Abstract
Measuring the quality of life of an animal in a production system is difficult, time-consuming, and expensive. We tested the ability of both NIR and MIR spectroscopy, each combined with machine learning, to predict the prior exposure of pigs to long- and short-term life challenges when they are raised in an intensive system. Samples were obtained post-mortem from two locations in the prefrontal cortex. The analysis showed a clear separation between the gray and white matter from the prefrontal cortex with MIR spectroscopy. Exposure to long-term challenge was poorly predicted by the MIR or NIR spectra (< 45% correct classifications). By contrast, the correct classification of samples according to the exposure to a short-tern challenge before death was higher than 65%. These rates of classification, considering the complexity of the stimulus and the small sample size, support that vibrational spectroscopy could be used to assess the exposure to challenging events of an animal on post-mortem brain tissue.
Similar content being viewed by others
Introduction
During the last few decades, some segments of society, including consumers in general, have expressed concern about the quality of life that animals experience when they are used to produce our proteins1. The concept of a “life worth living” has been proposed by prominent animal welfare scientists to conceptualise the welfare of farmed animals2,3. A criticism of such an approach is that measuring the quality of life of animals raised in either extensive or intense systems is difficult, time-consuming, and expensive. Assessment of the quality of life relies on the frequent recording of many observations as well as the measurement of behavioural and physiological indicators, on every single animal4. In recent years, such assessment of complex systems has become more manageable with the advancement of digital technologies and mathematics, such as different types of sensors (e.g. biosensors, optical devices, radio frequency), machine learning (ML) and artificial intelligence (AI) algorithms in systems that are designed to collect and interpret extensive data as well as develop models that can be used in both research and in field applications4. But in general, the concept of a “life worth living” remains a theoretical construct.
The perception of life events and the resulting experience of an animal, that can range from positive to negative experience, is the product of the central processing of physiological and behavioural events. To complicate any assessment, it is highly probable that in non-human animals, as it is in humans, individual variability due to both past experiences and genetics can influence the experiential state5. In humans, the experiential state can be assessed qualitatively by asking a person how they feel. That is not possible in non-human animals and consequently, the experiential state is very difficult to measure in those animals. While, for example, biomarkers of other physiological or behavioural responses to stress have been validated, there are no biomarkers for the full range of experiential state in animals6. Even in humans, the peripheral indicators of experience are not well identified. A way around this problem would be a technique that could measure biochemical changes in brain regions that are involved in the mechanisms of perception and / or expression of an experiential state, such as emotion.
Among the several brain structures, such as the hippocampus and the amygdala, that are part of the “emotional brain”, in humans, the biochemistry and organisation of the prefrontal cortex (PFC) is impacted by the perception of life events, the processing of information, the formation of emotions, and decision-making7,8. For example, the prefrontal cortex undergoes biochemical and structural changes when human and non-human animals are exposed to stressors9. Another example is that two cell populations in the prefrontal cortex that respond to experiences of both positive and negative-valence differ in their connectivity, profile of gene expression, and their impact on behaviour, at least in mice10. An integrative measure of such changes could be a reliable indicator of the experiential status of an animal.
Interrogating the central nervous system of an animal during its life is not an easy task. Methodologies that are commonly used in humans to assess these changes, such as surface electrodes, functional magnetic resonance imaging (fMRI), and more recently functional near-infrared (fNIR) spectroscopy, are considered state-of- the art11. However, these methods are impractical for use in the field or in large animals such as those utilized by the meat industries12. These techniques have led to valuable insights in human neuroscience, revealing that exposure to either chronic or acute stressful events is associated with changes in the neurochemical balance, the anatomical structure, and the metabolic activity of the prefrontal cortex13. While some of these changes in the brain tissue, like neurotransmission, are short-lived, others, such as structural changes, are long-lasting13.
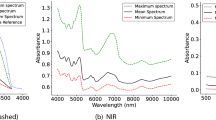
Infra-red spectroscopy is sometimes called vibrational spectroscopy because it is a way to measure the vibrations of atoms and their bonds. Stronger bonds vibrate at a higher frequency than weaker bonds, permitting the determination of the functional groups that are present in a sample. Techniques such as near-infrared (NIR) and mid-infrared (MIR) spectroscopy use light to measure molecular vibrations that are associated with changes in chemical bonds between atoms as well as variations in the structure of a sample14,15. Absorption bands in the NIR region (13,000–4,000 cm− 1) correspond to the so-called overtones and combinations of fundamental molecular vibrational modes of chemical components that are present in a sample15. The interpretation of NIR spectra can be challenging due to the presence of these broad and overlapping bands16. On the other hand, the peaks observed in the MIR region (4,000–400 cm− 1) represent the fundamental vibrational modes of the main molecules that are present in a sample17. A narrow band within the MIR region (1,800–900 cm− 1) is known as the fingerprint region because it provides a signal that is related to the unique fundamental vibrations of many molecules of biological and biochemical interest, including amide groups derived from protein, lipids and fatty acids, ester groups, and carbohydrates14. Therefore, spectroscopy in both the NIR and MIR regions could reflect structural changes that are induced by the experience that an animal has, either in the short- or long-term.
This study evaluated whether either or both NIR and MIR spectroscopy, combined with machine learning tools, could be harnessed to analyse samples obtained from two locations in the prefrontal cortex of pigs. We used pigs as a model because we had the opportunity to access brains that were collected as part of another experiment. The major aim was to test whether the spectra could identify differences in the brains of several groups of pigs that were raised in an intensive system and exposed to validated long-term and short-term stimuli that induced contrasting levels of stress in the pigs, and therefore different experiences18,19.
Results
Analysis and interpretation of NIR spectra
The first two principal components (PC) of the collected Fourier Transform NIR (FT-NIR) scans accounted for more than 85% of the total variance between the NIR spectra of the two brain locations analysed (i.e. anterior prefrontal cortex and the lateral region of the prefrontal cortex). PC1 and PC2 explained 82.4% and 3.1% of the total variance in the data set, respectively (Fig. 1).
The loadings derived from the PCA were investigated further as they provided with the relevant information (main frequencies or wavenumbers) that was used by an algorithm to separate the two brain locations (Fig. 2). The highest loadings in both PC1 and PC2 were observed at wavenumbers around 7,200 cm− 1 (O-H bonds), a region that is associated mainly with water, and around 5,400–5,800 cm− 1 (C-H, C-H2 and C-H3) a region that is associated mainly with lipids, fatty acids, and proteins15. A high negative loading at around 4,200 cm− 1 was observed in PC1 (C-H combination tones and C-C stretching combinations), and was likely associated with different chemical groups such as lipids, fatty acids, or amino acids15. In addition, other shifts were observed at wavenumbers associated with the water region (O-H) as well as opposite loadings in the absorbance values in the region around 5,300–5,340 cm− 1 15. The region around 5,300 cm− 1 is associated mainly with the second overtone of O-H stretching and C = O vibrations in water and other compounds that contain carbon and oxygen (e.g. lipids and fatty acids)20.
The brain samples were classified according to anatomical location, sex, exposure to and long-term challenge (by manipulation of the environmental conditions - see M&M)), and exposure to an acute stressor prior to death (Table 1). 78% of the brain samples from the lateral prefrontal cortex were correctly classified as females, and 70% of the same samples were correctly classified as males, a rate significantly better than chance (Chi2 for sex; p = 0.013). Brain samples obtained from the anterior prefrontal cortex were classified correctly 65% and 60% as female and male samples, which was no better than chance (Chi2 for sex; p = 0.48). The classification models correctly identified the environmental condition in fewer than 50% of the samples from either the anterior or the lateral prefrontal cortex (Chi2; p = 0.48). The samples obtained from the anterior prefrontal cortex were 58%, 60%, and 45% correctly classified as positive, control, or negative, respectively (Chi2; p = 0.02). While the raw percentages suggest that the models did better at identifying the brain samples from the pigs that had undergone the acute stimulus just prior to death than they did at identifying differences in brains due to long-tern environmental conditions, correctly identifying at least 56% of the brains correctly, from both the anterior and lateral regions of the prefrontal cortex, the rate of correct classification was no better than chance (Chi2; lateral PFC: p = 0.8, anterior PFC: p = 0.29).
Analysis and interpretation of MIR spectra
The first two PC’s derived from the MIR analysis of both gray and white brain matter samples explained more than 98% of the total variance, where PC1 and PC2 explained 93% and 5% of the variance, respectively. A clear separation between the gray and white matter samples that were sourced from the lateral prefrontal cortex (Fig. 3) was observed along PC2.
Principal component scores plot of pre-processed mid-infrared spectra of gray (green triangle) and white matter (blue square) from the lateral prefrontal cortex of pigs (n = 36). The red circle identified a reading from the buffer that the buffer the pig brains were stored in, given here as a control sample (red circle).
The analysis of the PCA loadings revealed that the highest loading in PC1 (98% variance) was observed in frequencies around 2,840–3,000 cm− 1 (Fig. 4). This region is associated with the presence of C-H2 and C-H3 groups related to lipids and fatty acids14. High loadings were also observed at frequencies around 1,600–1,760 cm− 1, a region related to the amide I band (protein or compounds containing nitrogen) and the C = O stretching bond of fatty acid ester21,22. The highest loadings in the region between 980 and 1,270 cm− 1 can be associated with different compounds such as carbohydrates, lipoproteins, and proteins14. The highest loadings in PC2 showed similar patterns, representing the mirror image of the loadings derived from the PC1. However, a shift in the region between 3,000 and 2,850 cm− 1 from those reported in PC1 was observed, mainly associated with fatty acids14.
Loading plots for the principal components, PC-1 (red line) and PC-2 (blue line) of the pre-processed mid-infrared (MIR) spectra obtained from MIR spectroscopy of the gray and white matter of the lateral prefrontal cortex of pigs. The pre-processing method consisted of detrend, standard normal variant (SNV), and second derivatives order (Savitzky-Golay) filtering with 11 moving windows.
The results from the LDA classification for the samples sourced from either gray or white brain tissues and analysed using MIR spectroscopy are shown in Table 2. The samples were correctly classified as female (correct rate of 62.5%) or male (correct rate of 61%) in the gray brain tissue no better than chance (Chi2; p = 0.35), while 57% and 61% of the samples were correctly classified as female and male in the white matter tissue, again no better than chance (Chi2; p = 0.52). The gray matter samples from pigs that had been exposed to the acute stimulus, or not, just prior to death were classified correctly 68% (for presence of the stimulus) and 65% (for absence of the stimulus) of the time, a rate no better than chance (Chi2; p = 0.11). The classification rate for the acute stimulus in the white matter was 74% and 70% for presence and absence, respectively, and significantly better than chance (Chi2; p = 0.02). For the exposure to the three environment conditions in the white brain tissue, the LDA classification rates were correct for 60% of the samples from pigs in the positive treatment, 44% for pigs in the negative treatment, and 50% for pigs in the control treatment, again a rate than was significantly better than chance (Chi2; p = 0.03). The rate of correct classification was lower than 45% for the samples from the gray matter tissue, and was no better than chance (Chi2; p = 0.65).
Discussion
Our exploratory study showed a clear separation between brain samples consisting of gray and white matter that had been collected from the prefrontal cortex of pigs and analysed using MIR spectroscopy as seen on the PCA score plot (Fig. 3). Information contained in the PCA loadings from the MIR spectra indicated that specific frequencies that are associated with biochemical and chemical compounds (e.g. amide groups, fatty acids, lipoproteins and lipids) contributed to the separation between these tissues. It is well known that the white matter is comprised of a large amount of myelin, as well as being rich in lipids and proteins32. By contrast, the gray matter is mainly comprised of cell bodies (neurons, glia, astrocytes) and very few myelinated axons23. Loadings around 2,840-3,000 cm− 1 are associated with lipids and proteins and those regions contribute to explain the observed separation between gray and white tissue14.
Our results from the LDA classification indicated that the MIR spectra captured sex-specific characteristics in the prefrontal cortex (with successful classification rates between 57 and 65%) and the NIRS spectra successfully classified samples in the lateral PFC on sex. In some cases, the analysis of the brain samples using the NIR spectra provided similar results as those obtained from the MIR spectra in both PCA and LDA. While the literature that is available on the classification of sex from MIR and NIR is inconclusive, several studies in human and laboratory animals have identified sexual dimorphism in brain microanatomy of the gray matter24. It has been reported that the volume of the gray matter in the prefrontal cortex is higher in female than in male individuals25 where there is more dendritic arborisation in female, but neuronal density and the number of neurons is higher in males25. Such sexual dimorphism in structure is known to be induced by exposure to sex steroids, especially during gestation, because they affect neurodevelopment and associated sex differences at the level of neurotransmitters25. Such mechanisms were at play in our study, because the immunocastration treatment started long after the critical phase in neurodevelopment of the animals, and the fact that immunocastration might not have been fully efficient by the time that the brains were collected. In humans, the volume of white matter is higher in men than in women and sex affects the microstructure of the white matter26. Such results can help to explain why we obtained better classification rates for the prediction of sex using the white brain tissue than we did with the gray brain tissue.
Exposure to the chronic environmental conditions was not well predicted by the MIR spectra (with classification rates below 45% in most tissues) but was classified successfully in the white matter. We obtained quite good classification rates for the classification of samples according to the presence or absence of the aversive stimulus a few hours before death, with rates higher than 65% in all cases, a rate that was statistically better than chance for the white matter tissue. The prediction (classification rates) could be related to the perception of challenge that was induced by the two protocols used in this experiment, even if both challenges had been validated to induce a stress response in pigs18,27. The prediction level is not as high as those previously reported for the identification of more simple biological outcomes like foodborne pathogens or bloodstains28,29. Our values for the predictability need to be considered in the light of the complexity of nervous tissues in the brain and the number of other factors that could affect the activity and the structural composition of the prefrontal cortex of each animal. Given the relative simplicity of discriminating spectra based on bloodstains or foodborne pathogens, we submit that a 65% success rate for the brain is encouraging for the future of MIR in assessing animal welfare. It must also be noted that the signal measured by NIR and MIR could have been attenuated because the brain tissue was fixed and fixatives such as formaldehyde can denature some molecules such as DNA and RNA30.
Our results also demonstrated that NIR and MIR spectroscopy have the potential to detect biochemical changes in the brain tissue of animals exposed to different events that elicit a stress response. These techniques could be used as post-mortem tools to assess some aspects of the quality of life of an animal, at least from acute negative experiences before slaughter. While, ultimately it would be more adequate to assess the quality of life of animals ante- rather than post-mortem, the use of vibrational spectroscopy could help to address some societal concerns about the welfare of farm animals, and provide feedback to producers and consumers about the life of those animals31.
While this exploratory study provided some encouraging results, it had several limitations, mainly because the brain samples were obtained opportunistically. The combination of vibrational spectroscopy (NIR and MIR) with machine learning tools (PCA, LDA) produced adequate classification results. However, the relatively small sample size that was used during the development of the models requires, ideally, a larger dataset to properly validate the models. Large-scale studies that would involve more animals, as well as the scanning of fresh tissue rather than fixed brains, should be considered and evaluated before we extend the use of this technique to a practical application (e.g. to evaluate the effect of stunning methods on animal stress)32. Furthermore, a validation using an independent set of animals, or a different experiment should be required to test the reliability of the method proposed.
Overall, our results show that information in the infrared spectra (e.g. biochemical and chemical signatures) can be used to identify changes in the brain tissue that are associated with different stimuli. The study has also shown that the IR spectra have the potential to detect structural change in tissue from the prefrontal cortex of the brain after the exposure of an animal to an acute stressor.
Methods
All animal procedures were conducted and reported in accordance the ARRIVE guidelines (https://arriveguidelines.org) and the Australian code for the care and use of animals for scientific purposes (National Health and Medical Research Council: Canberra, 2013; 8th edition’), approved by the CHM Alliance Pty Ltd Animal Ethics Committee (PP 151/22) and ratified by The University of Queensland Animal Ethics Committee.
Animals and housing
This experiment was conducted at Westbrook Research Facility in Queensland, Australia. A total of 36 Large White x Landrace x terminal pigs (PIC Australia, Grong Grong, NSW, Australia) were used in the experiment, beginning at 12 weeks of age. The experimental animals were part of a larger cohort of 144 pigs, all housed in six pens in a naturally ventilated shed, with 24 mixed-sex individuals in each pen (2.6 × 6.5 m) that had partially slatted and solid concrete flooring. Pens were sex balanced and randomly assigned to chronic treatments. The animals were fed ad libitum with access to feed (commercial grower diet) and water throughout the experiment. The male pigs were immunocastrated using two injections of the GnRH analogue (Improvac®, Zoetis Australia, Parkville, VIC, Australia) at 8 and 12 weeks of age. Health and welfare checks were conducted twice daily and, if required, pigs were treated with approved medications.
Experimental design and treatments
Animals were randomly assigned to one of two pens in each of the three environmental conditions. Six of the 24 pigs in each pen were selected randomly for the brain collection experiment from in the original 144 pigs in the larger experiment. The sub-groups were balanced for sex and body mass. Each condition exposed the animals to a validated paradigm that induced either positive, neutral, or negative long-term experiences18,19,33. There was no visual or physical contact between pigs from the different conditions. Exposure to the three conditions started when the pigs were 12 weeks old and lasted for four weeks. The conditions were:
-
1.
Positive: standard husbandry conditions with the addition of enrichment in the form of commercially available toys that were changed weekly18, the daily provision of hay in a hayrack18, and daily positive interaction from the stockperson, following a protocol similar to that described by other authors33;
-
2.
Neutral or Control: standard husbandry conditions.
-
3.
Negative: standard husbandry conditions with a weekly rotation of pen mates by moving the six experimental pigs to a new group of 18 pen mates27,34.
An hour before euthanasia and the collection of the brains at 16 weeks of age, the focal pigs from each pen were removed from their home pen and subjected to one of two additional acute aversive treatments:
-
1.
The application of a snout rope for five minutes (an acute aversive stimulus; +AC):
-
2.
Or, no additional handling or stressor (no aversive stimulus; -AC):
Brain collection
Each animal was removed from its pen and held by a trained operator. The animal was sedated with an injection of a mixture of Zoletil (4 mg.kg− 1, Virbac, Milperra, NSW, Australia) and Xylazine (2 mg.kg− 1, Randlab, Milperra, NSW, Australia) in the ear vein. Once unconscious, the animal was injected intravenously into a jugular vein with an overdose of pentobarbital (140 mg.kg− 1, Virbac, Milperra, NSW, Australia). A veterinarian confirmed death by the absence of cardiac activity. The head was then severed using a sharp knife, the skin on the top of the skull was removed, and the skull was opened using a bone saw (Hebu, Tek equipment, Preston, VIC, Australia). The dura mater was cut and removed using a scalpel and the brain was lifted from the skull and placed on a cutting board. A coronal slice of about 15 mm of the frontal lobes was cut and immersed in 10% paraformaldehyde in PBS within 8 to 15 min after euthanasia (Fig. 5). The segment containing the prefrontal cortex was kept in the fixative at 4 °C for 1 week and was transferred and stored in PBS at 4 °C until analysis. Any large blood vessels or pieces of dura mater or pia mater were removed from the brain before scanning. The NIR and MIR scans were performed approximately one month after the brains were collected.
Near infrared and mid infrared analysis
The NIR spectra of the brain samples were collected from two anatomical regions (see Fig. 5). The first scan was taken on the anterior prefrontal cortex across the two hemispheres, while the second scan was taken at the side of a block taken from the lateral prefrontal cortex of the right hemisphere. The NIR scans of the anterior PFC was obtained from 33 brains (out of 36) because three brains were damaged during collection, and the quality of the surface of the coronal sections was not good enough to allow scanning.
The NIR spectra of the two brain sections were obtained using a Fourier transform (FT) NIR Bruker Tango-R spectrophotometer (Bruker Optics GmbH, Ettlingen, Germany). An integrating sphere with a gold-coating (diffuse reflection) was used to collect the FT-NIR spectra. The spectral data were collected in reflectance mode and recorded using the OPUS© software (version 8.5, Bruker Optics GmbH, Ettlingen, Germany, https://www.bruker.com/en/products-and-solutions/infrared-and-raman/opus-spectroscopy-software.html) with 64 interferograms at a resolution of 4 cm− 1 in the wavenumber range between 11,550 and 3,950 cm− 1. Samples were scanned using a borosilicate cuvette that was cleaned between samples with 70% (v/v) water-ethanol and dried with laboratory Kimwipes® ( Acacia Ridge, QLD, Australia) between samples.
The MIR spectra of the brain samples were obtained using slice samples that were cut into 1 mm thick sections starting from the lateral part of the PCF of the right hemisphere. Sections that contained the white and gray matter were separated using a sterilized razor blade (Wilkinson Sword) and scanned separately. The MIR spectra were recorded using a Bruker Alpha instrument fitted with an attenuated total reflectance (ATR) platinum diamond, single reflection, module (Bruker Optics GmbH, Ettlingen, Germany). The spectra of the brain samples were recorded in the spectral region between 4,000 and 400 cm− 1 (average of 24 interferograms, resolution 4 cm− 1) (OPUS software, version 8.5, Bruker Optics GmbH, Ettlingen, Germany). The region between 2,400 and 1,900 cm− 1 was removed from the analysis due to the interference of water vapour and CO2. Air was used as the reference background spectra and collected every 10 samples. The ATR cell was cleaned with a mixture of 70% ethanol in water (v/v) and dried with laboratory Kimwipes® before the measurement of each sample.
Data analysis
Interpretation of the spectra and the development of a principal component analysis (PCA) was performed using Solo v9.2 (Eigenvector Research, Inc., WA, USA). Linear discriminant analysis (LDA) classification models were performed using Unscramble X (version 11, Oslo, Norway) and figures were plotted using in R using the ggplot package.
Pre-processing of the NIR and MIR spectra was applied to remove or at least minimize background noise using the second-order derivative Savitzky-Golay filtering with second degree of polynomial and 11 smoothing window points35. The MIR spectra region was removed (in the region between 2,400 and 1,900 cm− 1) before processing, where the spectra was pre-processed using detrend, standard normal variate (SNV) and second-order derivative Savitzky-Golay filtering with second degree of polynomial and 11 smoothing window points35.
The PCA is one of the most widely used techniques for data reduction during the exploratory analysis of spectral data. PCA can reduce a large spectral dataset into a few dimensions (principal components) by identifying orthogonal components that capture most of the variance between samples in the data, allowing for easier visualisation, interpretation, and understanding of the underlying patterns and relationships between samples36. PCA was used on all pre-processed spectra (NIR and MIR) to compare the two scanning locations.
To evaluate the effects of sex, environmental condition, and exposure to an acute aversive stimulus the NIR and MIR spectra of the brain samples was assessed by identifying distinct sample groupings. In this study, the spectra of the brain samples were classified using a supervised classification method (LDA) to distinguish between pre-defined groups or classes37. The LDA method was used to classify the brain samples into different categories or groups such as the type of tissue (e.g. gray and white matter), the sex of the animal, the three environmental conditions that the pigs were exposed to, and the presence or absence of exposure to an acute aversive stimulus before death. The LDA models attempt to build a linear relationship between the dependent variable (categories or group) and the independent variable (NIR or MIR spectra)38. This method takes the different variances of each variable into account as well as the correlation between the variables39. To construct a classification model, the NIR and MIR spectra of the brain samples were assigned as male or female, environmental exposure as positive, negative, or control, and acute aversive stimulus as yes or no. The NIR classification models were based on the spectra from the prefrontal cortex and the lateral region of the prefrontal cortex, while the MIR classification models were based on the spectra from the gray and white matter samples. The PCA and LDA models were validated using cross-validation (Venetian blinds with 10 data splits and one sample thickness)35. The ability of the LDA model to classify the samples according to the predefined groups was evaluated using the % of correct classification, and a Chi2 analysis to test whether the rate of correct classification was different from random chance.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Porcher, J. Animal welfare and society—part 2, the viewpoints of a sociologist. Anim. Front. 12, 48–52. https://doi.org/10.1093/af/vfac005 (2022).
Webster, J. Animal Welfare: Freedoms, dominions and A Life Worth living. Animals. 6, 35. https://doi.org/10.3390/ani6060035 (2016).
Mellor, D. Updating Animal Welfare thinking: moving beyond the Five Freedoms towards a Life Worth living. Animals. 6, 21. https://doi.org/10.3390/ani6030021 (2016).
Manteuffel, C., Spitschak, M., Ludwig, C. & Wirthgen, E. New perspectives in the objective evaluation of animal welfare, with focus on the domestic pig. J. Appl. Anim. Welf. Sci., 1–12 (2021).
Jones, M. Why the recognition of sentience is so important for animal welfare. Anim. Sentience. 6, 12 (2022).
Babington, S. et al. Finding biomarkers of experience in animals. J. Anim. Sci. Biotechnol. 15, 28. https://doi.org/10.1186/s40104-023-00989-z (2024).
Dixon, M. L., Thiruchselvam, R., Todd, R. & Christoff, K. Emotion and the prefrontal cortex: an integrative review. Psychol. Bull. 143, 1033–1081. https://doi.org/10.1037/bul0000096 (2017).
Chafee, M. V. & Heilbronner, S. R. Prefrontal cortex. Curr. Biol. 32, R346–r351. https://doi.org/10.1016/j.cub.2022.02.071 (2022).
McEwen, B. S. & Morrison, J. H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29, doi:S0896-6273(13)00544-8 [pii]. https://doi.org/10.1016/j.neuron.2013.06.028 (2013).
Ye, L. et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell 165, 1776–1788. https://doi.org/10.1016/j.cell.2016.05.010 (2016).
Blache, D., Terlouw, E. M. C. & Maloney, S. K. in In Animal Welfare. 181–212 (eds Appleby, M. C., Olsson, A. & Galindo, F.) (CAB International, 2017).
Blache, D. & Maloney, S. K. in In Advances in Sheep Welfare. 73–104 (eds Ferguson, D., Lee, C. & Fisher, A.) (Elsevier, 2017).
McEwen, B. S., Nasca, C. & Gray, J. D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 41, 3–23 (2016).
Talari, A. C. S., Martinez, M. A. G., Movasaghi, Z., Rehman, S. & Rehman I. U. advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 52, 456–506 (2017).
Workman, J. & Weyer, L. Practical Guide and Spectral Atlas for Interpretive near-infrared Spectroscopy 2nd edn (CRC, 2012).
Wetzel, D. L. & LeVine, S. M. in In Infrared and Raman Spectroscopy of Biological Materials. 101–142 (eds Gremlich, H. U. & Yan, B.) (Marcel Dekker Inc., 2000).
Petrich, W. Mid-infrared and Raman spectroscopy for medical diagnostics. Appl. Spectrosc. Rev. 36, 181–237 (2001).
Godyń, D., Nowicki, J. & Herbut, P. Effects of environmental enrichment on pig welfare—A review. Animals. 9, 383 (2019).
Terry, R., Nowland, T. L., van Wettere, W. H. E. J. & Plush, K. J. Synthetic olfactory agonist use in the Farrowing House to reduce sow distress and improve piglet survival. Animals. 11, 2613 (2021).
Serdyukov, V. I., Sinitsa, L. N. & Poplavskii, Y. A. Detection of the absorption spectra of water clusters under atmospheric conditions. JETP Lett. 89, 10–13 (2009).
Dovbeshko, G. I., Gridina, N. Y. & Kruglova, E. B. Pashchuk, O. P. FTIR spectroscopy studies of nucleic acid damage. Talanta. 53, 233–246 (2000).
Mossoba, M. M. et al. Printing microarrays of bacteria for identification by infrared microspectroscopy. Vib. Spectrosc. 38, 229–235 (2005).
O’Brien, J. S. & Sampson, E. L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 6, 545–551 (1965).
Luders, E. & Toga, A. W. Sex differences in brain anatomy. Prog. Brain Res. 186, 2–12 (2010).
Luders, E. et al. Mapping cortical gray matter in the young adult brain: effects of gender. NeuroImage. 26, 493–501. https://doi.org/10.1016/j.neuroimage.2005.02.010 (2005).
Kanaan, R. A. et al. Gender differences in white matter microstructure. PloS One. 7, e38272 (2012).
Hemsworth, P. H. Key determinants of pig welfare: implications of animal management and housing design on livestock welfare. Anim. Prod. Sci. 58, 1375–1386 (2018).
Pan, W., Zhao, J. & Chen, Q. Classification of foodborne pathogens using near infrared (NIR) laser scatter imaging system with multivariate calibration. Sci. Rep. 5, 9524 (2015).
Pereira, J. F. et al. Evaluation and identification of blood stains with handheld NIR spectrometer. Microchem. J. 133, 561–566 (2017).
Srinivasan, M., Sedmak, D. & Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 161, 1961–1971. https://doi.org/10.1016/s0002-9440(10)64472-0 (2002).
Bayer, E., Spiller, A. & Kühl, S. Consumer expectations towards slaughtering: are there different expectations towards the slaughter of conventional and organic animals? J. Agric. Food Res., 100695 (2023).
Kanis, E., Groen, A. F. & De Greef, K. H. Societal concerns about pork and pork production and their relationships to the production system. J. Agric. Environ. Ethics. 16, 137–162 (2003).
Hemsworth, P. H., Barnett, J. L. & Hansen, C. The influence of handling by humans on the behaviour, reproduction and corticosteroids of male and female pigs. Appl. Anim. Behav. Sci. 15, 303–314 (1986).
McGlone, J. J. Influence of resources on pig aggression and dominance. Behav. Processes. 12, 135–144. https://doi.org/10.1016/0376-6357(86)90052-5 (1986).
Rinnan, Å., Berg, F. & Engelsen, S. B. Review of the most common pre-processing techniques for near-infrared spectra. TRAC Trends Anal. Chem. 28, 1201–1222. https://doi.org/10.1016/j.trac.2009.07.007 (2009).
Bro, R. & Smilde, A. K. Principal component analysis. Anal. Methods. 6, 2812–2831 (2014).
Ruiz-Perez, D., Guan, H., Madhivanan, P., Mathee, K. & Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 21, 1–10 (2020).
Brereton, R. G. & Lloyd, G. R. Partial least squares discriminant analysis: taking the magic away. J. Chemom. 28, 213–225 (2014).
Mika, S., Ratsch, G., Weston, J., Scholkopf, B. & Mullers, K. R. Fisher discriminant analysis with kernels. Proceedings of the 1999 IEEE signal processing society workshop. 41–48 (1999).
Acknowledgements
The authors would like to thank the staff of the CHM Westbrook Research Facility for their help during the animal experimentation. This work was funded by the Australasian Pork Research Institute Ltd, The University of Queensland and The University of Western Australia.
Author information
Authors and Affiliations
Contributions
EAK and DC performed the vibrational spectroscopy and analysed the spectra. AJT, KT, KP, DD, DB designed the study. KT, KP, AJT and DB collected the tissues. EAK, DD, KP, DB, AT and SKM wrote the drafts of the manuscript. All authors contributed to the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Blache, D., Kho, E.A., Tilbrook, A.J. et al. Near or mid-infra-red spectroscopy of the prefrontal cortex to identify previous stressful experience in an animal. Sci Rep 14, 27594 (2024). https://doi.org/10.1038/s41598-024-79171-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79171-y