518–525 (2009).
Segers, V. F. M. & Lee, R. T. Stem-cell therapy for cardiac disease. Nature. 451, 937–942 (2008).
Khubutiya, M. S. et al. Effect of conditioned medium and bone marrow stem cell lysate on the course of acetaminophen-induced liver failure. Bull. Exp. Biol. Med. 159, 118–123 (2015).
Malik, S., Awan, S. J., Hashim, M., Farzand, A. & Nadeem, S. Regeneration potential of bone marrow-derived mesenchymal stromal cells lysate for H2O2 (In-Vitro) injured cells. Curr. Biotechnol. 10, 101–110 (2021).
Abkhooie, L. et al. Potential roles of MyomiRs in cardiac development and related diseases. Curr. Cardiol. Rev. 17, (2021).
Yannarelli, G. et al. OCT4 expression mediates partial cardiomyocyte reprogramming of mesenchymal stromal cells. PLoS One 12, e0189131 (2017).
Rousscau-Migneron, S., Tancrede, G. & Nadeau, A. Lidocaine improves survival rate in diabetic rats submitted to acute left coronary artery ligation. Basic. Res. Cardiol. 85, 404–410 (1990).
Behroozi, Z., Ramezani, F., Janzadeh, A., Rahimi, B. & Nasirinezhad, F. Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiol. Behav. 228, 113186 (2021).
Burnouf, T., Strunk, D., Koh, M. B. C. & Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 76, 371–387 (2016).
Hayon, Y., Dashevsky, O., Shai, E., Varon, D. & Leker, R. R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemost. 110, 323–330 (2013).
Businaro, R. et al. Platelet lysate-derived neuropeptide y influences migration and angiogenesis of human adipose tissue-derived stromal cells. Sci. Rep. 8, 1–12 (2018).
Bordin, A. et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell. Prolif. 55, e13312 (2022).
Guo, X. et al. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell. Res. Ther. 9, 1–12 (2018).
Shinmura, D. et al. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells. 29, 357–366 (2011).
Rui, Y. et al. Epigenetic memory gained by priming with osteogenic induction medium improves osteogenesis and other properties of mesenchymal stem cells. Sci. Rep. 5, 11056 (2015).
Li, B., Meng, X. & Zhang, L. microRNAs and cardiac stem cells in heart development and disease. Drug Discov Today 24, 233–240 (2019).
Norrby, K. & Ridell, B. Tumour-type‐specific capillary endothelial cell stainability in malignant B‐cell lymphomas using antibodies against CD31, CD34 and factor VIII. Apmis. 111, 483–489 (2003).
Chen, D. et al. Crosstalk between SDF-1/CXCR4 and SDF-1/CXCR7 in cardiac stem cell migration. Sci. Rep. 5, 16813 (2015).
Kumar, P. et al. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor. Rev. 46, 1–9 (2019).
Xu, Y., Chen, C., Hellwarth, P. B. & Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact Mater. 4, 366–379 (2019).
Moghadam, F. H., Tayebi, T. & Barzegar, K. Differentiation of rat bone marrow mesenchymal stem cells into adipocytes and cardiomyocytes after treatment with platelet lysate. Int. J. Hematol. Stem Cell. Res. 10, 21 (2016).
Baghaei, K. et al. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. bed Bench. 10, 208 (2017).
Salem, M. L., El-Bakry, K. A., Moubark, E. H., Sobh, A. & Khalil, S. M. Beneficial modulatory effects of treatment with bone marrow lysate on hematopoietic stem cells and myeloid cells in tumor-bearing mice. Br. J. Biomed. Sci. 79, 10328 (2022).
Shih, D. T. B. & Burnouf, T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 32, 199–211 (2015).
Rostamzadeh, F. et al. PEGylated graphene quantum dot improved cardiac function in rats with myocardial infarction: morphological, oxidative stress, and toxicological evidences. Oxid. Med. Cell. Longev. (2021).
Wang, L. et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am. J. Physiol. Circ. Physiol. 297, H1020–H1031 (2009).
Sarazan, R. D., Kroehle, J. P. & Main, B. W. Left ventricular pressure, contractility and dP/dtmax in nonclinical drug safety assessment studies. J. Pharmacol. Toxicol. Methods 66, 71–78 (2012).
Downey, J. M. Measuring infarct size by the tetrazolium method. ISHR Handb. Exp. Lab. Proced. Downey JM, ed. Int. Soc. Hear. Dis. (2000).
Bertinchant, J. P. et al. Comparison of the diagnostic value of cardiac troponin I and T determinations for detecting early myocardial damage and the relationship with histological findings after isoprenaline-induced cardiac injury in rats. Clin. Chim. acta. 298, 13–28 (2000).
Wei, Q. et al. Ramipril attenuates left ventricular remodeling by regulating the expression of activin A-follistatin in a rat model of heart failure. Sci. Rep. 6, 33677 (2016).
Kondo, T. et al. Immunohistochemical analysis of CD31 expression in myocardial tissues from autopsies of patients with ischemic heart disease. Leg. Med. 59, 102127 (2022).
Kim, T. H., Kim, S. H., Leong, K. W. & Jung, Y. Nanografted Substrata and triculture of human pericytes, fibroblasts, and endothelial cells for studying the effects on Angiogenesis. Tissue Eng. Part. A 22, 698–706 (2016).
Hu, Y. et al. MicroRNA-1 downregulation induced by carvedilol protects cardiomyocytes against apoptosis by targeting heat shock protein 60. Mol. Med. Rep. 19, 3527–3536 (2019).
Xu, Z. et al. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci. Rep. 5, 12276 (2015).
Wang, X., Yang, C., Liu, X. & Yang, P. Ghrelin alleviates angiotensin II-induced H9c2 apoptosis: Impact of the miR-208 family. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 24, 6707 (2018).
Ebrahim, N. et al. Functional recellularization of acellular rat liver scaffold by induced pluripotent stem cells: Molecular evidence for wnt/b-catenin upregulation. Cells. 10, 2819 (2021).
Asif, Y. et al. Sustained cardiac programming by short-term juvenile exercise training in male rats. J. Physiol. 596, 163–180 (2018).
Alzahrani, F. A. et al. Potential effect of exosomes derived from cancer stem cells and MSCs on progression of DEN-induced HCC in rats. Stem Cells Int. 8058979 (2018).
Acknowledgements
This work was supported by grants from the Vice-Chancellor for Research and Technology at Kerman University of Medical Sciences, Kerman, Iran (Grant No. IR.KMU.REC.98000777) and Rafsanjan University of Medical Sciences (Grant No. IR.RUMS.REC. 98193).
Author information
Authors and Affiliations
Contributions
HN, ZB, and FR designed the research. FR and SJ conducted the experiments. FR analyzed the data. HN, FR, SJ, and ZB wrote the manuscript. EJ observed and interpreted the histopathological findings. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Najafipour, H., Rostamzadeh, F., Jafarinejad-Farsangi, S. et al. Human platelet lysate combined with mesenchymal stem cells pretreated with platelet lysate improved cardiac function in rats with myocardial infarction. Sci Rep 14, 27701 (2024). https://doi.org/10.1038/s41598-024-79050-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79050-6