Abstract
Targeted vasopeptide therapies have significantly advanced the management of pulmonary arterial hypertension (PAH). However, due to insufficient preclinical evidence regarding the involvement of the endothelin-1 (ET-1) pathway in chronic thromboembolic pulmonary hypertension (CTEPH) pathophysiology, the potential of ET-1 receptor antagonism in treating CTEPH remains uncertain. In this study, we investigated the role of the ET-1 pathway in CTEPH microvasculopathy using a multifaceted approach. Plasma ET-1 levels were measured in a cohort of 59 CTEPH patients and 41 healthy controls. Additionally, we evaluated the expression of key ET-1 pathway members in pulmonary explants from CTEPH, idiopathic PAH, and control patients. We used an in vitro system to test the hypothesis that the turbulent flow, observed near the vascular obstructions pathognomonic of CTEPH, enhances ET-1 expression. Our findings were further validated in vivo using a CTEPH piglet model that contains distinct regions representing pre- and post-thrombus lung territories. We found a twofold increase in circulating ET-1 levels in CTEPH patients compared to healthy subjects. Pulmonary explants from CTEPH patients exhibited pronounced overexpression of ET-1, endothelin receptor A (ETA), and phosphorylated myosin light chain (p-MLC) in muscularized pulmonary microvessels, suggesting heightened vascular contraction. In vitro experiments showed that turbulent flow facilitates ET-1 secretion compared to laminar flow regions. Additionally, in the CTEPH piglet model, elevated plasma ET-1 levels were observed compared to controls. Immunofluorescence and confocal microscopy analyses confirmed increased ETA and p-MLC in remodeled arteries from both pulmonary territories. However, ET-1 protein elevation was exclusively observed in the obstructed territory. These findings collectively indicate impaired vascular tone in microvessels of CTEPH patients and the CTEPH piglet model. Furthermore, our data implicates the ET-1 pathway in microvasculopathy, with turbulent flow playing a pathological role. These insights underscore the potential utility of ET-1 receptor antagonists as a promising therapeutic approach for CTEPH treatment.
Similar content being viewed by others
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a debilitating and deadly cardiopulmonary disease1. This condition is defined by the presence of pulmonary arterial occlusion by intraluminal unresolved fibrotic thrombi leading to elevated mean pulmonary arterial pressure (mPAP) and increased pulmonary vascular resistance (PVR)2. Alongside these occlusive changes, CTEPH patients often present with microvasculopathy akin to the changes observed with idiopathic pulmonary arterial hypertension (iPAH), featuring intimal, medial, and adventitial hyperplasia leading to luminal reduction3,4. Although pulmonary thromboendarterectomy (PTE) often can cure CTEPH by surgically removing proximal occlusive fibro-thrombotic material, many patients are not eligible for this procedure or experience residual pulmonary hypertension after PTE. Balloon pulmonary angioplasty (BPA) and medical therapy with drugs like the oral guanylate cyclase agonist riociguat or the prostacyclin analog treprostinil offer approved alternatives or adjunctive options to surgery. However, these therapies often fail to fully meet the needs of patients who are not cured by PTE1. Given the shared microvasculopathy between CTEPH and iPAH, therapies targeting endothelin (ET)-1, which have shown efficacy in iPAH treatment, present a promising avenue for medical intervention in CTEPH.
While there is currently no approved ET-1 receptor antagonist (ERA) for treating CTEPH, this class of medication holds potential as a new therapy for affected patients. Small-scale studies have reported elevated plasma concentrations of ET-1 in CTEPH patients compared to healthy controls (n = 5 and 25)5,6, suggesting a potential clinical significance of this pathway in the disease. Additionally, small scale clinical trials investigating CTEPH treatment have suggested a potential benefit of ERAs789. Despite active clinical investigation of ERA treatment, little is known about their effect on CTEPH pathophysiology. ET-1 is a 21–amino acid peptide secreted by vascular endothelial cells (ECs) in response to stimuli that leads to vasoconstriction and pulmonary artery smooth muscle cell (PA-SMC) proliferation. It signals through two G protein-coupled receptors: endothelin receptor A (ETA), present in PA-SMCs, which induces increased intracellular calcium levels consequently activating downstream targets such as myosin light-chain kinase (MLCK) and endothelin receptor B (ETB), expressed in other cell types including ECs, which is responsible for ET-1 clearance. The contrasting impacts of ET-1 in the pulmonary vasculature necessitate a nuanced understanding of the contribution of this pathway in CTEPH pathophysiology to guide the appropriate clinical use of ERAs10,11.
In this study, we investigated the ET-1 pathway within CTEPH. To establish the clinical relevance of ET-1 signaling in CTEPH, we measured circulating venous plasma ET-1 levels in CTEPH patients and control subjects and conducted staining for pathway components in lung tissue. To test the hypothesis that disturbed flow around obstructions could induce ET-1 expression, we cultured human pulmonary microvascular endothelial cells (PMECs) under variable flow conditions and examined ET-1 expression. Furthermore, we validated our hypothesis using a CTEPH piglet model, allowing us to analyze ET-1 pathway activation in obstructed and non-obstructed lung regions. Collectively, these findings offer valuable insights into the role of ET-1 in CTEPH pathophysiology.
Results
An overabundance of ET-1 was observed in both plasma and endothelium of remodeled pulmonary microvessels of patients with untreated CTEPH
To investigate the clinical relevance of the ET-1 pathway in CTEPH pathophysiology, we initially examined the ET-1 concentration in plasma samples from 59 CTEPH patients, compared to 41 healthy controls. While the control group exhibited a younger age profile (47 [33–54] versus 65 [56–72]), the gender distribution was similar between the two cohorts (37% female and 42% female) (Supplementary Table S1). No patients in either cohort were treated with endothelin receptor antagonists (ERAs). ET-1 levels were significantly elevated in CTEPH patients, displaying a twofold increase compared to controls (2.6 ± 0.2 versus 1.3 ± 0.1 pg/mL) (Fig. 1A).
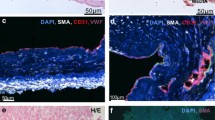
The Endothelin-1 (ET-1) Pathway Demonstrates Heightened Expression in Both the Plasma and Lung Microvessels of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Patients. (A) The concentration of ET-1 in venous plasma is elevated in CTEPH patients (n = 59) compared to control subjects (n = 41). (B) Co-immunofluorescent staining for ET-1 (red), von Willebrand factor (vWF, white), alpha-smooth muscle actin (α-SMA, green), and DAPI (blue) within lung microvessels from control subjects, CTEPH patients, and idiopathic pulmonary arterial hypertension (iPAH) patients, and the quantification of ET-1 fluorescent mean intensity (FMI) in the pulmonary endothelium (n = 5). (C) Co-immunofluorescent staining for ET-1 receptor A (ETA) (red), α-SMA (green), and DAPI (blue) in controls, CTEPH, and iPAH lungs, along with quantification of ETA FMI in pulmonary artery smooth muscle cells (PA-SMCs) (n = 5). (D) Co-immunofluorescent staining for phospho-myosin light chain (p-MLC) (red), α-SMA (green), and DAPI (blue) in controls, CTEPH, and iPAH lungs, along with quantification of p-MLC FMI in PA-SMCs (n = 5). All data are presented as mean with standard error of the mean (SEM). Statistical significance is denoted as * for P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001 and “ns” for not significant. The scale bar in all staining images is set at 100 µm.
Next, to discern the expression patterns of ET-1, ETA, and phosphorylated myosin light chain (p-MLC), we conducted co-immunofluorescent staining and quantification of these targets using human lung tissue samples from control patients, CTEPH patients, and individuals diagnosed with iPAH (Fig. 1B–D). Co-immunostaining for ET-1, von Willebrand Factor (vWF), and α-smooth muscle actin (α-SMA), followed by confocal analysis, revealed elevated ET-1 expression in lung tissues of both CTEPH and iPAH patients compared to control subjects (Fig. 1B). Quantitative analyses confirmed a twofold increase in fluorescence mean intensity (FMI) of the endothelial ET-1 signal in small pulmonary arteries (< 500 µm in diameter) of CTEPH and iPAH patients compared to control samples (n = 5) (Fig. 1B). Similar expression patterns were observed in downstream targets of ET-1, with ETA (Fig. 1C) and p-MLC (Fig. 1D) staining showing increased FMI in remodeled microvessels from CTEPH samples (ETA 2.9-fold increase and p-MLC 3.7-fold increase) and iPAH samples (ETA 3.1-fold increase and p-MLC 2.2-fold increase). The effect of ET-1 on MLC phosphorylation was confirmed in vitro using cultured human PA-SMCs (Fig. S1). Figure S2 illustrates ETB staining, indicating no changes in expression. Collectively, these findings reveal elevated circulating ET-1 levels in CTEPH patients, alongside increased expression of ET-1 pathway components and targets in the remodeled microvessels of CTEPH patients.
Human pulmonary microvascular endothelial cells (PMECs) ET-1 protein expression increased in vitro when cells were exposed to disturbed flow
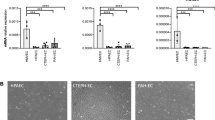
To explore the relationship between hemodynamic forces and ET-1 production, we cultured human PMECs on a Y-shaped ibidi slide under unidirectional low flow conditions for 48 h (Fig. 2A). This ibidi slide configuration comprises two distinct flow regions: laminar flow and disturbed flow at the branch point simulating a vessel obstruction (Fig. 2B). Co-immunostaining for ET-1 and VE-cadherin revealed varying ET-1 expression levels in each region of the slide (Fig. 2C). Notably, ET-1 expression was most pronounced at the site of channel obstruction (Fig. 2C – panel ii) and diminished progressively as the shear stress increased to 2.5 dyn/cm2 (Fig. 2C – panels iii and iv) and 5 dyn/cm2 (Fig. 2C – panels i and v). These findings showed that areas characterized by disturbed flow exhibit heightened ET-1 secretion.
Human Pulmonary Endothelial Cells (PMECs) Exhibit Increased ET-1 Expression in Regions of Disturbed Flow. (A) A schematic representation of the experimental setup for cell culture under flow within a Y-shaped ibidi plate. (B) The diagram indicates the expected shear stress distribution and highlights the image locations (i-v) for subsequent analyses. This schematic was generated using Biorender. (C) Immunofluorescent staining of PMECs at specified regions within the plate, showing ET-1 expression (red), VE-cadherin (green) and DAPI (blue). The scale bar in all staining images is set at 100 µm.
The CTEPH piglet model exhibited elevated pulmonary vascular resistance, mean pulmonary artery pressure, and vascular wall thickness compared to sham controls
Despite the current lack of a truly representative animal model that mimics the pathophysiology of CTEPH, there are in vivo models that imitate aspects of CTEPH. We used CTEPH piglet model that comprises both a non-obstructed territory (right superior lobe; RSL) and an obstructed territory (left superior lobe; LSL) to facilitate the in vivo exploration of the relationship between flow dynamics and ET-1 signaling (Fig. 3A). Right heart catheterization confirmed the presence of altered hemodynamic parameters consistent with CTEPH in the piglet model compared to sham controls (n = 11). This analysis revealed elevated mPAP (33.7 ± 2.8 versus 13.3 ± 0.7 mmHg) and PVR (9.1 ± 1.2 versus 2.6 ± 0.2 WU), without a change in cardiac output (2.7 ± 0.2 versus 3.2 ± 0.2 L/min) (Fig. 3B).
The CTEPH Piglet Model Demonstrates Elevated Pulmonary Vascular Resistance, Mean Pulmonary Artery Pressure (mPAP), and Pulmonary Vascular Wall Thickness Compared to Sham Controls. (A) A diagram illustrating the CTEPH piglet model with labeled territories. This schematic was generated using Biorender. (B) The mPAP, cardiac output (CO), and pulmonary vascular resistance (PVR) of the CTEPH piglet model compared to sham controls (n = 11). (C) Immunohistochemistry staining for α–smooth muscle actin and quantification of wall thickness in pulmonary arteries with diameters of < 50 µm in lungs of the CTEPH piglet model and sham controls (n = 5). (D) Immunohistochemistry staining for α–smooth muscle actin and quantification of wall thickness in pulmonary arteries with diameters of 50–200 µm in lungs of the CTEPH piglet model and sham controls (n = 5). All data are presented as the mean ± SEM. Statistical significance is denoted as * P < 0.05, **P < 0.05, ****P < 0.0001, and “ns” for not significant. Scale bars 50 µm. LSL: left superior lobe; RSL: right superior lobe.
To further validate the relevance of this in vivo CTEPH model, we next assessed the microvascular structure of the pulmonary arteries, focusing on arteries under 50 µm and those between 50-200 µm in diameter. Quantification of arterial wall thickness (n = 5) confirmed microvascular alterations in the CTEPH piglet model; wall thickness was increased in arteries in both the RSL and LSL regions compared to sham controls (Fig. 3C-D). Overall, the CTEPH piglet model demonstrated pulmonary hemodynamic and microvascular changes consistent with clinical pathophysiology.
The CTEPH piglet model exhibited region-specific elevation in ET-1 production compared to sham controls
Consistent with our human findings, circulating ET-1 concentration was higher in the CTEPH piglet model compared to sham controls (n = 11) (2.1 ± 0.2 versus 1.3 ± 0.1 pg/mL) (Fig. 4A). To validate our hypothesis that increased ET-1 signaling occurs in lung regions with disturbed flow, we compared ET-1 staining in the RSL and LSL regions of the CTEPH piglet model to sham controls. ET-1 staining revealed that only the obstructed LSL territory exhibited enhanced ET-1 expression in the endothelium of small pulmonary arteries compared to the sham control lung (n = 5) (Fig. 4B). Notably, the non-obstructed RSL territory displayed nearly identical ET-1 expression compared to that of the control animal lung (Fig. 4B). Despite the absence of change in ET-1 expression in the non-obstructed RSL territory, both the RSL and LSL territories demonstrated higher expression of ETA in the arterial media compared to control expression, a 2.1- and 2.3-fold increase respectively (34.7 ± 1.5 and 38.1 ± 4.2 versus 16.6 ± 1.1 AU) (Fig. 4C). Furthermore, the level of p-MLC was increased in the media of small pulmonary arteries in both the RSL and LSL regions compared to the sham (Fig. 4D). These findings indicate increased circulating ET-1 in CTEPH piglet model, with high expression in the LSL region. While ET-1 production was region specific, the pathway appears to be activated in both territories due to heightened levels of ETA and p-MLC.
The Endothelin-1 (ET-1) Pathway Demonstrates Increased Expression in both the Plasma and Lung Microvessels of the CTEPH Piglet Model. (A) The concentration of ET-1 in venous plasma in the CTEPH piglet model compared to sham controls (n = 11). (B) Co-immunofluorescent staining for ET-1 (red), von Willebrand factor (vWF, white), alpha-smooth muscle actin (α-SMA, green), and DAPI (blue) within lung microvessels from the sham controls, non-obstructed (RSL), and obstructed (LSL) territories, along with quantification of fluorescent mean intensity (FMI) in the pulmonary endothelium (n = 5). (C) Co-immunofluorescent staining for ET-1 receptor A (ETA) (red), a-SMA (green), and DAPI (blue) in sham lungs, and the RSL and LSL territories, along with quantification of FMI in pulmonary artery smooth muscle cells (PA-SMCs) (n = 5). (D) Co-immunofluorescent staining for phospho-myosin light chain (p-MLC) (red), α-SMA (green), and DAPI (blue) in sham lungs, and the RSL and LSL territories, along with quantification of FMI in PA- SMCs (n = 5). All data are presented as mean values with standard error of the mean (SEM). Statistical significance is denoted as * for P < 0.05, **P < 0.01, and ***P < 0.001. The scale bar in all staining images is set at 100 µm. LSL: left superior lobe; RSL: right superior lobe.
Discussion
Despite the similarities between CTEPH and iPAH microvasculopathy, the role of ET-1 signaling in CTEPH pathophysiology remains unclear, representing a critical area for further investigation1,4,12. While clinical trials like MERIT-1 (NCT02021292)13 and IMPACT-CTEPH (NCT04780932) are actively exploring treatment options for CTEPH, understanding the ET-1 involvement is essential for optimizing patient selection and management strategies for ERAs. Our study contributes valuable evidence supporting the role of ET-1 in CTEPH progression. We confirmed ET-1 upregulation in untreated CTEPH patients and localized its expression in the remodeled microvessels of diseased lungs. Additionally, we hypothesized that disturbed flow near thrombus obstructions could trigger elevated ET-1 secretion and validated this hypothesis in vitro and in vivo. Expanding our investigation to an in vivo CTEPH piglet model further strengthened our findings, revealing region-specific upregulation of ET-1 in obstructed lung. This work cements the importance of ET-1 signaling in CTEPH pathophysiology and provides novel human, in vitro, and in vivo data suggesting the role of obstructed flow in this process.
Our discovery of elevated plasma ET-1 concentration in a substantial cohort of 59 untreated CTEPH patients aligns with and supports previous findings from smaller and less well-defined patient cohorts (n = 5 and 25)5,6. Although our patient cohorts had similar gender distributions, it is important to note that our controls were not perfectly age matched. Previous work examined the age- and sex-dependent differences of ET-1 plasma concentration in healthy individuals and found that the sex-dependent effect is more pronounced than the age-dependent increase14, with the expected difference on a smaller scale than the change observed in our data (< 0.3 pg/mL versus 1.3 pg/mL found in our study). Our work is the first evidence indicating elevated ETA protein expression in pulmonary vessels of CTEPH patients and demonstrating its microvascular localization. Distal remodeled arteries also displayed high phosphorylation status of MLC, suggesting pronounced vasoconstriction. The iPAH samples showed lower levels of p-MLC protein compared to those from CTEPH patients. This difference may be linked to the pharmacological effects of guideline-recommended PDE5 inhibitors that are currently prescribed to iPAH patients. Previously, Southwood et al. demonstrated high ETAprotein levels in fibrinous clot material extracted during PTE, these specimens were obtained from proximal areas and may not be representative of pulmonary vasculature15. The presence of ETA in both the remodeled microvessels and fibrinous clot material suggests that ET-1 signaling plays a dual role in dysregulated thrombus progression to fibrinous obstruction and microvascular contraction.
Unlike other types of pulmonary hypertension, CTEPH is characterized by pulmonary vascular obstruction secondary to fibrotic thrombi2; disturbed flow at branch points, curvatures, and obstacles is a notable feature16. Since reduced shear stress in areas of disturbed flow is known to stimulate ET-1 expression17, we hypothesized that disturbed flow at vascular obstructions contributes to the exaggerated endothelial secretion of ET-1 in CTEPH. Using a Y-shaped ibidi slide as an in vitro system to explore the effect of flow on human PMECs, we showed elevated ET-1 protein expression in areas of disturbed flow and suppressed ET-1 expression in areas of laminar flow. This suggests that disturbed flow in obstructed regions of CTEPH lungs is one mechanism driving ET-1 production, among others factors such as hypoxia may also contribute to ET-1 expression, particularly in areas of reduced blood flow post-obstruction, as hypoxia is known to affect ET-1 signaling. For instance, a rat model of obstructive sleep apnea demonstrated that intermittent hypoxia increased ET-1 expression, leading to enhanced ETAexpression in vascular smooth muscle and vasoconstriction18. Therefore, the etiology of ET-1 expression in CTEPH may be multifactorial, warranting further investigation into the interplay between hypoxia, disturbed flow, and ET-1 signaling to provide deeper insights into how this pathway influences CTEPH.
To validate our human and in vitro findings in vivo, we employed a CTEPH piglet model initially described by Mercier and colleagues. This model involves ligating the left main pulmonary artery (LSL region) and subsequently inducing serial embolization of the distal right lower pulmonary artery, leaving the right superior lobe (RSL region) non-obstructed and subjected to pulmonary overflow19. While previous animal models have focused on either pulmonary obstruction or pulmonary overflow separately, our model uniquely encompasses both aspects, allowing for the study of both the obstructed and the high shear stress regions involved in CTEPH physiology19. Our results confirmed the replication of key CTEPH features in this model, including elevated mPAP and PVR, indicative of pulmonary hypertension consistent with CTEPH2. Histological analysis of small pulmonary arteries in both LSL and RSL territories revealed increased wall thickness due to medial hyperplasia, akin to microvascular changes observed in human CTEPH3. The similarity of these microvascular changes with human disease validated this model’s relevance to CTEPH research, and the presence of these changes in both the obstructed and non-obstructed region facilitated the investigation of the impact of disturbed and laminar flow on CTEPH pathophysiology. Consistent with findings in our cohort of CTEPH patients and previous literature5,6, the CTEPH piglet model exhibited elevated plasma levels of ET-1 compared to the sham group. Stam and colleagues previously demonstrated increased ET-1 expression in a CTEPH piglet model20. Our study builds upon this existing data by elucidating region-specific differences. ET-1 overexpression was observed only in the endothelium of distal remodelled pulmonary arteries in the LSL region, not in the non-obstructed RSL pulmonary arteries. Although previous studies measuring ET-1 mRNA in total lung homogenates reported higher levels in the right upper lobe21, our analysis, which focused on small pulmonary vessels in these regions, did not confirm these findings at the protein level. Since shear stress can repress ET-1 expression, the reduced ET-1 levels in the RSL region may be due to the increased shear stress in this region resulting from the entire cardiac output being directed towards a small portion of the lung vasculature17. ETA overexpression was present in both RSL and LSL regions. This result is consistent with our human lung microvascular data and previously published results demonstrating elevated ETAin fibrinous clot material extracted during PTE15. Although endothelial ET-1 overexpression was confined to the LSL region, elevated levels of downstream pathway targets were observed in both the RSL and LSL regions. This observation raises the possibility that obstructed areas may influence non-obstructed regions through paracrine or endocrine mechanisms. However, it is crucial to consider that other factors, such as chronic hypoxia and inflammation, may also play a significant role to these effects. Moreover, ET-1 production is not limited to a single cell type; it can be synthesized by various lung cells, including endothelial, epithelial, smooth muscle, club, neuroendocrine cells, and alveolar macrophages22. In addition, the regulation of MLC phosphorylation and vascular smooth muscle cell proliferation involves multiple signaling pathways beyond ET-1. Thus, while the hypothesis of paracrine or endocrine influence is valid, it is essential to approach this with caution, as these processes are likely multifactorial.
Our results capitalize on a large sample size to confirm the increase in ET-1 plasma expression in patients with CTEPH. This study is the first to evaluate the expression of the ET-1 pathway in distal arteries of CTEPH patients. A limitation of our human lung sample histology is that the tissue was explanted from patients during lung transplantation, so these results may not represent patients in earlier stages of disease progression. Our animal model of CTEPH contained distinct regions, allowing us to study these regions separately. However, in the vasculature of CTEPH patients, obstructed and non-obstructed territories are intermixed and may exhibit substantial paracrine interactions lost in our model, leading our model to underestimate the paracrine effect of ET-1 on CTEPH pathophysiology. Furthermore, we did not test an ET-1 pathway-targeting agent in the piglet CTEPH model. Future studies should explore this approach, especially in patients, given the availability of such therapeutic agents. Lastly, while our work strongly implicates the ET-1 pathway in CTEPH pathophysiology, it does not exclude the involvement of other mechanisms in CTEPH microvascular pathology including hypoxia-induced vasoconstriction18, Smad2/3 activation by low shear triggering inward remodeling23, pathologically high shear stress promoting endothelial-to-mesenchymal transition and vessel hyperplasia24, or inflammatory cell involvement25,26. These alternative mechanisms represent intriguing avenues for future research.
Our findings confirmed the involvement of ET-1 signaling in the pathophysiology of CTEPH (Fig. 5). We observed elevated ET-1 in the plasma of a substantial cohort of CTEPH patients, along with increased expression of its downstream pathway components, ETA and p-MLC. In vitro culture of human PMECs revealed that areas of disturbed flow are associated with heightened ET-1 expression. In our CTEPH piglet model, which features both obstructed and non-obstructed territories, we found elevated plasma ET-1 concentration, with a localized increase in cellular ET-1 protein observed exclusively in the obstructed territory. However, despite localization of this increase, both regions showed elevated ETA and p-MLC levels and increased cellular accumulation in the intima and media. These findings support the consideration of ET-1 receptor antagonists as a therapeutic approach for CTEPH treatment.
Study diagram: The obstruction of a vascular territory by the persistence of a fibro-thrombotic sequestrum leads to local disruptions in blood flow and removes a brake on ET-1 synthesis in endothelial cells surrounding this lesion. Subsequently, this ET-1 is released into circulation, exerting a paracrine action on its ETA receptor. This receptor is crucial for inducing vasoconstriction through the phosphorylation of myosin light chain (p-MLC) and promoting the proliferation of vascular smooth muscle cells (PA-SMCs). The abnormal overexpression of ETA by these downstream microvessels creates a vicious cycle that promotes the accumulation of resident pulmonary vascular cells in the walls, leading to a reduction in pulmonary vascular lumens in these unobstructed areas. Furthermore, the pulmonary endothelium of vessels downstream from the obstructed zones also contributes to the elevation of ET-1 levels in CTEPH, thereby contributing to CTEPH microvasculopathy.
Methods
Human blood samples
Human blood samples were obtained from 59 CTEPH patients eligible for PTE who had not previously received endothelin receptor antagonists (Table S1). Plasma was collected days prior to surgery. Plasma samples from 41 control patients were acquired from the “Etablissement Français de Sang” (EFS, Paris, France). This study was approved by the local ethics committee (CPP EST-III n°18.06.06, Le Kremlin-Bicêtre, France). All patients gave informed consent before the study.
ELISA assay
To quantify the concentration of circulating ET-1, an ELISA (R&D systems Quantikine DET100) was performed on human and piglet venous plasma according to the manufacturer’s specifications.
Human lung tissue
This study was approved by the local ethics committee (CPP EST-III n°18.06.06, Le Kremlin-Bicêtre, France), and all research was performed in accordance with relevant guidelines/regulations. All patients gave informed consent before the study. Human lung specimens were collected from iPAH and CTEPH patients at the time of lung transplantation. Control samples were obtained during lobectomy or pneumectomy for localized lung cancer. Pulmonary hypertension was ruled out in control patients through preoperative echocardiological evaluations, and the sample was collected from a region free of tumor. Surgical lung specimens were processed according to standard histological techniques, including fixation in formalin, paraffin embedding, and preparation of tissue sections for staining with hematoxylin, eosin, and saffron trichrome. The absence of tumor infiltration was verified by histopathological analysis.
Paraffin lung immunostainings
Immunofluorescent staining for ET-1 (Abcam for human lung staining, Abcam and Origen for piglet lung staining), ETA (Novus Biologicals for human staining, Abcam for piglet model staining), phosphorylated MLC (Cell signaling), alpha-smooth muscle actin (α-SMA, Novus Biologicals) were performed in human and piglet lung paraffin sections. The following process was used: 5 µm thick lung sections were deparaffinized, permeabilized when needed, incubated with antigen retrieval buffer, and saturated with a 5% bovine serum albumin (BSA) blocking buffer. Then, they were incubated overnight with the appropriate dilution of primary antibodies, followed by their respective fluorescent secondary antibodies (Thermo Fisher Scientific). Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole, Thermo Fisher Scientific). Mounting was realized with ProLong Gold antifade reagent (Thermo Fisher Scientific). Antibodies references are detailed in supplementary Table S2.
In vitro study
Human PMECs were isolated from two control lung specimens and cultured as previously described27,28. PMECs were seeded at a concentration of 500,000 cells/mL in a Y-shaped ibidi slide and exposed to low flow at 5 dyn/cm2 for 48 h using an ibidi pump system (ibidi, Munich, Germany). The pump system was set up according to the manufacturer’s instructions and incubated at 5% CO2 and 37 °C. PMECs were stained for ET-1 (Abcam) and VE-cadherin (Santa Cruz). Antibodies references are detailed in supplementary Table S2.
Immunofluorescence image acquisition
Images were acquired using an LSM900 confocal microscope (Zeiss, Marly-le-Roi, France) with Zen (software v3.8). The fluorescence intensity was quantified by measuring the mean intensity in the media of 5–10 arteries per tissue.
Animal model
The study was approved by both our local ethics committee on animal experiments, Comité d’éthique CEEA 26, and the French Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation (MESRI) (Ethical approval number: APAFIS#27670). Animal experimentation was conducted according to the Principles of Laboratory Animal Care developed by the National Society for Medical Research. The study is reported in accordance with ARRIVE guidelines. The experimental CTEPH model was previously described3,19,21. Briefly, piglets were randomly divided into two groups of eleven animals each. The model consists of ligating the left principal artery before a six-week period of surgical glue injection into the right lower lobe causing progressive embolization mimicking disease. This consequently triggers a blood overflow in the right upper lobe and ischemia in the left lobe. Thus, the model approximates the pre- and post-thrombus arterial territory, as shown in Fig. 3A. Hemodynamic and echocardiographic analyses were performed to validate the model.
Statistics
All data are expressed as the mean ± SEM. Normality of the data was assessed with Shapiro–Wilk test. Statistical differences between two groups were determined with a t-test for parametric data. Comparison between more than two groups was assessed with ANOVA followed by Holm-Sidak’s post-hoc test. Differences were considered significant when P < 0.05. Analyses were done with Prism software (GraphPad Software v9.5.1).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BPA:
-
Balloon pulmonary angioplasty
- CTEPH:
-
Chronic thromboembolic pulmonary hypertension
- ERA:
-
Endothelin receptor antagonist
- ET-1:
-
Endothelin-1
- ETA :
-
Endothelin receptor A
- ETB :
-
Endothelin receptor B
- iPAH:
-
Idiopathic pulmonary arterial hypertension
- LSL:
-
Left superior lobe
- MLC:
-
Myosin light-chain
- mPAP:
-
Mean pulmonary arterial pressure
- PAH:
-
Pulmonary arterial hypertension
- PAWP:
-
Pulmonary arterial wedge pressure
- PDE5i:
-
Phosphodiesterase type-5 inhibitor
- PMEC:
-
Pulmonary microvascular endothelial cell
- PTE:
-
Pulmonary thromboendarterectomy
- PVR:
-
Pulmonary vascular resistance.
- RSL:
-
Right superior lobe
References
Kim, N. H. et al. Chronic thromboembolic pulmonary disease. Eur Respir J https://doi.org/10.1183/13993003.01294-2024 (2024).
Humbert, M. et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 61, https://doi.org/10.1183/13993003.00879-2022 (2023).
Dorfmuller, P. et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 44, 1275–1288. https://doi.org/10.1183/09031936.00169113 (2014).
Guignabert, C. et al. Pathology and pathobiology of pulmonary hypertension: current insights and future directions. Eur Respir J https://doi.org/10.1183/13993003.01095-2024 (2024).
Reesink, H. J. et al. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J 70, 1058–1063. https://doi.org/10.1253/circj.70.1058 (2006).
Stewart, D. J., Levy, R. D., Cernacek, P. & Langleben, D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease?. Ann Intern Med 114, 464–469. https://doi.org/10.7326/0003-4819-114-6-464 (1991).
Ghofrani, H. A. et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 5, 785–794. https://doi.org/10.1016/S2213-2600(17)30305-3 (2017).
Escribano-Subias, P., Bendjenana, H., Curtis, P. S., Lang, I. & Vonk Noordegraaf, A. Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH). Pulm Circ 9, 2045894019846433. https://doi.org/10.1177/2045894019846433 (2019).
Jais, X. et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. Journal of the American College of Cardiology 52, 2127–2134. https://doi.org/10.1016/j.jacc.2008.08.059 (2008).
Giaid, A. et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328, 1732–1739. https://doi.org/10.1056/NEJM199306173282402 (1993).
Adam, L. P., Milio, L., Brengle, B. & Hathaway, D. R. Myosin light chain and caldesmon phosphorylation in arterial muscle stimulated with endothelin-1. J Mol Cell Cardiol 22, 1017–1023. https://doi.org/10.1016/0022-2828(90)91041-5 (1990).
Chaumais, M. C. et al. Clinical pharmacology of endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension. Am J Cardiovasc Drugs 15, 13–26. https://doi.org/10.1007/s40256-014-0095-y (2015).
Ghofrani, H. A. et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 12, e21–e30. https://doi.org/10.1016/S2213-2600(24)00027-4 (2024).
Miyauchi, T. et al. Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects. Am Heart J 123, 1092–1093. https://doi.org/10.1016/0002-8703(92)90734-d (1992).
Southwood, M. et al. Endothelin ETA receptors predominate in chronic thromboembolic pulmonary hypertension. Life Sci 159, 104–110. https://doi.org/10.1016/j.lfs.2016.02.036 (2016).
Dessalles, C. A., Leclech, C., Castagnino, A. & Barakat, A. I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun Biol 4, 764. https://doi.org/10.1038/s42003-021-02285-w (2021).
Wang, G. X. et al. Shear-induced changes in endothelin-1 secretion of microvascular endothelial cells. Microvasc Res 63, 209–217. https://doi.org/10.1006/mvre.2001.2387 (2002).
Wang, Z. et al. Effects of cyclic intermittent hypoxia on ET-1 responsiveness and endothelial dysfunction of pulmonary arteries in rats. PLoS One 8, e58078. https://doi.org/10.1371/journal.pone.0058078 (2013).
Mercier, O. & Fadel, E. Chronic thromboembolic pulmonary hypertension: animal models. Eur Respir J 41, 1200–1206. https://doi.org/10.1183/09031936.00101612 (2013).
Stam, K. et al. Pulmonary microvascular remodeling in chronic thrombo-embolic pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology 315, L951-L964, https://doi.org/10.1152/ajplung.00043.2018 (2018).
Mercier, O. et al. Piglet model of chronic pulmonary hypertension. Pulm Circ 3, 908–915. https://doi.org/10.1086/674757 (2013).
Abraham, D. J. et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol 151, 831–841 (1997).
Deng, H. et al. Activation of Smad2/3 signaling by low fluid shear stress mediates artery inward remodeling. Proc Natl Acad Sci U S A 118, https://doi.org/10.1073/pnas.2105339118 (2021).
Shinohara, T. et al. High Shear Stress Reduces ERG Causing Endothelial-Mesenchymal Transition and Pulmonary Arterial Hypertension. bioRxiv, https://doi.org/10.1101/2024.02.02.578526 (2024).
Viswanathan, G. et al. Single-Cell Analysis Reveals Distinct Immune and Smooth Muscle Cell Populations that Contribute to Chronic Thromboembolic Pulmonary Hypertension. Am J Respir Crit Care Med 207, 1358–1375. https://doi.org/10.1164/rccm.202203-0441OC (2023).
Miao, R. et al. Cell landscape atlas for patients with chronic thromboembolic pulmonary hypertension after pulmonary endarterectomy constructed using single-cell RNA sequencing. Aging (Albany NY) 13, 16485–16499. https://doi.org/10.18632/aging.203168 (2021).
Eddahibi, S. et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113, 1857–1864. https://doi.org/10.1161/CIRCULATIONAHA.105.591321 (2006).
Tu, L. et al. Selective BMP-9 Inhibition Partially Protects Against Experimental Pulmonary Hypertension. Circulation research 124, 846–855. https://doi.org/10.1161/CIRCRESAHA.118.313356 (2019).
Acknowledgements
The authors thank all pathologists and technicians from the Centre de Recherche Biologique at Marie Lannelongue Hospital—Groupe Hospitalier Paris Saint Joseph for their valuable expertise and continued support throughout the research. The authors extend their thanks to the French PAH patient association (HTaP France) and express their appreciation to all participants of the French Pulmonary Hypertension Network PulmoTension for their valuable contributions to the study. The authors also thank Fabien Robert for his help with the Western blot analysis. A.C. benefits from a Heart Failure Association (HFA) scholarship of the European Society of Cardiology (ESC), facilitating exchange opportunities between the Department of Translational Medical Science, University of Naples Federico II (Italy) and the Université Paris-Saclay. D.J.G benefits from the Internal Medicine Program at the Department of Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA, in collaboration with Université Paris-Saclay.
Funding
This work has benefited from a state funding managed by the National Research Agency according to the Investments for the Future program integrated into France 2030, under the reference ANR-18-RHUS-0006. This research was also supported by grants from the Fondation pour la Recherche Médicale (FRM) grants no. EQU202203014670 (Equipe FRM 2022).
Author information
Authors and Affiliations
Contributions
L.T, M.H, and C.G designed the initial concept. F.B., A.C., D.J.G., C.N, R.T., M.O., A.A., J-B.M., G.F., J.G., M-R.G., L.T., L.S., M.H., and C.G. participated in acquisition and interpretation of data. F.B., D.J.G., C.G. wrote the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Over the last three years, C.G. reports grants from Acceleron Pharma (Cambridge, MA, USA), a wholly-owned subsidiary of Merck & Co., Inc. (Rahway, NJ, USA), MSD, Corteria, Structure therapeutics, Diagonal Therapeutics, Gossamer, outside the submitted work. M.H. reports grants and personal fees from Acceleron, Aerovate, Altavant, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Merck, MorphogenIX and United Therapeutics, outside the submitted work. All the other authors declare that there is no conflict of interest regarding the publication of this original article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feriel, B., Alessandra, C., Deborah, G.J. et al. Exploring the Endothelin-1 pathway in chronic thromboembolic pulmonary hypertension microvasculopathy. Sci Rep 14, 28308 (2024). https://doi.org/10.1038/s41598-024-79623-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79623-5