Abstract
This study aims to reduce engine emissions while maintaining engine performance and providing a sustainable fuel source for long-term use. It introduces a novel approach by combining pine oil (PO) and lemon grass oil (LGO) with diesel fuel in a specific ratio (10% PO + 10% LGO + 80% Diesel). This work is innovative in that it employs these two distinct low-viscosity biofuel blends in conjunction with diesel fuel in an agricultural engine, resulting in reduced carbon footprints in the tailpipe. The blend tested in a single-cylinder diesel engine showed that using PO and LGO together reduced UHC emissions by 42.96%, CO emissions by 20.79%, and smoke emissions by 26.26%, while keeping the BTE the same. However, there was a 7.16% rise in NOx emissions. To decrease NOx emissions, antioxidants—250 mg of p-phenylene diamine (10% PO + 10% LGO + 80% + 250 mg PPDA) and 100 mg of butylated hydroxy toluene (10% PO + 10% LGO + 80% + 100 mg BHT) were added. The blends were injected at different injection pressures of 300 bar, 450 bar, and 600 bar, which can result in a reduction in NOx emissions of up to 11.78% and 10.99% for B2 + 300 bar and B3 + 300 bar, respectively. We used the SWOT study to evaluate the advantages and disadvantages of the mixed fuel, and employed the PUGH Matrix decision tool to carry out the sustainability assessment. The results showed that the blend (10% PO + 10% LGO + 80% Diesel + Antioxidant + 600 bar) is a sustainable fuel, considering environmental, social, and economic factors to be more feasible than pure diesel.
Similar content being viewed by others
Introduction
The transportation industry is essential to many areas, including globalization, the economy, the environment, society, and technology. Automobile engines cause air pollution, noise pollution, and greenhouse gas pollution1. Furthermore, the need for fossil fuels was growing daily. Therefore, in order to lessen its reliance on fossil fuels, the transportation industry needs to replace its energy sources in a sustainable way. In order to lessen our reliance on fossil fuels, biofuels provide a possible substitute. The use of biofuel has a number of potential benefits. It promotes agriculture’s sustainable expansion, lowers global warming, and provides energy security for the transportation sector. It can be extracted from a variety of sources, including crops, municipal waste, vegetable oils, animal fats, waste cooking oil, waste engine oil, waste plastics, algae, and so on.
Researchers have found that biofuels are more efficient and environmentally friendly than traditional fossil fuels. Further research is underway to explore the potential of plant-based sources for biofuel production. There are different methodologies adopted for the extraction of biofuel from feed stroke, but the major techniques are the grind of feed stroke and the steam distillation process. The extraction of fuel from the grinding process necessitates a secondary process due to the biofuel’s higher density and viscosity. Despite this secondary process, it presents certain challenges2. During the transesterification process, the addition of alcohol leads to the formation of fatty acids. This leads to a decrease in the fuel’s stability and the alcohol’s corrosive nature, which damages engine components like the fuel tank, fuel supply line, and injector, necessitating coating to lessen the corrosive effect.
Therefore, it may be necessary to abandon the steam distillation technique to address the aforementioned issue. Here are some examples of biofuels extracted from the steam distillation process, as they offer comparable properties to fossil fuels. Examples of these biofuels include orange peel oil, lemon peel oil, lemon grass oil, pine oil, camphor oil, winter green oil, and eucalyptus oil, among others. Every fuel has unique characteristics that improve engine performance and emission characteristics3.
The 5.2 kW Kirloskar diesel engine was used to examine the combustion behavior of pine oil at standard injection pressure using different diesel fuel preparations, ranging from 0 to 100% with a 25% variation. The findings demonstrated that all other indicators exhibited increasing tendencies, with the exception of NOxemissions. Given that pine oil and diesel fuel have similar fuel qualities, In particular, the calorific value was higher than that of diesel fuel, and the kinematic viscosity was lower, causing fuel to penetrate the combustion chamber. The BTE increased by 10% and reduced HC, CO, and smoke emissions by 65%, 30%, and 70%, respectively. According to the study, pine oil can serve as a more suitable substitute for diesel fuel4. Similar to diesel fuel, we mix pine oil in different ratios with CPME and CPMEP biofuels and then use it straight in the single-cylinder engine without any modifications. Reduced energy content in the CPME and CPMEP led to lower BTE and higher BSFC, according to the data. This combination has the benefit of lowering NOx emissions5.
Because of the pine’s natural oxygen level and energy content, NOx emissions may increase. The fuel was mixed with decanal in different ratios ranging from 10 to 50% in order to lower the NOx emissions, and the engine was tested without any changes. The decanal fuel weakened the blend’s characteristics, resulting in a decrease in BTE and an increase in BSFC. Operating on lower-calorie fuel impacts the engine’s performance, leading to decreased NOx emissions6. The aforementioned features of lemon grass oil are present in it; the calorific value of diesel fuel was 42,100 kJ kg, whereas that of lemon grass oil was 37,000 kJ kg. Mixing LGO fuel with varying amounts of fossil fuel, from 5 to 40%, results in a decrease in BTE and an increase in BSFC. This blend has the benefit of significantly lowering NOx emissions. The 20% mix, which is ideal for engine use, showed a 48% increment in BSFC, 14.75%, 49.99%, and 46.80% decreased BTE, HC, and CO emissions7. Combining LPO with diesel fuel led to an increase in the engine’s BTE. In order to extend the ignition delay period, LPO gasoline with a cetane number similar to diesel fuel causes a reduction in CO, HC, and BTE emissions, along with a fine for NOx emissions. An extended ignition delay interval improves fuel atomization, leading to a more premixed combustion phase. Additionally, the BTE rose when the injection pressure increased from the usual injection pressure to 600 bar injection pressure increases both fuel penetration and combustion chamber pressure, resulting in a BTE of 36.12%. 20% of the combination demonstrates LPO fuel’s greater performance. Several techniques were used, including pilot injection, EGR, and the blend’s use of anti-oxidants, to lower NOxemissions8.
According to the studies, fuel injection pressure is essential for raising BTE and lowering emissions of smoke, HC, CO, and BSFC. Injecting the palm biodiesel blend at 450 and 600 bar lowers the emissions of CO and HC while also reducing the BSFC by roughly 2.43%. The higher injection pressure, however, facilitates the fuel blend’s evaporation and atomization, resulting in complete combustion and raising the combustion chamber’s temperature, which raises the blend’s NOxemissions9. Comparable outcomes were observed when a diesel fuel blend consisting of 10% to 30% castor oil methyl esters was injected at 300, 400, and 500 bar. Plotting the results against the fuel injection pressure reveals that, with the exception of NOxemissions, every engine performance and emission behaviour were improved. The temperature of the combustion chamber rose because the oxygenated fuels inject at a higher injection pressure, which encourages the premixed combustion phase10.
Antioxidants can be added to fuel to lower NOx emissions, according to the study. The NOx generation for Annona biodiesel blends including several antioxidants, including PPDA, AT, and LA, was evaluated at varied preparation levels ranging from 50 to 450 mg. The findings demonstrated that, compared to the A20 mix, 250 mg of PPDA decreased NOx emissions by roughly 24.7%. This was attributed to the PPDA’s ability to inhibit the production of free radicals within the combustion chamber. The evaluation was further broadened to include a reduction in emissions from the Jamun biodiesel blend. The same antioxidants were combined with J20 biofuel in several compositions ranging from 150 to 450 mg, with a 100 mg variation. According to the study, there is a significant decrease in the NOx emission at 250 mg of PPDA, along with a decrease in the CO, HC, and smoke emissions. Reduced emissions of HC, CO, and smoke are achieved by the antioxidant’s control over the oxidation of carbon molecules and by increasing the quantity of OH radicals inside the combustion chamber. The study concludes that including 250 mg of PPDA in the blend reduces emissions of smoke, HC, CO, and NOx11.
Antioxidants including BHA, BHT, TBHQ, and 2EHN were added to the canola methyl esters at different concentrations of 500 ppm, 750 ppm, and 1000 ppm to see how they affected the emission behaviour of the plant. The findings demonstrate that the chemical reaction is controlled by the inclusion of antioxidants, which limit NOxemissions since the antioxidants include NO molecules. However, the presence of oxygen inhibitors in the fuel caused a considerable rise in CO emissions12. Similarly, less NOx would be released when BHT and BHA were combined with a palm biodiesel blend. This is because antioxidant surprises in chemical molecular interactions would reduce the amount of NOx. The Alexandrian laurel biofuel blend showed reduced NOxemissions with the addition of BHT and increased BTE as compared to diesel fuel. But the HC, CO, and smoke emissions increased13.
To maximize the engine’s output, the optimal mix of inputs was found through trials using the optimization process. The majority of engine studies used Taguchi optimization to optimize the input parameters14. All of this was done using the Minitab program. Taguchi optimization was utilized to optimize the input parameters for Karanja biodiesel validation. In addition to the injection angle, mix, and compression ratio, the engine’s load was taken into consideration as a controlling component in this investigation. Taguchi optimization was used to optimize the impact of EGR on the biodiesel mix. The components included in this study were the blend ratio, engine load, EGR percentage, and L16 orthogonal array15. An L18 orthogonal array was utilized in a similar fashion to determine the ideal ratio of hexane to water mix diesel fuel in the ceramic coated engine. The studies show that the Taguchi optimization techniques yield superior outcomes16. We are grateful to the authors who conducted these investigations and generated comprehensible findings. The SWOT analysis helpful to know about the fuel advantage and disagrees considering the internal and external factors. The components of SWOT analysis are Strength, Weakness, Opportunities and Threats17.
Meeting present needs without jeopardizing the ability of future generations to meet their own is the concept of sustainability. Achieving equilibrium between social, economic, and environmental aspects is necessary to preserve the long-term well-being of people and the environment. Sustainability aims to promote harmony between human activities and natural ecosystems while acknowledging the planet’s finite resources and the interdependence of all living things. This usually means taking steps to reduce waste, reduce carbon emissions, protect the environment, and promote social justice and equity26. Table 1 displays Summary of Literature Survey.
Based on the research mentioned earlier, the research gap was identified that the no research was done to test the tertiary fuel combination with antioxidants in the engine application. Numerous authors concluded that adding 20% pine oil to diesel fuel would improve engine performance while increasing NOx emissions. But when combined with 20% diesel fuel, the orange oil has the reverse effect. This work is novel in that it uses these two unique low-viscosity biofuel mixes in combination with diesel fuel in an agricultural engine. We injected the blends at several injection pressures: 230 bar, 300 bar, 450 bar, and 600 bar. The addition of two distinct antioxidants, PPDA and BHT, can reduce the amount of NOx emissions produced. So, the main goal of this study is to find out how to use the antioxidant-blended tertiary fuel blend in engines that are run at different injection pressures. The Taguchi optimization methodology was used to optimize the results. Furthermore, the study expanded to examine the pros and cons of the fuel blend using SWOT analysis. The research also looks at fuel blend sustainability while taking performance, cost, and environmental aspects into account.
Materials and methodology
The primary goal of this research is to better understand how tertiary fuel blends such as diesel fuel, pine oil, and lemon grass oil affect engine performance and emissions. Lemon grass oil is extracted from the leaves of the lemon grass plant, and pine oil is extracted from the seeds of the pine tree. The steam distillation method yielded both fuels27. These fuels are acquired from our suppliers and examined for characteristics in accordance with ASTM guidelines. Table 2 lists the characteristics of lemon grass oil and pine oil.
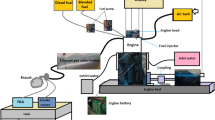
Pine oil has a marginally higher heating value than diesel fuel, whereas LGO was found to have a lower heating value than diesel fuel. However, compared to pine oil, LGO has a greater cetane number, which helps to lessen the influence of cetane number in blends of diesel fuel and pine oil rather than adding additives to raise cetane number. Additionally, the blends are blended with specific antioxidants at levels of 250 mg and 100 mg, respectively, such as BHT and PPDA. The blend preparation is shown in the Fig. 1.
Table 3 lists the attributes and mix ratio in tabular form. PPDA is an organic component that is created as a by-product of the chemical and plastic industries. Its molecular formula is C6H18N2. Eight open hydrogen bonds are visible in the PPDA’s molecular structure, and these bonds would react with the oxygen in the combustion chamber, which would control the oxidation of fuel molecules. BHT is an organic component that is lipophilic and is generated from phenol. The BHT’s molecular structure, C15H24O, reveals the 24 free hydrogen atoms that regulate the rate of burning by absorbing oxygen atoms. The properties of BHT and PPDA listed in the Table 4.
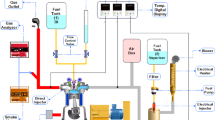
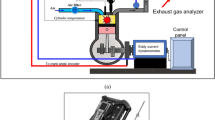
The mixes are tested in a single-cylinder farm diesel engine that runs at a constant speed of 1500 rpm and generates 5.2 kW of brake power at peak load conditions. As seen in the Fig. 2, the engine was configured with an eddy current dynamometer for loading applications.
Table 5 displays the experimental setup specification. The AVL DI Gas 444N was used to measure the emissions of HC, CO, CO2, and NOx. The AVL 437C smoke meter was used to monitor smoke emissions. The NDIR principle is used by the AVL DI gas analyser to measure emissions of HC, CO, and CO2; the electrochemical principle is used to monitor emissions of NOx. A smoke meter’s working concept is based on the attenuation of a light beam brought on by exhaust gases in a measuring chamber with a defined measurement length and a uniformly filled, non-reflecting surface. The measuring range and accuracy of the emission measurement device are listed in Table 6.
Using an open ECU setup, the blends were injected at various injection pressures of 230 bar (standard injection pressure), 300 bar, 450 bar, and 600 bar. The outcomes are displayed in relation to the applied load.
The goal of this work was to reach the ideal operating condition; to that end, the fuel blend and injection pressure were optimized using Taguchi optimization. Taguchi optimization is a direct and easy way to maximize machine operating conditions for maximum performance. Three components with four levels—blend, load, and injection pressure—were selected for Taguchi optimization listed in Table 7.
The engine loading application was used to determine the levels, which ranged from 25% load to 100% load with a 25% load standard deviation. The Taguchi optimization recommended the L16 orthogonal array, and all performance and emission value data are entered into the plan and summarized in Table 8.
The S/N ratio was chosen for this analysis using two distinct criteria: a greater ratio is preferable for BTE and CO2 emissions, while a smaller ratio is preferable for BSFC, HC, CO, and smoke emissions. In a similar manner, the elements and levels were incorporated into the Taguchi methodology.
Uncertainty analysis
To determine the error in the experimental data, one crucial parameter was the uncertainty analysis. Errors can be classified into two categories: random and systematic. In contrast to the random element, which is unexpected and uncontrollable, the systematic mistake may be foreseeable and under control. One can compute the cumulative uncertainty by applying the root mean squares approach. Table 9 lists the uncertainties of the various parameters, and Eq. 1 shows the equation that can be used to compute the uncertainties. After all the data are entered into the formula, the percentage of uncertainty is determined to be 2.07.
Result and discussion
Brake thermal efficiency
The braking power produced for a given amount of energy is known as the BTE, which is a measure of an engine’s efficiency in turning fuel power into usable work28. Figure 3a illustrates the BTE under all loading scenarios, whereas Fig. 3b shows the % increase in BTE in mixes above diesel fuel. The BTE if diesel fuel is 30.24% where as B1, B2 + 600 bar and B3 + 600 bar are 30.5%, 30.95% and 30.96% respectively. It is evident that the engine generates more BTE under all circumstances for a given blend. Blend 1’s latent heat characteristic lengthens the ignition delay time, which mixes fuel and air inside the combustion chamber and raises Blend 1’s BTE29. Blends 2 and 3 yield consistent BTE under all circumstances when they inject at greater injection pressures; nevertheless, when compared to diesel fuel, their BTE increases. This is because the greater IP allows fuel to penetrate the combustion chamber. Because of that highest atomization that can be accomplished, which represents greater BTE, the injections of blends 2 and 3 at higher injection pressure become more turbulent and spread inside the combustion chamber in comparison to diesel fuel and blend 1. For all loading scenarios, the BTE rose even with the inclusion of PPDA and BHT. Given that the IP significantly affects the fuel blend’s penetration, evaporation, and atomization, the blends’ inherent oxygen makes up for the oxygen shortage and guarantees full combustion when the anti-oxidants in the blends react with the air inside the combustion chamber10. Table 1 illustrates blend’s calorific value, which is similar to diesel fuel, guarantees an improvement in the BTE under all loading scenarios.
Brake specific fuel consumption
As the amount of fuel required to generate one unit of brake power, the BSFC is defined. The BSFC demonstrates that for higher engine output, engine efficiency and lower BSFC values are preferred30. The amount of BSFC for each loading is displayed in Fig. 4a, and the percentage drop in BSFC when compared to diesel fuel is shown in Fig. 4b. The graph makes it clear that as engine load increased, the BSFC decreased. The BSFC of Diesel, B1, B2 + 600 bar and B3 + 600 bar are 0.25 kg/kWhr, 0.25 kg/kWhr, 0.29 kg/kWhr and 0.29 kg/kWhr respectively. Blend fuels assure the atomization of the fuel blend because they have a lower flash point than diesel fuel. Fuel mixtures would evaporate more quickly as a result of the combustion chamber’s temperature rising due to an increase in engine load31. As Blend 1 illustrates, higher atomization and evaporation rates result in lower BSFC. Blends 1, 2, and 3 have lower viscosities, which improve vaporization and hasten the mixing of fuel and air. Nonetheless, the engine would not experience any performance barriers as a result of the combination of BHT and PPDA. This result is caused by excess oxygen in the combustion chamber, which makes up for the oxygen needed for antioxidants. While uniform mixing of air and fuel mixtures is achieved by the increased injection pressure of low viscosity fuel blends, which encourages fuel blend penetration throughout the combustion chamber, By decreasing droplet size, the higher injection pressure aids in atomization and ensures that the fuel and air. It is also clear that lowering the blend’s BSFC is significantly influenced by its calorific value. As a result, the BSFC trend was favourable when using a blend with higher injection pressure32.
Emission characteristics
UHC emission
The amount of UHC emissions present in the exhaust gas indicates that the fuel combination with air was not burned efficiently33. Figure 5a illustrates the UHC concentration in relation to engine load, whereas Fig. 5b shows the percentage reduction in UHC emissions when diesel fuel is used. The UHC of Diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar B3 + 450 bar, and B3 + 600 bar are 105 ppm, 60 ppm, 63 ppm, 62 ppm, 60 ppm, 62 ppm, 61 ppm, and 60 ppm respectively. It was evident from the data that the UHC concentration rose as loading increased, which was brought on by injecting more fuel to keep the engine running at a consistent speed. On the other hand, Blend 1’s UHC emissions significantly dropped. This is a result of PO and LGO’s latent heat vaporization properties, which raise evaporation rates, increase atomization, and encourage complete combustion. While blend 2 and 3 UHC emissions are higher than those of blend 1 due to the addition of BHT and PPDA, they are still lower than those of diesel fuel. Because there is more single bond hydrogen in BHT and nitrogen in PPDA, the chemical reaction is suppressed by boosting UHC production and decreasing oxidative radicals in the chamber. A higher IP would hasten the oxidation of HC molecules, which would minimize HC emissions. However, with the increase in injection pressure from 300 to 600 bar, the generation of UHC decreased due to the increased load.
CO emission
The results of fuel richness would increase the amount of CO emissions34. The kinetics of CO emission formation are as follows: initially, all the carbon in the chamber is converted into CO; if there are oxygen molecules, a partial amount of CO is converted to CO2; if there is excess oxygen present in the combustion zone, all the CO is converted to CO235. Figure 6a illustrates the changes in CO emissions as a function of engine load, whereas Fig. 6b shows the percentage drop in CO emissions when diesel fuel is used. The CO emission of Diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar B3 + 450 bar, and B3 + 600 bar are 0.1603%, 0.127%, 0.08%, 0.09%, 0.1%, 0.08%, 0.093%, and 0.098% receptively. Fuel was injected into the structure in the shape of a cone, with three distinct zones: the rich zone was formed near the injector nozzle, the stoichiometric zone was formed near the middle of the combustion zone, and the lean zone was formed near the wall of the combustion chamber. The lack of available oxygen causes the rich zone to emit more CO36. Because fuels naturally contain oxygen, which is utilized to convert CO into CO2 emissions in the rich zone, blend fuels exhibit lower CO emissions. However, the combination of BHT and PPDA exhibits a detrimental impact on CO emissions due to a decrease in free OH radicals. However, the increase in IP results in increased CO emissions as a consequence of the increased in-cylinder temperature rise caused by the previous cycle heat release rate. Although the values are still lower, CO emissions.
CO2 emission
The complete combustion produces CO2 and H2O molecules as by-products37. Figures 7a and 7b illustrate the variation in CO2 emissions and the percentage rise in CO2 emissions relative to diesel fuel, respectively, in the event that there is a higher amount of CO2 emissions in the exhaust gas, which indicates the full combustion of the fuel and air mixture. The CO2 emission of Diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar B3 + 450 bar, and B3 + 600 bar are 67.88%, 69.88%, 73.36%, 72.26%, 71.18%, 73.52%, 72.46%, and 71.38% respectively. Based on the data, it is known that the integrated oxygen in the PO and LGO is the reason why all of the blends exhibit higher CO2 emissions. The cetane number is important in addition to oxygen concentration because it prolongs the ignition delay interval, which enhances the premixed combustion phase.
The addition of antioxidants BHT and PPDA shows an advantage effect on CO2 emissions, but the values are constantly decreasing with an increase in IP. As the diesel engines work with a lean mixture, the BHT and PPDA attract the free OH radicals in the chamber, which will have an impact on the conversion of CO2 from CO. Also, during the expansion stroke, the temperature was reduced by the latent heat characteristics of the fuel blend and the expansion of compressed gas, which reduced CO2 emissions. However, the values are still higher than those of diesel, which means the blends promote combustion.
NOx emission
The nitrogen present in the air oxidizes the nitrogen at higher temperatures and yields the oxides of nitrogen38. Figure 8a shows the variation of NOx emissions for all loadings, and Fig. 8b displays the percentage increase in NOx emissions as compared to diesel fuel. The NOx emission of Diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar B3 + 450 bar, and B3 + 600 bar are 1147 ppm, 1229 ppm, 1012 ppm, 1039 ppm, 1097 ppm, 1021 ppm, 1048 ppm, and 1090 ppm respectively. From the data, Blend 1 produces a higher amount of NOx emissions as compared to diesel fuel and other blends. Even though the engine operates at a lean mixture near stoichiometric, which produces a higher amount of NOx, this is caused by the latent heat characteristics of LPO and PO, which reduce the overall combustion temperature and increase the ignition delay period, which would affect the larger stoichiometric mixture zone. In addition to the integrated oxygen molecules present in the LGO and PO, they supply sufficient oxygen during the adiabatic constant pressure combustion. All to get the engine to yield a higher amount of NOx emissions. But these yields are controlled by the addition of BHT and PPDA antioxidants. The nitrogen present in the PPDA acts as an inert gas and controls the oxidation rate, and BHT reduces the formation of free OH radicals in the chamber, which has an effect on reduced NOx. But this nature is not valid when there is an increase in injection pressure. When the fuel injects at a higher injection rate, it becomes turbulent, leading to more region being covered by the stoichiometric zone, which increases the NOx emission, but values are lower than those of diesel and blend 1. So, the use of antioxidants has an advantage in reduction of NOx emissions.
Smoke emission
When the engine runs with a reduced amount of oxygen, it will produce a higher amount of smoke emissions39. Figure 9a depicts the rate of smoke emission in relation to load, whereas Fig. 9b indicates the percentage reduction in smoke emission when compared to diesel fuel. The NOx emission of Diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar B3 + 450 bar, and B3 + 600 bar are 69.7%, 51.4%, 54%, 53.5%, 52.7%, 54.4%, 53.1%, and 52.8%, respectively. The production of smoke emissions depends on the ratio of HC. Blend 1 shows an advantage in reducing smoke emissions for all engine loads. The oxygen present in the LGO and PO supplies sufficient oxygen and reduces smoke emissions. Moreover, smoke emissions would be reduced in comparison to diesel fuel if the lower aromatic components of LGO and PO were substituted for the higher aromatic components. Although the amount of smoke produced would increase if BHT and PPDA were added, the amounts would still be less than those of diesel fuel. This is because free radicals, which regulate the oxidation of O and H molecules, are diminished by both antioxidants. By raising the IP, it can make up for it by lowering the smoke. Smoke emissions are reduced as a result of the improved homogeneous mixture produced by the higher injection pressure.
The investigations mentioned above verified that a 20% mixed blend would improve engine performance, and the only drawback of this blend was increased NOx emissions. The inclusion of BHT and PPDA can regulate this. The appropriate combination for an engine operating at constant pressure is determined by further analytical validation of the data.
Validating the experimental results with literature survey
The experimental values are compared to the available literature survey in Table 10. The table indicates that blend 1 exhibits a higher sBTE and NOx due to the calorific value and inbuilt oxygen, which facilitates the release of more heat and the maximum adiabatic temperature rise during combustion. However, blend 1 exhibits a reduced level of HC, CO, and smoke emissions as a result of the fuel’s decreased viscosity and energy content, which results in the complete combustion of the air and fuel mixture. The engine was subjected to testing in order to reduce the NOx from blend 1. The antioxidants PPDA and BHT were introduced. The results showed that the PPDA and BHT assimilate the free radicals in the combustion chamber, resulting in a reduction in NOx emissions and a reduction in BTE. Increasing the injection pressure from 300 to 600 bar enhanced the engine’s performance and led to an increase in BTE under load conditions. The 600-bar emission was still lower than that of diesel fuel. The blend combination’s lower emissions, compared to previous work, are impacting the engine’s performance.
Statistical analysis
Taguchi optimization
The optimization process of statistical analysis entails gathering, organizing, analysing, interpreting, and processing data. While there are other approaches to system data analysis, Taguchi optimization is the most straightforward and reliable. Taguchi optimization is useful in determining the best set of input parameters to decrease system performance and maximize it41. The orthogonal array, which combines a number of levels and factors with a minimum number of runs, provides the experimental design. Three variables—blend, load, and IP—with four distinct load levels—25%, 50%, 75%, and 100%—have been selected for this investigation. Table 3 displays the L16 orthogonal array that was recommended by the Taguchi optimization.
Taguchi optimization results are displayed as a signal-to-noise curve, where the variability of operation is the noise and the mean performance is the signal. The engine’s signal-to-noise ratio must be maintained for reliable and steady functioning. Two outcome categories—bigger is better and smaller is better, for example—for BTE and CO2, the bigger is preferable; however, for BSFC, UHC, CO, smoke, and NOxemissions, the smaller is preferable42.
Figure 10 displays the Taguchi optimization findings. It is evident from BTE that all of the blends function well as IP is increased. It is said that as IP increases, droplets will get finer and more turbulent. As a result, the system’s performance improved. Antioxidants were added, but the engine’s performance remained unaffected because the fuel’s intrinsic oxygen content and calorific value would improve combustion and raise the BTE.
The changes in BSFC had the opposite effect on the rise of IP. Fresh air entering the chamber would increase due to the preceding cycle heat release rate raising the cylinder temperature. This would result in a decrease in density and volumetric efficiency at 600 bar injection pressure. The signal-to-noise ratio curve’s mean value is where B1 and B2 are located.
Figure 10 displays the UHC findings for blend combinations. Although the UHC emission increased somewhat in response to the increase in IP, the results were still relatively close to the mean values, and B1 and B2 displayed higher HC emissions than B1. This is because the antioxidants draw in OH radicals, yet the values are higher than the average UHC emission value. The natural oxygen in the blend would provide enough oxygen to meet the needs of a chemical reaction.
As the load grew, more CO emissions were produced. Fuel richness developed in the combustion chamber as a result of injecting more fuel when the load increased. When compared to other mixes, the B1 yields superior results. IP would yield a better result at 600 bars. Fuel richness causes CO emissions, mainly in the vicinity of the injector nozzle. The above cause is overcome by the latent heat vaporization of the fuel, which also lengthens the igniting delay period. The gasoline blend’s reduced aromatic content would facilitate the chemical reaction. Although there was a performance lag in this case, the 450 bar IP values are still quite close to the mean.
When the cycle period for complete combustion is shortened due to an increase in engine load, the soot particles resulting from incomplete combustion of the fuel mixture and air seem to have larger values. The fuel attribute, which guarantees a uniform combination of fuel and air, can help achieve this. Because the antioxidant mixes absorb free radicals and generate an increasing trend, they would enhance smoke emissions.
The rise in IP and load is counteracted by NOx emissions. Higher IP caused the fuel blend’s turbulent behaviour to become more uniform, which in turn caused the oxygen in the fuel and surrounding air to react with the nitrogen to increase NOxemissions43. Because the chemical reaction occurs at a greater temperature than in B1, B2, and B3, it causes more NOx emissions; however, the antioxidants have already begun to function at a lower temperature. Nitrogen now reacts with the extra oxygen in the combustion chamber. The excess oxygen present in the combustion chamber now reacts with nitrogen.
The engine’s efficiency is demonstrated by its CO2 emissions. The higher CO2 levels indicate improved engine performance when there is an input charge. The CO2 findings in this instance were chosen because they were greater, which is preferable for Taguchi optimization. The Blends fared well as their IP increased. Because the IP speeds up the chemical process from the ignition delay period to the end of combustion, it performs better at 600 bar. The system generates extra greenhouse gases as a result; however, these can be managed by after-treatment equipment. Rather than modifying fuel and engines, we might choose to reduce CO2 emissions from fuel and engines, which impacts the rise in UHC, CO, and smoke emissions. The Table 11 shows all of the parameter values that have been optimized.
SWOT analysis
SWOT analysis is a strategic method used to evaluate an individual or corporate organization. The fundamental elements of SWOT analysis include strengths, weaknesses, opportunities, and threats. The outcome is contingent upon two factors: internal and external. Strengths and weaknesses are categorized as internal elements, while opportunities and threats are classified as external factors. The strengths and opportunities are advantageous for the implementation of a new project; however, the weaknesses and threats have become detrimental, necessitating the necessity to rectify the project44. The SWOT analysis is a valuable tool for assessing the strengths and weaknesses of the current study. The current study utilized SWOT analysis to assess the potential risks and benefits. An analysis of SWOT is essential for grasping both internal and external elements, refining strategic choices, boosting competitive edge, and facilitating forward-thinking planning. The Fig. 11 shows the SWOT analysis of current study.
Strength
The expansion of the transportation industry is indicative of a rise in the utilization of fossil fuels. The substitution of fossil fuels diminishes reliance on fossil fuels. As part of this ongoing experiment, 20% of the diesel fuel was substituted with PO and LGO. The same characteristics of mixtures of PO and LGO would ensure energy stability for transportation, and they can be utilized in the engine without requiring any alterations. An additional benefit of utilizing combinations of PO and LGO is the mitigation of secondary procedures, which also contributes to stability. This leads to a more sustainable and efficient fuel alternative for transportation. Moreover, the fuels are sustainable and do no harm to the environment. In summary, using a combination of PO and LGO as a substitute for diesel fuel shows great potential for decreasing dependence on fossil fuels and mitigating environmental harm. This study emphasizes the possibility of a more environmentally friendly energy source in the transportation industry.
Weakness
Based on the experimental study, the combination of PO and LGO blends results in higher levels of NOx emissions compared to CO2 emissions, even when antioxidants are included. This is because a higher IP is crucial in facilitating the completion of the combustion process. Full combustion would result in the production of only water and CO2 emissions. Furthermore, the latent heat vaporization of the fuel ensures that the air–fuel combination becomes uniform. In addition, the presence of oxygen in the PO and LGO fulfils the necessary conditions for oxygen throughout the process of combustion. It should be emphasized that the availability of PO and LGO is limited due to the tiny supply chain involved in their production from raw materials. Furthermore, these oils are only manufactured for aromatherapy and medical applications. Introducing new biofuel legislation could improve crop productivity, oil extraction, and storage, perhaps addressing the aforementioned issues.
Opportunities
Additional guidance is available regarding the incorporation of biofuel in the transportation industry. The increased crop yield, production, and potency of the biofuel will create employment opportunities for graduates and ensure long-term, environmentally friendly economic development. Optimizing land utilization would boost agricultural productivity. The greater abundance of feedstocks would diminish reliance on fossil fuels. Global forestation has been enhanced due to the extraction of pine oil from pine trees. This has the potential to decrease the generated CO2 emissions from transportation. In summary, the incorporation of biofuel in the transportation industry not only tackles environmental issues but also has the capacity to generate economic prospects and foster sustainable growth. To decrease our dependence on fossil fuels and minimize carbon emissions, we should prioritize the development of efficient extraction and storage techniques while also diversifying the range of feedstocks utilized.
Threats
Introducing a blend of diesel fuel into the combustion chamber would result in a higher quantity of CO2 emissions. The technique requires a sophisticated post-treatment unit to decrease the carbon content of the exhaust gas. Compared to fossil fuels, the production cost of biofuel was greater due to the scarcity of feed sources. The oil extraction company is only focused on developing oils with supple properties for use in fragrances and medicinal products, rather than for use as engine feedstock. The government’s newly implemented strategy on the production of PO and LGO for energy purposes has the potential to resolve this issue. The government’s recent policy regarding the production of PO and LGO for energy purposes has the potential to enhance the supply of raw materials for biofuels, hence potentially lowering production expenses. In addition, allocating resources towards research and development to enhance extraction techniques could contribute to the long-term cost-effectiveness of biofuels.
Assessing sustainability
Sustainability refers to the ability to fulfil present requirements without jeopardizing the ability of future generations to fulfil their own needs. Sustainability operates by considering environmental, social, and economic concerns, which correspond to the three pillars of sustainability45. The internal combustion engine (IC engine) is of utmost importance in the transportation sector. Continuous advancements are being made to enhance the IC engine’s efficiency and minimize emissions. Despite the introduction of battery-powered vehicles and fuel cell operated vehicles, internal combustion (IC) engines will remain a prominent force in the transportation industry for the foreseeable future. Due to their lower cost, higher energy density, and faster refuelling times, IC engines have several advantages over other alternatives. However, the depletion of fossil fuel resources and environmental concerns may eventually prompt a transition towards more sustainable alternative fuels for IC engine .
The alternative fuel has to be a sustainable fuel for both present and future generations due to its potential to significantly decrease greenhouse gas emissions, conserve biodiversity, encourage sustainable land utilization, aid in water management, create employment opportunities, ensure energy security, decrease reliance on fossil fuels, and offer a new renewable energy option for the country. Considering all the aforementioned aspects, the current investigation was intricate. However, it was imperative to achieve stability and ensure continuous environmental, social, and economic stability. The Pugh matrix was employed as a decision-making tool to assess the sustainability of the file that was mentioned earlier. In 1960, Stuart Pugh discovered the Pugh matrix. This tool is extensively utilized in engineering, design, and other disciplines where decision-making is intricate and necessitates a systematic approach.
Depending on their level of significance, the technique uses a numerical scale that ranges from -2W to + 2W. The technique enables the simultaneous evaluation of several criteria and offers a quantitative method for comparing various solutions46. The procedure employed for the Pugh matrix is illustrated in Fig. 12. The initial stage involves determining the specific criteria that will be used to make the decision. Four factors have been selected, namely environmental impact, engine performance, engine emissions, and fuel cost. The aforementioned elements are inputted into the Pugh matrix for all types of fuel and their respective combinations of IP. The datum point was designated as zero for all the elements, with negative values denoting a disadvantage, positive values denoting an advantage, and zero values denoting neutrality.
The subsequent stage involves determining the weights for each fuel based on the conducted experiments, taking into account all the above parameters. The following step entails computing the comprehensive score for each fuel, taking into account these weights. The first rank denotes the optimal and enduring fuel choice for both present and future requirements. The second rank denotes the subsequent alternative that is considered more preferable47. Figure 13 depicts the sustainable factors, with higher values indicating advantageous possibilities and lower values indicating less sustainable options. Table 12 displays the design in the form of a Pugh matrix, encompassing all criteria. The allocation of weight to each criterion is discussed in the following sections.
Criteria 1: environment
Urban areas have higher levels of pollution from automobiles compared to rural areas. Based on the empirical data, it was determined that the engine generates emissions of UHC, CO, CO2, NOx, and smoke, which contribute to air pollution, have adverse effects on human health, and disrupt ecosystems48. Based on the data, it was determined that diesel fuel generates higher emissions. As a result, a weight of -2W was assigned to it. On the other hand, both LGO and PO contain oxygen, which aids in complete combustion and reduces emissions of UHC, CO, and smoke. However, these fuels have the drawback of producing higher NOx emissions, which led to a weight of -1W being assigned to them. The B2 and B3 inject at a pressure of 300 bar, resulting in increased emissions due to the presence of the anti-oxidants BHT and PPDA. Therefore, a weight of -2W was allocated. Increasing the injection pressure (IP) sped up the chemical reaction. This led to big drops in the emissions of UHC (unburned hydrocarbon), CO (carbon monoxide), and smoke. As a result, injection pressures of 450 bar and 600 bar produced values of -1 and 0, respectively. The increased amount of IP facilitates the attainment of homogeneity in the mixture and enhances the process of complete combustion. Consequently, the total efficiency of the combustion process was greatly enhanced.
Criteria 2: Performance of the engine
Brake thermal efficiency
The BHT shows how efficiently an engine converts the supplied fuel energy into useful work. Greater values indicate superior engine performance. Due to the decreased BTE produced by the diesel fuel in comparison to blends, a weight of zero was assigned. A weight of + 1W was assigned to B1 due to its stronger BTE production compared to diesel fuel. The higher calorific value and reduced viscosity improve combustion efficiency. The + 2W designation was allocated to B2 and B3 blends due to the influential impact of fuel characteristics and greater IP on enhancing engine performance.
BSFC
The BSFC is a quantitative measure that evaluates how much fuel an engine uses to produce one kW of brake power. An engine operates more efficiently when its BSFC value is lower. In the experimental analysis, the consumption of diesel fuel and B1 was higher in comparison to B2 and B3. Therefore, a weight with a value of -2W was given. Both blends B2 and B3 exhibit equivalent fuel consumption rates when injected at varying pressures. The fuel’s increased IP and lower latent heat of vaporization facilitate its entry into the combustion chamber, where it can readily evaporate and atomize.
Criteria 3: engine emission
UHC emission
The UHC was generated due to a lack of oxygen in the combustion chamber. This primarily occurs in areas of the combustion chamber where there is a high concentration of fuel and air. The larger amount of UHC emissions produced by diesel fuel is due to the rich air–fuel mixture near the injector nozzle. Therefore, a value of -2W was assigned. Whereas blend B1 exhibits reduced UHC emissions due to the oxygen content in the LGO and PO, the blended fuel adequately provides oxygen during the combustion process. Introducing the antioxidants PPDA and BHT would effectively neutralize the free radicals present in the combustion chamber. This would result in increased emissions of UHC; hence, + 1W was provided for both the 300 bar IP and the 450 bar IP. The injection pressure of 600 bars reduces the production of UHC emissions by causing the gasoline to flow more turbulently. As a result, a + 2W rating was assigned for this scenario.
CO emission
Incomplete combustion results in elevated levels of carbon monoxide (CO) emissions. Elevated levels of carbon monoxide emissions result in significant air pollution and pose substantial health risks to the surrounding area. Due to the greater carbon monoxide (CO) emissions produced by diesel fuel, a penalty of -2W was assigned. The use of oxygen in blend B1 results in significantly reduced levels of CO emissions. A total of + 2W was provided. Injecting blends B2 and B3 at 300 bars resulted in an increase in CO emissions due to the presence of antioxidants in the blend, leading to an assignment of + 1W weight. The elevation of the IP from 300 to 450 bar and 600 bar results in a greater quantity of carbon monoxide (CO) being emitted. Therefore, 0W and -1W were classified as 450 bar and 600 bar, respectively, due to the influence of the temperature from the preceding cycle on combustion.
CO 2 emission
Research has indicated that increased CO2 emissions are a result of the complete burning of fuel. While this is beneficial for engine performance, it is detrimental to environmental considerations. Carbon dioxide (CO2) emissions are widely recognized as a significant factor in driving climate change. The testing results demonstrated a significant decrease in CO2 emissions for diesel fuel, resulting in a + 2W rating. However, the presence of oxygen in LGO and PO enhances combustion, leading to a -1W rating. The weights of B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar, B3 + 450 bar, and B3 + 600 bar are -1, -2, -1, -1, -2, -1, and -1, respectively.
NO x emission
NOxemissions pose a significant threat to environmental well-being. It has an impact on both human health and ecosystems49. The diesel fuel exhibited lower NOx emissions in comparison to the blends, resulting in a score of + 1. However, as a result of the highest temperature reached during complete combustion, the mixture releases a greater amount of NOx, leading to a score of -2W. The weights for B2 and B3 blend injection at 300 bar, 450 bar, and 600 bar are + 2, + 1, and 0, respectively. PPDA and BHT in combination operate as antioxidants that eliminate free radicals during the initial stage of combustion, leading to a reduction in peak temperature. However, the rise in IP has a notable impact on combustion, resulting in an increase in NOx emissions. However, the NOx levels observed were considerably lower compared to those of diesel fuel and B1.
Smoke emission
The smoke was generated due to the process of incomplete combustion. Diesel fuel had higher smoke emissions due to inadequate oxygen, but blend B1 had lower smoke emissions due to its extended ignition delay period and inherent oxygen that ensures complete combustion. An increase in IP greatly reduces the smoke emissions caused by the BHT and PPDA in the chamber. The following values are assigned based on the experimental data: The values for diesel, B1, B2 + 300 bar, B2 + 450 bar, B2 + 600 bar, B3 + 300 bar, B3 + 450 bar, and B3 + 600 bar are -2, + 2, + 1, + 1, + 2, + 1, + 1, and + 2, respectively.
Criteria 4: Cost of the fuel
Currently, the process of extracting diesel fuel is firmly established in comparison to other fuel options. The processes of manufacturing, storing, and transporting diesel fuel, as well as the utilization of its products, are streamlined. Diesel fuel continues to be affordable and readily available to consumers. However, when it comes to alternative fuel, the processes of gathering raw materials, manufacturing, preserving, and distribution were still in the earliest phases of development. Therefore, a value of + 1 is assigned to diesel fuel, a weight of 0 is allocated to B1, and a value of -1 is assigned to B2 and B3 combinations due to the inclusion of PPDA and BHT.
Figure 14displays the cumulative values of sustainable evaluations for various fuels and their combinations. The B2 and B3 achieved the highest score while injecting at a pressure of 600 bars. Therefore, B2 + 600 bar and B3 + 600 bar were deemed more viable for both current and future requirements. In summary, the sustainable assessments suggest that the B2 and B3 combinations at 600 bars are the most feasible choices for fulfilling both present and future fuel requirements. This implies that additional progress and financial commitment to these alternative fuel sources could result in enhanced long-term sustainability.
Conclusion
The investigation confirms the effectiveness of a single-cylinder engine using mixtures of PO and LGO fuels at varying injection pressures, in addition to BHT and PPDA. The results are verified using a range of methodologies, including experimental, statistical, SWOT, and sustainability assessments. Empirical evidence consistently demonstrates that the utilization of blends yields a favourable impact on both performance and emissions.
-
The engine’s performance was marginally enhanced for all fuel blends in comparison to diesel fuel. The highest values of 30.96% and 30.95% were obtained for B2 and B3 mixtures, respectively, when injecting at an injection pressure of 600 bar. The blend’s comparable calorific value and latent heat vaporization contribute to enhanced combustion.
-
The blend’s inherent oxygen and decreased aromatic components in the PO and LGO resulted in significant reductions in UHC and CO emissions. The B2 + 600 bars experienced a maximum reduction of 42.86% of UHC, whereas the B2 + 300 bars had a maximum reduction of 50.10% of CO.
-
The NOx emissions for B1 increased by around 1229 ppm, while for diesel is 1147 ppm. By incorporating PPDA and BHT into the blend, the maximum reduction 1021 ppm and 1012 ppm was achieved for 300 bar injection pressure, respectively. The rise in IP would increase NOx emissions, but the levels are still significantly lower compared to diesel and B1.
-
The smoke output was decreased through the utilization of LGO and PO mixes. The B1 blend emitted 51.4% smoke, while diesel emitted 69.7%. Incorporating PPDA and BHT would result in a modest elevation in smoke levels; however, these values would still be lower than those observed in diesel and B1.
-
The statistical research proved helpful in determining the optimal engine operating state to achieve enhanced performance while minimizing emissions. The findings of the Taguchi analysis indicated that the B3 fuel had superior performance across all the independent operations.
-
The SWOT analysis demonstrates that the blend exhibits greater advantages in terms of strength and opportunity in comparison to its weaknesses and threats. The emission of NOx and CO2 can be decreased by utilizing after treatment devices in the exhaust pipe.
-
B2 + 600 bar and B3 + 600 bar achieved the highest scores in the sustainability research. Therefore, these fuel mixtures meet both current and future requirements.
Utilizing the blends B2 and B3 at an injection pressure of 600 bar resulted in maximum engine performance and minimal emissions. Furthermore, these fuels offer a durable solution for ensuring energy security in the long run. In summary, the study indicates that using blends B2 and B3 at an injection pressure of 600 bar can efficiently tackle the problems related to emissions and engine performance. These fuel mixtures present a hopeful resolution for attaining long-term energy security and sustainability. Therefore, it can be inferred that B2 and B3 were viable and effective fuel options for both present and future utilization in terms of their performance and environmental consequences.
Future work
In the present work LGO and Pine oil acted as energy source for engine. The LGO oil may replaced with Orange Peel Oil because of the India is securing second place in the production of orange and the waste peels are mostly dumped in the yard. Hence the peel oil may serve as waste to energy or repurposing. The extracted orange peel oil was blend with various amount and the significant will be studied in the future.
Data Availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AT:
-
A-tocopherol acetate (AT)
- BHA:
-
2, 6-Di-tert-butyl-4-methylphenol
- BHT:
-
2(3)-Tert-butyl-4-methoxy phenol
- BSFC:
-
Brake specific Fuel Consumption
- BTE:
-
Brake Thermal Efficiency
- CB:
-
Calophyllum inophyllum
- CMO:
-
Camphor oil
- CO:
-
Carbon Monoxide
- CO2 :
-
Carbon-di-oxide
- CWO:
-
Cedarwood oil
- JA:
-
Jamun biodiesel
- LA:
-
L-ascorbic acid
- LGO:
-
Lemon Grass Oil/citronella biofuel
- LPO:
-
Lemon Peel Oil
- MBEBP:
-
2,20-Methylenebis (4-methyl-6-tert-butyphenol)
- NOx :
-
Oxides of Nitrogen
- PME:
-
Palm biodiesel
- PO:
-
Pine Oil
- PPDA:
-
P-phenylenediamine
- SN:
-
Soapnut Oil
- UHC:
-
Unburned Hydrocarbon
- WGO:
-
Wintergreen Oil
References
Dhairiyasamy, R., Gabiriel, D., Bunpheng, W., & Choon kit, C. (2024). Optimization of ethanol and methanol blends for enhanced performance and reduced emissions in BS-VI compliant motorbike engines. In Multiscale and Multidisciplinary Modeling, Experiments and Design (Vol. 8, Issue 1). Springer Science and Business Media LLC. https://doi.org/10.1007/s41939-024-00655-5
Kanthavelkumaran, N.; Mathu Kumar, S.; Iyyappan, S.; Suthan Raja Prasad, N. N. Utilizing manilkara zapota seed oil for biodiesel production and conducting an investigation into its properties and characteristics. Global Nest Journal. https://doi.org/10.30955/gnj.005387 (2024)
Ramalingam, K. et al. An Assessment on Production and Engine Characterization of a Novel Environment-Friendly Fuel. Fuel 279, 118558. https://doi.org/10.1016/J.FUEL.2020.118558 (2020).
Vallinayagam, R. et al. Combustion Performance and Emission Characteristics Study of Pine Oil in a Diesel Engine. Energy https://doi.org/10.1016/j.energy.2013.05.061 (2013).
Panneerselvam, N. et al. Effect on Direct Injection Naturally Aspirated Diesel Engine Characteristics Fuelled by Pine Oil, Ceiba Pentandra Methyl Ester Compared with Diesel. Transp Res D Transp Environ https://doi.org/10.1016/j.trd.2016.08.023 (2016).
Madhu babu, K.; Sivanagaraju, N.; Nagendra babu, R. An experimental investigation on characteristics of a diesel engine with pine oil blends and by adding decanol as an additive. Mater Today Proc https://doi.org/10.1016/J.MATPR.2023.04.401. (2023)
Kumar, P. et al. Effect of Synthesized Lemongrass Biodiesel on the Performance and Emission Characteristics of a CI Engine. Sustainable Energy Technologies and Assessments 57, 103221. https://doi.org/10.1016/J.SETA.2023.103221 (2023).
Ashok, B. et al. A Novel Study on the Effect Lemon Peel Oil as a Fuel in CRDI Engine at Various Injection Strategies. Energy Convers Manag https://doi.org/10.1016/j.enconman.2018.07.037 (2018).
Yoon, S. K., Ge, J. C. & Choi, N. J. Influence of Fuel Injection Pressure on the Emissions Characteristics and Engine Performance in a CRDI Diesel Engine Fueled with Palm Biodiesel Blends. Energies (Basel) https://doi.org/10.3390/en12203837 (2019).
Fayad, M. A.; Omran, S. H.; Ali, M. H.; Al Salihi, H. A. Emission Characteristics and Engine Performance from Castor Oil Methyl Ester Blends in Diesel Engine under Various Injection Pressures. Journal of Advanced Research in Fluid Mechanics and Thermal Sciences https://doi.org/10.37934/arfmts.94.1.4559 (2022)
Rajendran, S. & Ganesan, P. Experimental investigations of diesel engine emissions and combustion behaviour using addition of antioxidant additives to jamun biodiesel blend. Fuel https://doi.org/10.1016/j.fuel.2020.119157 (2021).
Ileri, E. & Koçar, G. Effects of Antioxidant Additives on Engine Performance and Exhaust Emissions of a Diesel Engine Fueled with Canola Oil Methyl Ester-Diesel Blend. Energy Convers Manag 76, 145–154. https://doi.org/10.1016/J.ENCONMAN.2013.07.037 (2013).
Rizwanul Fattah, I. M., Masjuki, H. H., Kalam, M. A., Mofijur, M. & Abedin, M. J. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers Manag 79, 265–272. https://doi.org/10.1016/J.ENCONMAN.2013.12.024 (2014).
Ryu, S. P., Yoon, K. S., Park, C. W. & Lee, I. S. Study on the optimization of ci engine simulation using taguchi method. Transactions of the Korean Society of Automotive Engineers https://doi.org/10.7467/KSAE.2022.30.3.231 (2022).
Ayhan, V. et al. Optimization of the factors affecting performance and emissions in a diesel engine using biodiesel and EGR with taguchi method. Fuel https://doi.org/10.1016/j.fuel.2019.116371 (2020).
Vural, E., Özer, S., Özel, S. & Binici, M. Analyzing the Effects of Hexane and Water Blended Diesel Fuels on Emissions and Performance in a Ceramic-Coated Diesel Engine by Taguchi Optimization Method. Fuel 344, 128105. https://doi.org/10.1016/J.FUEL.2023.128105 (2023).
Nguyen, V. N. et al. Performance and Emission Characteristics of Diesel Engines Running on Gaseous Fuels in Dual-Fuel Mode. International Journal of Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2023.09.130 (2024).
Huang, H.; Huang, R.; Guo, X.; Pan, M.; Teng, W.; Chen, Y.; Li, Z. Effects of pine oil additive and pilot injection strategies on energy distribution, combustion and emissions in a diesel engine at low-load condition. Appl Energy https://doi.org/10.1016/j.apenergy.2019.05.028 (2019)
Asokan, M. A. et al. Emission and performance behaviour of blends of diesel/lemongrass oil in di diesel engine. Materials Today: Proceedings https://doi.org/10.1016/j.matpr.2021.03.038 (2021).
EdwinGeo, V.; Fol, G.; Aloui, F.; Thiyagarajan, S.; Jerome Stanley, M.; Sonthalia, A.; Brindhadevi, K.; Saravanan, C. G. Experimental Analysis to Reduce CO2 and Other Emissions of CRDI CI Engine Using Low Viscous Biofuels. Fuel. https://doi.org/10.1016/j.fuel.2020.118829. (2021)
Ashok, B.; Thundil Karuppa Raj, R.; Nanthagopal, K.; Krishnan, R.; Subbarao, R. Lemon Peel Oil – A Novel Renewable Alternative Energy Source for Diesel Engine. Energy Convers Manag https://doi.org/10.1016/j.enconman.2017.02.049 (2017)
Venkatesan, V. & Nallusamy, N. Pine oil-soapnut oil methyl ester blends a hybrid biofuel approach to completely eliminate the use of diesel in a twin cylinder off-road tractor diesel engine. Fuel https://doi.org/10.1016/j.fuel.2019.116500 (2020).
Kotaiah, K.; Periyasamy, P.; Prabhahar, M. Performance and emission characteristics of small agricultural diesel engine using lemongrass oil and its diesel blends. In Materials Today: Proceedings https://doi.org/10.1016/j.matpr.2020.05.773 (2020)
Rashedul, H. K. et al. Effect of antioxidant on the oxidation stability and combustion-performance-emission characteristics of a diesel engine fueled with diesel-biodiesel blend. Energy Convers Manag https://doi.org/10.1016/j.enconman.2015.10.024 (2015).
Rajendran, S. Effect of antioxidant additives on oxides of nitrogen (NOx) emission reduction from annona biodiesel operated diesel engine. Renew Energy https://doi.org/10.1016/j.renene.2019.10.104 (2020).
Joshua, P. J. T., Kandasamy, A., Venkatesan, E. P. & Saleel, C. A. Experimental Study on Sustainability Involving the Pugh Matrix on Emission Values of High-Temperature Air in the Premixed Charged Compression Ignition Engine. ACS Omega 8(44), 41243–41257. https://doi.org/10.1021/acsomega.3c04694 (2023).
Dhinesh, B.; Isaac JoshuaRamesh Lalvani, J.; Parthasarathy, M.; Annamalai, K. An assessment on performance, emission and combustion characteristics of single cylinder diesel engine powered by cymbopogon flexuosus biofuel. Energy Convers Manag. https://doi.org/10.1016/j.enconman.2016.03.049 (2016)
Natarajan, A., Kandasamy, A., Perumal Venkatesan, E. & Saleel, C. A. Experimental Investigation on the Effect of Graphene Oxide in Higher Alcohol Blends and Optimization of Injection Timing Using an ANN Method. ACS Omega 8(44), 41339–41355. https://doi.org/10.1021/acsomega.3c04895 (2023).
Kumar, A. N.; Brahma Raju, K.; Srinivas Kishore, P.; Narayana, K. Some Experimental Studies on Effect of Exhaust-Gas Recirculation on Performance and Emission Characteristics of a Compression-Ignition Engine Fuelled with Diesel and Lemon-Peel Oil Blends. In Materials Today: Proceedings https://doi.org/10.1016/j.matpr.2017.12.22 (2018)
Backiyaraj, A., Parthasarathy, M., Murugu Nachippan, N., Senthilkumar, P. B. & Kumaran, T. Influence of nano AL2O3 on compression ignition engine characteristics fuelled with mahua biodiesel. Mater Today Proc https://doi.org/10.1016/j.matpr.2022.09.210 (2023).
Dubey, A.; Prasad, R. S.; Singh, J. K. An analytical and economical assessment of the waste cooking oil based biodiesel using optimized conditions on the process variables. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects https://doi.org/10.1080/15567036.2020.1839600 (2020)
Dhahad, H. A. & Fayad, M. A. Role of Different Antioxidants Additions to Renewable Fuels on NOX Emissions Reduction and Smoke Number in Direct Injection Diesel Engine. Fuel 279, 118384. https://doi.org/10.1016/j.fuel.2020.118384 (2020).
Dubey, A., Prasad, R. S., Kumar Singh, J. & Nayyar, A. Optimization of Diesel Engine Performance and Emissions with Biodiesel-Diesel Blends and EGR Using Response Surface Methodology (RSM). Clean Eng Technol 8, 100509. https://doi.org/10.1016/j.clet.2022.100509 (2022).
Dubey, A., Prasad, R. S., Singh, J. K. & Nayyar, A. Response Surface Methodology (RSM) Based Experimental and Model Analysis of Diethyl Ether-Biodiesel-Diesel Blends with Exhaust Gas Recirculation (EGR) on Stationary Diesel Engine. Pet Sci Technol 41(12), 1272–1291. https://doi.org/10.1080/10916466.2022.2092136 (2023).
John B. Heywood. John B. Heywood - Internal Combustion Engine Fundamentals-McGraw-Hill Education (2018); 2012.
Kanthavelkumaran, N.; Seenikannan, P.; Raj, R. R.; Kumaresh, E. Improving the Environmental Performance of Di Diesel Engine Using Environment Support Fuel. Journal of the Balkan Tribological Association 23 (2). (2017)
Nanthagopal, K. et al. Experimental investigation on engine parameters variation in common rail direct injection engine fueled with biodiesel. Clean Technol Environ Policy https://doi.org/10.1007/s10098-019-01796-9 (2020).
Dubey, A., Prasad, R. S., Singh, J. K. & Nayyar, A. Combined Effects of Biodiesel − ULSD Blends and EGR on Performance and Emissions of Diesel Engine Using Response Surface Methodology (RSM). Energy Nexus 7, 100136. https://doi.org/10.1016/j.nexus.2022.100136 (2022).
Kanthavelkumaran, N., Seenikannan, P. & Kumaresh, E. Measuring and controlling the exhaust emission from di diesel engine using biofuel as an environment fuel. Measurement and Control (United Kingdom) https://doi.org/10.1177/0020294014565636 (2015).
Sathiyamoorthi, R. & Sankaranarayanan, G. Effect of Antioxidant Additives on the Performance and Emission Characteristics of a DICI Engine Using Neat Lemongrass Oil-Diesel Blend. Fuel 174, 89–96. https://doi.org/10.1016/j.fuel.2016.01.076 (2016).
Manigandan, S. et al. Influence of injection parameters on NOx emission from biodiesel powered diesel engine by taguchi technique. International Journal of Ambient Energy https://doi.org/10.1080/01430750.2018.1492447 (2020).
Mishra, S. S.; Mohapatra, T.; Sahoo, S. S.; Mishra, P. Overall performance investigation and optimization of a multi-fuel operated compression ignition engine using coupled Taguchi and grey relational analysis. ACS Omega https://doi.org/10.1021/acsomega.2c03566 (2022)
Kanthavelkumaran, N.; Mathu Kumar, S.; Iyyappan, S.; Suthan Raja Prasad, N. N. Characteristics and evolution of bioethanol from manila tamarind (pithecellobium dulce) leaf through fermentation. Global Nest Journal https://doi.org/10.30955/gnj.005634 (2024)
Dash, S. K. et al. Effect of injection pressure adjustment towards performance, emission and combustion analysis of optimal nahar methyl ester diesel blend powered agricultural diesel engine. Energy https://doi.org/10.1016/j.energy.2022.125831 (2023).
Ross, N. S.; Mia, M.; Anwar, S.; G, M.; Saleh, M.; Ahmad, S. A hybrid approach of cooling lubrication for sustainable and optimized machining of Ni-based industrial alloy. J Clean Prod https://doi.org/10.1016/j.jclepro.2021.128987. (2021)
Gürbüz, H.; Gönülaçar, Y. E. Experimental and Statistical Investigation of the Effects of MQL, Dry and Wet Machining on Machinability and Sustainability in Turning of AISI 4140 Steel. Proceedings of the Institution of Mechanical Engineers, Part E: Journal of Process Mechanical Engineering https://doi.org/10.1177/09544089221076243 (2022)
Kurpiela, S. & Teuteberg, F. Strategic Planning of Product-Service Systems: A Systematic Literature Review. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2022.130528 (2022).
Kanthavelkumaran, N.; Seenikannan, P.; Rethan Raj, R.; Kumaresh, E. Improvement in the Environment Health and Engine Performance on Diesel Engine Using Biofuel as a Substitute. Journal of the Chinese Society of Mechanical Engineers, Transactions of the Chinese Institute of Engineers, Series C/Chung-Kuo Chi Hsueh Kung Ch’eng Hsuebo Pao 42 (2). (2021)
Kanthavelkumaran, N.; Jayaram, R. S.; Brabin Nivas, M. L.; Prasanth, P. V. Bioethanol (environment support fuel) production and optimization from pineapple peel and banana peel. Global Nest Journal https://doi.org/10.30955/gnj.005251 (2023)
Acknowledgements
The authors extend their appreciation to University Higher Education Fund for funding this research work under Research Support Program for Central labs at King Khalid University through the project number CL/CO/A/1.
Author information
Authors and Affiliations
Contributions
JP Conceptualization, Data curation, Investigation, Methodology, Writing—original draft. A.K- Supervision, Validation. , E.P.V, S.S, L.Z- Validation, Methodology, —Investigation, C.A.S, N.H Formal analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Padmanaban, J., Kandasamy, A., Venkatesan, E.P. et al. Sustainability study and SWOT analysis of mixed biofuel blends in engine at various injection pressure analysed by experimentally and statistically. Sci Rep 14, 31574 (2024). https://doi.org/10.1038/s41598-024-79073-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79073-z
Keywords
This article is cited by
-
Sustainable RCCI engine operation with an ANN based novel tri-fuel approach
Scientific Reports (2025)
-
ML techniques increasing the power factor of a compression ignition engine that is powered by Annona biodiesel using SATACOM
Scientific Reports (2025)
-
Machine learning and experimental emission assessment in high temperature air premixed charged compression ignition engines using the Pugh matrix
Scientific Reports (2025)
-
Combustion and emission analysis of hydrogen–microalgae biodiesel dual-fuel CI engine with cold EGR
Journal of Thermal Analysis and Calorimetry (2025)