Abstract
To investigate whether we can rule out the left atrial appendage (LAA) thrombus in patients with atrial fibrillation (AF) using different scanning protocol by Cardiovascular Computed Tomography (CCT). We retrospectively reviewed the CCT images of 138 patients with AF to assess LAA thrombus. Patients with no thrombosis diagnosed by preoperative CCT should be confirmed using intraoperative findings as the reference standard. Patients diagnosed with thrombosis were then compared with TEE examination. Different CT scanning protocol were used to assess LAA thrombus according to individual differences. In 126 cases, there was no thrombus in the LAA evaluated by preoperative CCT, and it was confirmed in the subsequent radiofrequency operation. CCT has the accuracy of 100% in confirming the absence of thrombus in the LAA. Twelve thrombi were detected by CCT, eleven thrombi were detected by TEE. Only one patient had different results between CCT and TEE. We could use different CT scanning protocol to rule out LAA thrombus according to individual differences. In some special patients, LAA thrombus can be evaluated by CCT instead of TEE.
Similar content being viewed by others
Introduction
LAA is a clinically important anatomic structure which has diverse and different morphological appearances1. LAA is the main location of thrombus formation, predominantly in patients with nonvalvular AF. The presence of a thrombus in LAA is considered an absolute contraindication to catheter ablation (CA), because the navigation of catheters inside the left atrium may dislodge the thrombus, resulting in thromboembolic complications2. Therefore, assessment of LAA thrombus is very important before the various endovascular interventions and surgical procedures in patients with AF.
Currently, TEE is considered the gold standard technique for the detection of LAA thrombus. It is accessible and has no special risks. However, the performance of TEE can prove difficult or may be impossible in some patients, such as those who cannot tolerate TEE process, those have severe esophageal lesions, esophageal obstruction or anatomical abnormalities. Physicians have longed for an alternative examination technique for these special groups.
Over the past dozen years, the role of CCT in assessing LAA thrombus has been supported by a growing number of reports3,4,5,6,7,8,9,10,11,12,13,14,15,16. However, all the reports use TEE as the reference standard. The patient underwent both CCT and TEE at meantime. In this study, we want to investigate whether CCT could rule out LAA thrombus using different scanning protocol.
Methods
This study was approved by the Institutional Ethics Committee of Yantai Yuhuangding Hospital. Requirement of informed consent was waived by the Institutional Ethics Committee of Yantai Yuhuangding Hospital, because it was a retrospective study. All methods were performed in accordance with the relevant guidelines and regulations.
Patient selection
From June 2022 to August 2023, 138 patients were enrolled in this study. Inclusion criteria: 1. Patient had a clear diagnosis of non-valvular AF 2. The patient underwent CCT and subsequent CA (If there was no thrombus in the LAA). Exclusion criteria: 1. Patients were excluded if they had an allergy to the contrast agent, renal dysfunction, or severe mitral valve disease. 2. Patients without the necessary clinical data.
Computed tomography examination
Computed tomography was performed by a third generation dual-source CT (SOMATOM Force; Siemens Healthineers, Forchheim, Germany). The CT scan parameters were as follows: 0.75 mm × 192 mm × 0.6 mm acquisition collimation with z-flying focal spot technique. An automated tube current modulation (Care Dose 4D, Siemens Healthcare) was used in scanning. Tube voltage and tube current were adjusted according to the body mass index. CT scans were performed in cranio-caudal direction at a supine position during a mid-inspiratory breath-hold. No betablockers were used for regulation of heart rate in any of the enrollees, and whether patients were in normal sinus rhythm or atrial fibrillation during image acquisition was not recorded.
Contrast media were injected by a dual-syringe injector (Stellant D-CE) using an 18-gauge intravenous needle placed in the right antecubital vein. Firstly, 75 mL of contrast agent (Loversol Injection, 320 mg/ml, Heng Rui Pharmaceuticals, Jiangsu, China) was administered. Then, 50 ml of saline was administered. The injection rate was 5 ml/s. Contrast agent application was controlled by a bolus tracking technique. A region of interest was placed in LA, and image acquisition was automatically started 6 s after the attenuation reached the predefined threshold of 100 HU. The standard scan was performed using a test bolus technique for scan timing. To minimize unnecessary radiation exposure, delayed scans were limited to the LA; the 1-min scan was performed only when a LAA filling defect was found at a quick review of the obtained images on the angiographic phase, the 3-min scan when it persisted at 1-min.
Image analysis
The acquired images were transferred to the workstation, CT post-processing was performed by a dedicated clinical radiology workstation (Syngo Via, VB10_HF07, Siemens Healthcare). All CT images were assessed by two independent radiologists (> 10 years of experience in cardiovascular imaging) who were blinded to echocardiographic and clinical information. Disagreements between readers were settled by consensus.
For CT, we defined a thrombus as a filling defect with an oval or convex shape. For TEE, a thrombus was defined as a well-circumscribed, uniformly consistent echogenic mass with texture different from that of the LAA wall17. If no thrombus was observed in the LAA by CCT, CA was performed as planned. If the LAA thrombus was diagnosed by CCT, TEE was subsequently performed to verify it.
Radiation dose
An effective radiation dose for the CT examination was calculated for all patients. The dose–length product (DLP, measured in milligray–centimetres) is defined as the volume CT dose index multiplied by the image length and is an indicator of the integrated radiation dose of the entire CT examination. The DLP displayed on the CT console was recorded. A reasonable approximation of the effective CT radiation dose was calculated by multiplying DLP by a conversion coefficient for the chest (κ = 0.017 mSv mGy−1 cm−118.
Statistical analysis
All statistical analyses were performed using SPSS, version 26.0 (https://www.ibm.com/cn-zh/spss). Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as absolute values and percentages.
Results
Study population
A total of 138 patients were assessed (80 men and 58 women, 67.8 ± 9.1 years). In all cases, image quality was considered acceptable for evaluation. The average duration between CT scanning to catheter ablation was 4.4 ± 1.5 days.
CT scanning methods
Thirty-five patients (25.4%) had only a standard early phase. No thrombus was detected in the LAA in all the patients (Fig. 1).
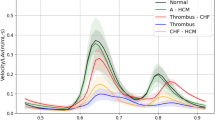
Eighty-nine patients (64.5%) had a two-phase CCT protocol (a standard early scan plus a 1-min delayed scan). Of the eighty-nine patients, seventy-seven (86.5%) patients had a filling defect in the LAA in the early phase, which disappeared in the delayed scan (Fig. 2). Twelve thrombi were detected by CCT, 11 thrombi were detected by TEE (Fig. 3). Only one patient had different results between CCT and TEE (Fig. 3).
Left atrial appendage thrombus and pseudo-thrombus. There was a filling defect in the LAA (arrow) in the early phase (A) which persisted in the delayed scan (B) and was confirmed on the TEE (C) (arrow). There was a filling defect in the LAA (arrow) in the early phase (D) which persisted in the delayed scan (E) and was not detected on the TEE (F) (arrow). LAA Left atrial appendage. TEE transesophageal echocardiogram.
Fourteen patients (10.1%) had an additional delayed scan in supine position (10 patients, 3-min delayed scan) or prone-position (4 patients, uncertain delayed time), if a filling defect was showed persistently in a 1-min delayed scan, which disappeared in the additional delayed scan (Fig. 4). No thrombus was detected in the LAA in all 14 patients. The different scanning methods and results are summarized in Table 1.
Filling defect changes in association with the CT position and scanning phase. A filling defect (arrow) was detected in both the early and delayed phases (A) and (B) in the supine position, which disappeared in the prone position (C). A filling defect (arrow) was detected in both the early phase (D) and the second phase (E 1-min delayed) in the supine position, which disappeared in the third phase (F 3-min delayed) in the supine position. LA left atrium, LAA Left atrial appendage.
Diagnostic accuracy of CCT
Of the 138 enrolled patients, CCT demonstrated thrombus in 12 (8.7%) and no abnormality in 126 (91.3%) patients. Among the 126 patients without a filling defect, CA was performed on schedule. The absence of thrombus was reconfirmed. CCT has the accuracy of 100% in confirming the absence of thrombus in the LAA. Among 11 patients, thrombi were also observed by TEE after the CT scanning. Only one filling defect in CT was not depicted as a thrombus by TEE.
Radiation effective dose
The mean estimated radiation effective dose was 4.2 ± 0.6 mSv for an early phase scan. The mean estimated radiation effective dose for the dual-phase protocol was 7.3 ± 1.0 mSv. The mean estimated radiation effective dose for the three-phase scan was 10.7 ± 1.3 mSv.
Discussion
AF is among the major risk factors for intracardiac thrombi that can lead to cerebral or systemic embolism19. The majority of intracardiac thrombi are formed inside the LAA due to turbulence and lower blood flow rates20. CA has become the first AF treatment choice in the last few decades. Before CA, whether a thrombus is present in the LAA must be evaluated21.
It is well known that the absence of a LAA filling defect on CCT allows confident exclusion of thrombus with a sensitivity and negative predictive value of 100%, thus obviating the need for further TEE assessment at the time of intervention22,23. Our study demonstrates that we can get the accuracy of 100% to exclude LAA thrombus by using different CT scanning protocol according to individual differences.
If no filling defect is found in the LAA on standard early scan, thrombus can be ruled out definitively and additional delayed scanning is not necessary. In our study, no filling defect was detected in thirty-five patients at the standard early phase. As a result, the additional radiation dose associated with delayed scanning is avoided. The mean estimated radiation effective dose was 4.2 ± 0.6 mSv. This is similar to some previous studies6,8,10.
Conversely, filling defects on CCT do not always correspond to a thrombus and may represent sludge due to circulatory stasis. The addition of a delayed acquisition is useful if filling of the LAA for the early phase is incomplete (called stasis, a frequent situation) and if the presence of a thrombus in the LAA cannot be ruled out. Both stasis and thrombus appear as filling defects in the early scan, whereas only thrombus is seen as a persistent filling defect in the delayed scan. Diagnostic specificity is much improved by this strategy. Notably, for the two-phase scan protocol, an optimal acquisition time for the second scan is most crucial but difficult aspects because the circulation time depends on the individual involved. If the acquisition time is not properly chosen, the contrast medium will run out or will not be fully filled in LAA. We adopted the 1 and 3-min delayed phase technology implemented by most centers24. In our study, seventy-seven patients had a filling defect in the LAA in the early phase, which disappeared in the 1 min delayed scan, while ten patients had an additional 3-min delayed scan in supine position, if a filling defect was showed persistently in a 1-min delayed scan. An early study thought a 3-min delay might be appropriate for optimal CT for detection of LAA thrombus, however, there are no scientific pilot data from human or animal studies about the basis for 3-min delay for the late phase for detection of LAA thrombus8. A CCT protocol adding a 6-min delayed phase to the angiographic phase can be considered optimized for the diagnosis of LAA thrombi in a recent study14. Li et al.16 suggest that early contrast-enhanced CT scanning with 1 and 3-min delay is necessary for the diagnosis of LAA thrombus, which could significantly improve the diagnostic efficiency. There is a controversy over the scan time of the late phase. The optimal time for the late phase for detection of LAA can vary according to individual differences, the kind of contrast medium, intravenous injection rate, total injection volume, and so on. Therefore, further study about the optimal acquisition time for the delayed phase for the detection of LAA thrombus is needed.
When the filling defect is relatively large after the second delayed scan, CT performed in the prone position could also be tried. Some reports have shown that CT performed in the prone position is more useful in the differentiation of thrombi from stasis than scans performed in the supine position, as gravity can increase the spread of contrast dye into the top area of the LAA25,26. Four patients in our study were suspected of thrombus in the supine position, and the absence of thrombus was confirmed by prone position. However, prone scanning, which requires changing the patient’s position, is a bit difficult to operate. Moreover, the contrast medium will run out because of the long operation time. Therefore, this type of scanning should not be used as a routine scan.
To minimize radiation, an alternative dual-enhanced protocol was proposed by only one scan after two separate bolus injections of contrast agent (a 50 ml timing bolus followed by a 70 ml bolus) with a 180 s delay between injections8. On the basis of this approach, excellent diagnostic performance in detecting LAA thrombus has been reported. Although average radiation exposure is acceptable with this technique (4.11 mSv), the contrast dosing nearly doubles the common single-dosing clinical protocol, therefore, it is unsuitable for patients with impaired renal function. This protocol was not used in our study.
Of the 138 enrolled patients, twelve thrombi were detected by CCT, 11 thrombi were detected by TEE. Only one patient had different results between CCT and TEE. Possible explanation is prominent muscle tissue in the LAA with misinterpretation as thrombus (Fig. 3). Prior to CA, if LAA thrombus is suspected by CCT, TEE can be further selected for definitive diagnosis.
Our study had some limitations. First, our study was conducted retrospectively with data from a single institution. As a confirmatory experiment to improve diagnostic accuracy, the retrospective research results are relatively reliable. However, the results are encouraging, multicenter and multiethnic research is still needed to improve regional and ethnic representation. The second limitation is radiation exposure. In our study, the total radiation dose was calculated to be approximately 10 mSv for the three-phase scan. In the future, we will do our best to reduce the radiation dose while maintaining image quality. Furthermore, Transesophageal ultrasound can identify a patent foramen ovale before an atrial fibrillation ablation, which allows avoiding a transseptal puncture; the measurement of the velocities in the left ear allows establishing some prognostic data in the patient with atrial fibrillation. CCT does not have these advantages.
Conclusions
CCT is a useful and noninvasive modality for detecting LAA thrombus. We can use different CT scanning protocol to rule out LAA thrombus according to individual differences. In some special populations, such as patients with esophageal lesions and those who cannot tolerate TEE process, LAA thrombus can be evaluated by CCT instead of TEE.
Data availability
All data generated or analysed during this study are included in this article.
Abbreviations
- AF:
-
Atrial fibrillation
- LAA:
-
Left atrial appendage
- CCT:
-
Cardiovascular computed tomography
- TEE:
-
Transesophageal echocardiogram
- CA:
-
Catheter ablation
- LA:
-
Left atrial
References
Erol, B. et al. Analysis of left atrial appendix by dual-source CT coronary angiography: Morphologic classification and imaging by volume rendered CT images. Eur. J. Radiol. 80, e346-350. https://doi.org/10.1016/j.ejrad.2010.11.008 (2011).
January, C. T. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 64, e1–e76. https://doi.org/10.1016/j.jacc.2014.03.022 (2014).
Feuchtner, G. M. et al. Diagnostic accuracy of cardiac 64slice computed tomography in detecting atrial thrombi. Comparative studywith transesophageal echocardiography and cardiac surgery. Invest. Radiol. 43, 794–801. https://doi.org/10.1097/RLI.0b013e318184cd6c (2008).
Hur, J. et al. Left atrial appendage thrombi in stroke patients: Detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology 251, 683–690. https://doi.org/10.1148/radiol.2513090794 (2009).
Kapa, S. et al. ECG-gated dual-source CT for detection of left atrial appendage thrombus in patients undergoing catheter ablation for atrial fibrillation. J. Interv. Card. Electr. 29, 75–81. https://doi.org/10.1007/s10840-010-9505-5 (2010).
Hur, J. et al. Cardioembolic stroke: Dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology 263, 688–695. https://doi.org/10.1148/radiol.12111691 (2012).
Choi, B. H. et al. Detection of left atrial thrombus in patients with mitral stenosis and atrial fibrillation: Retrospective comparison of two-phase computed tomography, transoesophageal echocardiography and surgical findings. Eur. J. Radiol. 23, 2944–2953. https://doi.org/10.1007/s00330-013-2944-5 (2013).
Hur, J. et al. Dual-enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. AM. J. Cardiol. 112, 238–244. https://doi.org/10.1016/j.amjcard.2013.03.018 (2013).
Lazoura, O. et al. A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int. J. Cardiovasc. Imag. 32, 347–354. https://doi.org/10.1007/s10554-015-0776-x (2016).
Wang, L. et al. CTA detection of left atrial stasis and thrombus in patients with atrial fibrillation. PACE 39, 1388–1393. https://doi.org/10.1111/pace.12959 (2016).
Li, W. H. et al. Detection of left atrial appendage thrombi by third-generation dual-source dual-energy CT: Iodine concentration versus conventional enhancement measurements. Int. J. Cardiol. 292, 265–270. https://doi.org/10.1016/j.ijcard.2019.04.079 (2019).
Schlett, C. L. et al. Value of dual-energy computed tomography for detection of left atrial appendage thrombus. Radiologe 60, 1162–1168. https://doi.org/10.1007/s00117-020-00774-3 (2020).
Guha, A. et al. Accuracy of contrast-enhanced computed tomography for thrombus detection prior to atrial fibrillation ablation and role of novel Left Atrial Appendage Enhancement Index in appendage flow assessment. Int. J. Cardiol. 318, 147–152. https://doi.org/10.1016/j.ijcard.2020.06.035 (2020).
Spagnolo, P. et al. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: Delayed contrast-enhanced cardiac CT. Eur. Radiol. 31, 1236–1244. https://doi.org/10.1007/s00330-020-07172-2 (2021).
Li, W. et al. Detection of left atrial appendage thrombus by dual-energy computed tomography-derived imaging biomarkers in patients with atrial fibrillation. Front. Cardiovasc. Med. 9, 809688. https://doi.org/10.3389/fcvm.2022.809688 (2022).
Li, X. N. et al. Diagnostic value of delayed contrast-enhanced cardiac computed tomography for detecting left atrial appendage thrombus in patients with atrial fibrillation. Front. Cardiovasc. Med. 9, 847163. https://doi.org/10.3389/fcvm.2022.847163 (2022).
Hur, J. et al. Thrombus in the left atrial appendage in stroke patients: Detection with cardiac CT angiography—A preliminary report. Radiology 249, 81–87. https://doi.org/10.1148/radiol.2491071544 (2008).
Geleijns, J. et al. A workshop on quality criteria for computed tomography held in Arhus, Denmark, November 1998. Eur. Radiol. 10, 544–545. https://doi.org/10.1007/s003300050095 (2000).
Benjamin, E. J. et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 98, 946–952. https://doi.org/10.1161/01.cir.98.10.946 (1998).
Kaski, J. C. & Arrebola-Moreno, A. L. Inflammation and thrombosis in atrial fibrillation. Rev. Esp. Cardiol. 64, 551–553. https://doi.org/10.1016/j.rec.2011.03.014 (2011).
Nakamura, R. et al. Prone-position computed tomography in the late phase for detecting intracardiac thrombi in the left atrial appendage before catheter ablation for atrial fibrillation. J. Cardiovasc. Electr. 32, 1803–1811. https://doi.org/10.1111/jce.15062 (2021).
Singh, N. K. et al. Left atrial appendage filling defects on 64-slice multidetector computed tomography in patients undergoing pulmonary vein isolation: Predictors and comparison to transesophageal echocardiography. J. Comput. Assist. Tomogr. 33, 946–951. https://doi.org/10.1097/RCT.0b013e31819cabc3 (2009).
Martinez, M. W. et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc. Imaging 2, 69–76. https://doi.org/10.1016/j.jcmg.2008.09.011 (2009).
Romero, J. et al. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: A meta-analysis. Circ. Cardiovasc. Imaging 6, 185–194. https://doi.org/10.1161/CIRCIMAGING.112.000153 (2013).
Kantarci, M. et al. Circulatory stasis or thrombus in left atrial appendage, an easy diagnostic solution. J. Comput. Assist. Tomogr. 43, 406–409. https://doi.org/10.1097/RCT.0000000000000853 (2019).
Kawaji, T. et al. Real-time surveillance of left atrial appendage thrombus during contrast computed tomography imaging for catheter ablation: The Reliability of Computed tomography beyond Ultrasound in THROMBUS detection (THROMBUS) study. J. Thromb. Thrombolysis 47, 42–50. https://doi.org/10.1007/s11239-018-1742-y (2019).
Funding
This work was supported by Shandong Province Natural Science Foundation [ZR2022MH274] and Taishan Scholars [tsqn202103197].
Author information
Authors and Affiliations
Contributions
1 Study concepts and design:Chunjuan Sun, Junwei Lv, Ming Liu, Heng Ma 2 Literature research:Chunjuan Sun, Junwei Lv, Ming Liu, Heng Ma 3 Clinical studies:Chunjuan Sun, Junwei Lv 4 Statistical analysis:Chunjuan Sun, Junwei Lv, Ming Liu, Heng Ma 5 Manuscript preparation:Chunjuan Sun, Junwei Lv 6 Manuscript editing:Ming Liu, Heng Ma.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, C., Lv, J., Liu, M. et al. Different scanning protocol to assess left atrial appendage thrombus in patients with atrial fibrillation by cardiovascular computed tomography. Sci Rep 14, 30414 (2024). https://doi.org/10.1038/s41598-024-81708-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81708-0
Keywords
This article is cited by
-
Advancements in Flexible and Wearable Echocardiograms for Real-Time Continuous Cardiovascular Monitoring
Current Treatment Options in Cardiovascular Medicine (2025)