Abstract
Urinary incontinence (UI) in females is a prevailing condition that affects individuals across various age groups and is not limited to older females. The presence of serum alpha-Klotho (α-klotho) serves as a reliable biomarker to indicate the effects of antiaging. Nevertheless, the scientific research on the association between α-klotho and UI remains limited. Therefore, the purpose of this study is to investigate and evaluate the connection between α-klotho levels and the UI among females in the US. We utilized data from the National Health and Nutrition Examination Survey 2007–2016 to investigate the potential connection between α-klotho levels and the UI among females aged 40 to 79. Weighted linear regression models and sensitivity tests were conducted to explore the correlation. 2628 females were involved in this study, representing 22,492,348 non-institutionalized residents in the US. The mean age was 53.9 ± 0.2 years and the mean level of α-klotho was 873.0 ± 8.9 pg/mL. After adjusting for relevant covariables, weighted linear regression models revealed that individuals with severe UI exhibited significantly lower serum α-klotho levels (β = − 100.66; 95% CI: − 156.31, − 45.01; P < 0.001) than those without UI. Furthermore, in stratified analyses, the correlation was not significant in individuals with normal weight, cardiovascular disease, or chronic kidney disease. We did not find a significant association between the type of UI and α-klotho levels. In the NHANES data from 2007 to 2016, a noteworthy inverse relationship was noted between α-klotho levels and the severity of UI among females aged 40 to 79.
Similar content being viewed by others
Introduction
Female urinary incontinence (UI) is a wildly prevalent condition affecting women across all age groups, with estimated prevalence rates ranging from 14 to 45%1,2. UI is characterized by the involuntary leakage of urine, which can occur during physical activities, coughing, sneezing, or even at rest. This condition greatly affects female’s well-being, leading to emotional distress and social isolation3,4. The etiology of UI is complex and involves a combination of physiological, anatomical, and lifestyle factors. Numerous risk factors have been identified, such as advancing age5, obesity6, pregnancy7, and weakness of the pelvic floor muscle8.
Alpha-klotho (α-klotho) is a transmembrane protein that is predominantly found in the distal tubule epithelial cells of the kidney, parathyroid gland, and choroid plexus of the brain9. It has been implicated in several physiological processes, including regulating calcium phosphate levels, insulin signaling, and aging9,10. As a co-receptor for fibroblast growth factor-23, α-klotho modulates the signaling pathways associated with FGF activation11. Additionally, α-klotho has been linked to various age-related diseases and conditions10, such as accelerated aging12, cognitive decline13, cardiovascular diseases (CVD)14, and chronic kidney disease (CKD)15.
The relationship between α-klotho and female UI remains largely unexplored. We hypothesize that lower levels of α-klotho may be associated with the development or severity of female UI. This hypothesis is based on the recognized role of α-klotho in renal function and its potential impact on factors such as hormonal regulation, muscle weakness, and aging12, all of which are implicated in the etiology of UI. Conducting a further investigation into the potential link between α-klotho and female UI could provide valuable insights into the underlying pathophysiology of this condition. In this cross-sectional study, we aim to examine the potential correlation between α-klotho levels and the presence, severity, and type of UI in a diverse group of middle-aged and older females.
Materials and methods
Study design and participants
The National Center for Health Statistics (NCHS) conducts the National Health and Nutrition Examination Survey (NHANES), a comprehensive US survey. NHANES utilizes a sophisticated probabilistic design to obtain samples representing individuals outside institutions. Its main objective is to assess the health and nutritional status of the population. NHANES data collection involves household interviews, performing physical examinations at a mobile examination center (MEC), and conducting laboratory testing. The ethics review board of the NCHS approved the research study using data from the NHANES, and all participants submitted written consent after being fully informed. The study procedures were structured in line with the Declaration of Helsinki.
The cross-sectional analysis included 7098 female participants aged 40–79 from the NHANES database (2007–2016). In order to measure serum α-klotho levels only those who provide surplus serum samples for future research. Initially, 1955 participants were excluded due to incomplete data on serum α-klotho or UI, hysterectomy (n = 1650), cancer (n = 319), pregnancy (n = 6), or other covariates (n = 540). Ultimately, the final sample that was considered for analysis consisted of 2628 participants. The selection process for participants can be visualized in Fig. 1.
Serum α-klotho level
The NHANES Procedures Manual for Laboratory/Medical Technologists was consulted for laboratory methodology and protocols. During the study conducted between 2019 and 2020, researchers investigated levels of serum α-klotho in frozen samples collected from 2007 to 2016. These samples were stored at a temperature of − 80 °C, and underwent analysis using an enzyme-linked immunosorbent assay (ELISA) kit manufactured by IBL International, Japan. Duplicate analysis was performed on each sample, and the calculated average value was determined. The average level of serum α-klotho was found to be 698.0 pg/mL, with a range spanning from 285.8 to 1638.6 pg/mL16. To assess the test’s accuracy, researchers computed the variation coefficients for intra-assay and inter-assay precision, utilizing two doses of purified α-klotho. The results indicated coefficients of variation of 2.3% and 3.3% for the intra-assay precision of the two samples, while the inter-assay coefficients of variation, determined through duplicate sample tests, were 3.4% and 3.8%17.
UI
The presence of stress UI (SUI) was determined by asking participants if they had any occurrence of urine leakage or loss of control, even in small amounts, within the past 12 months during activities like coughing, lifting, or exercising. Meanwhile, urge UI (UUI) was defined based on participants reporting leakage or loss of control accompanied by a strong need or pressure to urinate and an inability to make it to the toilet promptly. Participants who reported experiencing both SUI and UUI were categorized as having mixed UI (MUI).
To assess the severity of UI, we examined the data using the Incontinence Severity Index (ISI), an established measurement tool that demonstrated a significant association with the volume and frequency of incontinence18. The ISI includes a query about the frequency of episodes (less than once per month, a few times a month, a few times a week, or every day and/or night) as well as a query regarding the amount of leakage (drops/splashes, or more). The multiplication of the responses to these two questions yields a severity score ranging from 1 to 8. Scores 1 to 2 indicate slight severity, 3 to 4 indicate moderate severity, and 6 to 8 indicate severe severity.
Study covariates
The study collected data from female participants who self-reported their race/ethnicity, including Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and Others. The participants were categorized based on their ratio of family income to poverty (PIR) and divided into three groups: < 1.30, 1.30 − 2.99, and ≥ 3.00. Education level was also recorded, ranging from less than a high school diploma, a high school diploma or GED equivalent, to education beyond high school. Physical activity (PA) level was assessed and categorized as low, moderate, or vigorous. To determine weight classification, body mass index (BMI) (kg/m2) was utilized, with participants being classified as either normal weight (< 25.0), overweight (25.0–29.9), or obese (≥ 30.0). Smoking status was divided into three categories19: never smoker (less than 100 cigarettes consumed in a lifetime), former smoker (having consumed 100 or more cigarettes in a lifetime but currently not smoking), and current smoker (having consumed 100 or more cigarettes in a lifetime and currently smoking). Alcohol consumption status was categorized as follows20: (1) never drinker (having consumed fewer than 12 drinks in a lifetime); (2) former drinker (having consumed 12 or more drinks in a lifetime but not having consumed any in the last year); (3) light-to-moderate drinker (consuming less than 3 drinks per day for females and less than 4 drinks per day for males on average over the past year, or engaging in binge drinking [consuming 4 or more drinks on the same occasion for females and 5 or more drinks on the same occasion for males] on fewer than 5 days per month over the past year); (4) heavy drinker (consuming 3 or more drinks per day for females, 4 or more drinks per day for males, or engaging in binge drinking on 5 or more days per month over the past year). Diabetes was defined based on several criteria, including self-reported diagnosis, usage of anti-diabetic medication, a Hemoglobin A1c (HbA1c) level ≥ 6.5%, a fasting plasma glucose level ≥ 7.0 mmol/L, or random plasma glucose level ≥ 11.1 mmol/L. CKD was defined as eGFR < 60 ml/min/1.73m2 and/or urine albumin/creatinine ratio ≥ 30 mg/g21. The determination of the presence of CVD, which encompasses congestive heart failure, coronary heart disease, angina pectoris, heart attack, and stroke, was determined based on whether a medical professional had previously informed participants about their condition.
Statistical analysis
The data for this study was analyzed following the analytical procedures of the National Center for Health Statistics. Survey-weighted means ± Standard Error (SE) for continuous variables and survey-weighted proportions with a 95% confidence interval (CI) for categorical variables were used to characterize the characteristics of the sampled population. Weighted linear regression was used to compare the baseline characteristics of continuous variables, while weighted chi-square tests were used for categorical variables. Survey-weighted multiple linear regression models were utilized to examine the association between serum α-klotho levels and UI, including the severity and the different UI types. Four models were examined in accordance with the guidelines made by Strengthening the Reporting of Observational Studies in Epidemiology: Model 1 was unadjusted. Model 2 included minimal adjustments for demographic factors, including age and race/ethnicity. Model 3 incorporated additional adjustments for lifestyle variables, including BMI, marital status, education level, PIR, smoking status, alcohol consumption, and PA. Model 4 was further adjusted for clinical variables like diabetes, CKD, CVD, and vaginal delivery times. Moreover, stratified multivariate regression analyses were conducted to investigate any differences in the relationship between serum α-klotho levels and the severity of UI across various age groups (< / ≥ 60 years), BMI, PIR, diabetes, CKD, and CVD. Given that serum α-klotho levels decrease in individuals with CKD, CVD, or diabetes, which might affect the results, we conducted further sensitivity analyses after excluding individuals with CKD, CVD, or diabetes. All analyses were conducted using the R 4.3.2 software. Statistical significance was determined with a two-tailed P < 0.05.
Results
Population characteristics
In our study, we included a total of 2628 female participants aged between 40 and 79 years for the final analysis, representing 22,492,348 non-institutionalized females. The average age of the participants was 53.9 ± 0.2 years, and the average serum α-klotho level was 873.0 ± 8.9 pg/mL. Detailed baseline characteristics are summarized in Table 1. Among all participants, 1,186 individuals (weighted 58.17%) had UI. The slight, moderate, and severe UI percentages were 38.89%, 16.75%, and 2.53%, respectively.
Our findings indicate that participants with severe UI were more likely to be older, of Mexican American ethnicity, live alone, have a higher number of vaginal delivery, and have a higher prevalence of obesity and MUI. Additionally, these individuals had lower PIR, α-klotho levels, PA, and education levels compared to participants without UI. Furthermore, participants with severe UI had higher rates of cigarette smoking and comorbidities such as diabetes, CKD, or CVD and lower rates of alcohol consumption.
In our univariate analysis, we noticed a noteworthy connection between serum α-klotho levels and the severity of UI, but there is no apparent relationship with the type of UI. As the severity of UI increased, the serum concentration of α-klotho decreased. Variables such as age, race/ethnicity, BMI, education level, alcohol consumption, diabetes, and CKD were all associated with serum α-klotho levels. Detailed results can be found in Table S1.
Relationship between serum α-klotho level and UI
After adjusting for all covariates in model 4, our study revealed a significant finding regarding the link between serum α-klotho levels and the severity of UI. Specifically, individuals with severe UI had significantly lower serum α-klotho levels (β = − 100.66; 95% CI: − 156.31, − 45.01; P < 0.001) than those without UI. However, we did not detect any statistically significant correlation between serum α-klotho levels and either slight or moderate UI (β = 29.89; 95% CI: − 6.72, 66.50; P = 0.116; β = − 8.93; 95% CI: − 45.26, 27.41; P = 0.632, respectively; Table 2). In the unadjusted and each adjusted model, no statistically significant correlation was found between serum α-klotho levels and any UI type. For more detailed results, please refer to Table S2.
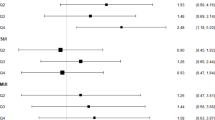
In addition, when conducting stratified analyses, we found that the association between serum α-klotho levels and the severity of UI remained robust across subgroups categorized by age group, PIR, and diabetes. Interestingly, we observed a stronger negative association in the 40–59 age group compared to those aged 60–79 (P for interaction = 0.031). However, it is important to note that we did not establish a significant connection in individuals with normal weight, CKD, or CVD (Fig. 2.). In the sensitivity analysis, after excluding individuals with CKD, CVD, or diabetes, the results remained consistent with those previously reported. Detailed findings are presented in Table S3 and Table S4.
Stratified analyses of the association between serum α-klotho levels (pg/mL) and the severity of UI. Each stratification adjusted for all factors (age, race/ethnicity, BMI, marital status, education level, PIR, smoking status, alcohol consumption, PA, diabetes, CKD, CVD, and vaginal delivery times) except the stratification factor itself. UI urinary incontinence, BMI body mass index, PIR ratio of family income to poverty, PA physical activity, CKD chronic kidney disease, CVD cardiovascular disease.
Discussion
In this study, we aimed to investigate the potential link between α-klotho levels and the UI in females. The outcomes demonstrated an inverse correlation between α-klotho levels and the severity of UI in middle-aged and older females in the US. However, this association was not statistically significant in females with normal weight, CKD, or CVD. Additionally, we found no significant association between slight or moderate UI and α-klotho levels. Similarly, we did not find any significant association between the type of UI and α-klotho levels. This investigation marks the first attempt to explore the connection between serum α-klotho levels and the UI in females, providing valuable insights into the plausible role of α-klotho in the pathophysiology of severe female UI. Understanding this relationship may have significant implications for preventive strategies, diagnostic approaches, and targeted therapies, ultimately improving the quality of life for females suffering from UI.
Extensive research has revealed the significant role of α-klotho protein in various physiological processes, particularly in the aging process12. Its involvement has been established in age-related conditions such as bone metabolism22, cardiovascular disease23, and cognitive decline24. Importantly, the aging process itself has been implicated in the development of UI25. However, the connection between α-klotho levels and UI in females remains relatively unexplored. Our study findings provide valuable insights by demonstrating a significant correlation between serum α-klotho levels and severe UI, while no significant association was found with slight or moderate UI. These findings propose a potential protective role of α-klotho in the development or progression of UI in females.
The relationship between α-klotho and UI is not yet fully understood. However, based on existing knowledge, several hypotheses can be proposed. Firstly, studies have shown a strong positive connection between α-klotho and muscular strength and function17,26,27, indicating that lower levels of α-klotho may weaken pelvic floor muscles and increase the risk of severe UI. Secondly, the α-klotho’s involvement in regulating oxidative stress11 suggests a potential connection to the development of UI, as oxidative stress is known to contribute to urinary dysfunction28,29. In addition, inflammation has been linked to reduced α-klotho protein levels30, which can affect the expression of connexins and lead to urinary bladder dysfunction31,32. Further investigation is warranted to gain a deeper understanding of the underlying mechanisms involved and determine if targeting α-klotho protein could prevent or manage UI in females.
Our study discovered no significant link between serum α-klotho levels and severe UI in individuals with normal weight. This is interesting considering prior investigations that have shown a connection between weight gain and a higher occurrence of UI31,32. Previous research has also found an inverse relationship between obesity in women and serum α-klotho levels, while lean mass index has been positively associated with serum α-klotho levels33. Therefore, our findings suggest that maintaining a low BMI may play a role in preserving adequate serum α-klotho levels and potentially reducing the occurrence of UI.
Our study also found no significant correlation between serum α-klotho levels and severe UI in individuals with CKD or CVD. However, the underlying mechanisms of this relationship are still unclear. Some studies suggest that CKD may result in a loss of muscle proteins, leading to decreased muscle strength and function34,35, which could possibly contribute to UI. Similarly, several studies have linked CVD, including coronary heart disease36, heart failure37, atrial fibrillation36, and stroke38, to low muscle strength and even sarcopenia. This decline in physical function, capability, and performance may also contribute to UI. Additionally, vascular disorders like atherosclerosis and dysfunction of the endothelium within the pelvic vascular system may potentially contribute to bladder dysfunction39. Furthermore, commonly prescribed medications for individuals with CKD or CVD, such as diuretics and beta-blockers, may increase urine production and excretion, potentially affecting urethral and pelvic floor muscles and influencing the occurrence and severity of UI40,41.
Strengths and limitations
Our study utilized the NHANES database, which is both extensive and representative of the nation. We made an unprecedented discovery that there is an inverse correlation between serum α-klotho levels and the severity of UI in middle-aged and older females. For the reliability of our findings, we conducted various sensitivity analyses alongside our large sample size. However, it is important to note several limitations of our study. Firstly, our study’s cross-sectional design restricts us from establishing a causal relationship between α-klotho levels and the severity of UI. Longitudinal studies are needed to determine causality. Secondly, our study relied on self-reported UI, which may lead to misreporting and introduce a bias towards the null hypothesis. Additionally, the symptoms of urinary incontinence may overlap with those of urinary tract infection, which could further contribute to recall bias. Thirdly, future studies should consider gathering additional information on medications or supplements that may affect UI levels. Fourthly, while we accounted for several potential confounding factors, it is important to acknowledge the possibility of unknown elements, such as antioxidants, oxidative stress indicators, and inflammation response, that could influence our results. Further investigation is necessary to understand the underlying mechanism by assessing antioxidant levels and inflammation response. Lastly, caution must be exercised when generalizing the findings of this study, as its Western population focus may not be applicable to other populations.
Conclusions
In conclusion, our study reveals a significant negative correlation between serum α-klotho levels and severe female UI. Our findings indicate that decreased α-klotho levels may play a role in developing or progressing severe UI. However, further research is needed to address the limitations of this study and explore the underlying mechanisms involved. This will provide valuable insights into the pathophysiology of UI and potential therapeutic strategies.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- ELISA:
-
Enzyme-linked immunosorbent assay
- ISI:
-
Incontinence severity index
- MEC:
-
Mobile examination center
- MUI:
-
Mixed urinary incontinence
- NCHS:
-
National Center for Health Statistics
- NHANES:
-
National Health and Nutrition Examination Survey
- PIR:
-
Ratio of family income to poverty
- PA:
-
Physical activity
- SUI:
-
Stress urinary incontinence
- UI:
-
Urinary incontinence
- UUI:
-
Urge urinary incontinence
References
Suskind, A. M. et al. Urinary incontinence in older women: The role of body composition and muscle strength: From the health, aging, and body composition study. J. Am. Geriatr. Soc. 65, 42–50. https://doi.org/10.1111/jgs.14545 (2017).
Batmani, S., Jalali, R., Mohammadi, M. & Bokaee, S. Prevalence and factors related to urinary incontinence in older adults women worldwide: A comprehensive systematic review and meta-analysis of observational studies. BMC Geriatr. 21, 212. https://doi.org/10.1186/s12877-021-02135-8 (2021).
Kaur, T. et al. A cross-sectional case-control study of depression in incontinent women. J. Midlife Health 12, 132–136. https://doi.org/10.4103/jmh.JMH_98_20 (2021).
Lim, Y. M., Lee, S. R., Choi, E. J., Jeong, K. & Chung, H. W. Urinary incontinence is strongly associated with depression in middle-aged and older Korean women: Data from the Korean longitudinal study of ageing. Eur. J. Obstet. Gynecol. Reprod. Biol. 220, 69–73. https://doi.org/10.1016/j.ejogrb.2017.11.017 (2018).
Linde, J. M., Nijman, R. J. M., Trzpis, M. & Broens, P. M. A. Urinary incontinence in the Netherlands: Prevalence and associated risk factors in adults. Neurourol. Urodyn. 36, 1519–1528. https://doi.org/10.1002/nau.23121 (2017).
Doumouchtsis, S. K., Loganathan, J. & Pergialiotis, V. The role of obesity on urinary incontinence and anal incontinence in women: A review. BJOG 129, 162–170. https://doi.org/10.1111/1471-0528.16848 (2022).
de Vasconcelos, V. S. & da Costa, A. A. R. Frequency and factors associated with urinary incontinence in pregnant adolescents: A cross-sectional study. J. Pediatr. Adolesc. Gynecol. 34, 366–376. https://doi.org/10.1016/j.jpag.2020.12.013 (2021).
Deegan, E. G., Stothers, L., Kavanagh, A. & Macnab, A. J. Quantification of pelvic floor muscle strength in female urinary incontinence: A systematic review and comparison of contemporary methodologies. Neurourol. Urodyn. 37, 33–45. https://doi.org/10.1002/nau.23285 (2018).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. https://doi.org/10.1038/36285 (1997).
Bergmark, B. A. et al. Klotho, fibroblast growth factor-23, and the renin-angiotensin system - An analysis from the PEACE trial. Eur. J. Heart Fail. 21, 462–470. https://doi.org/10.1002/ejhf.1424 (2019).
Xu, Y. & Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 36, 174–193. https://doi.org/10.1210/er.2013-1079 (2015).
Kuro, O. M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15, 27–44. https://doi.org/10.1038/s41581-018-0078-3 (2019).
Hanson, K., Fisher, K. & Hooper, N. M. Exploiting the neuroprotective effects of alpha-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction. Neuronal Signal 5, NS20200101. https://doi.org/10.1042/NS20200101 (2021).
Xu, J. P. et al. Associations between serum soluble alpha-klotho and the prevalence of specific cardiovascular disease. Front. Cardiovasc. Med. 9, 899307. https://doi.org/10.3389/fcvm.2022.899307 (2022).
Izquierdo, M. C. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant 27 Suppl 4, iv6–10. https://doi.org/10.1093/ndt/gfs426 (2012).
National Health and Nutrition Examination Survey 2015–2016 Data Documentation, Codebook, and Frequencies.https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/SSKL_I.htm. Accessed Apr 2021.
Sahu, A. et al. Age-related declines in alpha-klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859. https://doi.org/10.1038/s41467-018-07253-3 (2018).
Sandvik, H., Seim, A., Vanvik, A. & Hunskaar, S. A severity index for epidemiological surveys of female urinary incontinence: Comparison with 48-hour pad-weighing tests. Neurourol. Urodyn. 19, 137–145. https://doi.org/10.1002/(sici)1520-6777(2000)19:2%3c137::aid-nau4%3e3.0.co;2-g (2000).
Du, R. et al. Association between the duration of smoking cessation and alpha-Klotho levels in the US middle-aged and elderly population. Heliyon 10, e38298. https://doi.org/10.1016/j.heliyon.2024.e38298 (2024).
Jiang, M., Tang, X., Wang, P., Yang, L. & Du, R. Association between daily alcohol consumption and serum alpha klotho levels among U.S. adults over 40 years old: a cross-sectional study. BMC Public Health 23, 1901. https://doi.org/10.1186/s12889-023-16830-1 (2023).
Kidney Disease: Improving Global Outcomes Glomerular Diseases Work, G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1-S276. https://doi.org/10.1016/j.kint.2021.05.021 (2021).
Yilmaz, V. T. et al. FGF-23, alpha-klotho gene polymorphism and their relationship with the markers of bone metabolism in chronic peritoneal dialysis patients. Eur. J. Med. 47, 115–125. https://doi.org/10.5152/eurasianjmed.2015.93 (2015).
Sun, X., Chen, L., He, Y. & Zheng, L. Circulating alpha-klotho levels in relation to cardiovascular diseases: A Mendelian randomization study. Front. Endocrinol. (Lausanne) 13, 842846. https://doi.org/10.3389/fendo.2022.842846 (2022).
Kundu, P. et al. Serum levels of alpha-klotho are correlated with cerebrospinal fluid levels and predict measures of cognitive function. J. Alzheimers Dis. 86, 1471–1481. https://doi.org/10.3233/JAD-215719 (2022).
Sohn, K., Lee, C. K., Shin, J. & Lee, J. Association between female urinary incontinence and geriatric health problems: Results from Korean Longitudinal Study of Ageing (2006). Korean J. Fam. Med. 39, 10–14. https://doi.org/10.4082/kjfm.2018.39.1.10 (2018).
Semba, R. D. et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: The InCHIANTI study. Eur. J. Appl. Physiol. 112, 1215–1220. https://doi.org/10.1007/s00421-011-2072-3 (2012).
Ohsawa, Y. et al. Circulating alpha-klotho counteracts transforming growth factor-beta-induced sarcopenia. Am. J. Pathol. 193, 591–607. https://doi.org/10.1016/j.ajpath.2023.01.009 (2023).
Wu, Y. H. et al. Bladder hyperactivity induced by oxidative stress and bladder ischemia: A review of treatment strategies with antioxidants. Int. J. Mol. Sci. 22. https://doi.org/10.3390/ijms22116014 (2021).
Chen, W. H., Jiang, Y. H. & Kuo, H. C. Urinary oxidative stress biomarkers in the diagnosis of detrusor overactivity in female patients with stress urinary incontinence. Biomedicines 11. https://doi.org/10.3390/biomedicines11020357 (2023).
Fitzpatrick, E. A., Han, X., Xiao, Z. & Quarles, L. D. Role of fibroblast growth factor-23 in innate immune responses. Front. Endocrinol (Lausanne) 9, 320. https://doi.org/10.3389/fendo.2018.00320 (2018).
Ninomiya, S., Naito, K., Nakanishi, K. & Okayama, H. Prevalence and risk factors of urinary incontinence and overactive bladder in Japanese women. Low Urine Tract Symp. 10, 308–314. https://doi.org/10.1111/luts.12185 (2018).
Hagan, K. A. et al. A prospective study of the natural history of urinary incontinence in women. Am. J. Obstet. Gynecol. 218, 502 e501–502 e508. https://doi.org/10.1016/j.ajog.2018.01.045 (2018).
Amaro-Gahete, F. J. et al. Body composition and S-klotho plasma levels in middle-aged adults: A cross-sectional study. Rejuven. Res. 22, 478–483. https://doi.org/10.1089/rej.2018.2092 (2019).
Wang, X. H. & Mitch, W. E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 10, 504–516. https://doi.org/10.1038/nrneph.2014.112 (2014).
Huang, Y. et al. The impact of senescence on muscle wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 14, 126–141. https://doi.org/10.1002/jcsm.13112 (2023).
Xia, M. F. et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 40, 571–580. https://doi.org/10.1016/j.clnu.2020.06.003 (2021).
Zhang, Y. et al. Sarcopenia in heart failure: A systematic review and meta-analysis. ESC Heart Fail. 8, 1007–1017. https://doi.org/10.1002/ehf2.13255 (2021).
Gao, K. et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine 44, 101264. https://doi.org/10.1016/j.eclinm.2021.101264 (2022).
Ponholzer, A., Temml, C., Wehrberger, C., Marszalek, M. & Madersbacher, S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur. Urol. 50, 581–586. https://doi.org/10.1016/j.eururo.2006.01.031 (2006).
Patel, M. et al. Urinary incontinence and diuretic avoidance among adults with chronic kidney disease. Int. Urol. Nephrol. 48, 1321–1326. https://doi.org/10.1007/s11255-016-1304-1 (2016).
Tannenbaum, C. & Johnell, K. Managing therapeutic competition in patients with heart failure, lower urinary tract symptoms and incontinence. Drugs Aging 31, 93–101. https://doi.org/10.1007/s40266-013-0145-1 (2014).
Author information
Authors and Affiliations
Contributions
XY T: Conceptualization, Validation, Data Curation, Writing – Original Draft; YH S: Conceptualization, Writing – Original Draft Preparation; HL: Software, Data Curation, Visualization; WJ H: Software, Data Curation; ZL C: Data Curation, Validation; LY: Methodology, Opinion – Review; CY: Supervision, Project Administration, Writing – Review & Editing; RD: Conceptualization, Supervision, Project Administration, Writing – Review & Editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the participants from NHANES 2007–2016 gave documented signed consent. These studies were approved by the ethics reviews from the National Center for Health Statistics (NCHS) Ethics Review Board. The NCHS IRB/ERB protocol number for 2007–2010 was #2005-06 and the number for 2011–2016 was #2011-17.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, X., Song, Y., Liang, H. et al. The relationship between serum alpha-klotho levels and urinary incontinence in middle-aged and older females: insights from NHANES. Sci Rep 14, 28667 (2024). https://doi.org/10.1038/s41598-024-80231-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80231-6