Abstract
Observations of representatives of Trombidium at one locality over two subsequent years revealed the syntopic occurrence of three species: T. holosericeum, T. brevimanum, and T. heterotrichum. The separate identities of the species, which were initially supported by morphological evidence, were confirmed with a molecular approach (COI sequence data) complemented by molecular species delimitation tools (ABGD, ASAP) and phylogenetic inference. Laboratory rearing of ovigerous females of T. heterotrichum resulted in obtaining hitherto unknown larvae of the species. The first characteristic of the larvae is supplemented with extended biometrical data and data on the biology and ecology of the species. We hypothesize that at syntopic and synchronous occurrence of Trombidium spp., the host spectrum contributes to the niche segregation.
Similar content being viewed by others
Introduction
Terrestrial Parasitengona mites, together with Hydrachnidia, the latter also known as water mites, constitute one of the most diverse taxa of the prostigmatid mites (Actinotrichida: Prostigmata). Their distinctness refers to the complexity of life cycle, employing the alternation of active and calyptostatic instars, heteromorphism of larvae to active postlarval forms (deutonymphs and adults), and switching from parasitic larvae to predatory deutonymphs and adults1. Many of the terrestrial Parasitengona, also referred to as velvet mites, are comparably large arachnids with a dense, velvet-like bright red or orange cover of dorsal setae. Among them, the most characteristic and easiest to notice are red and trapezoidal in outline, some semi-synanthropic species of Trombidium spp. already observed in early spring in home gardens and cultivated areas.
Trombidium, a nominate genus of Trombidiidae known for its Holarctic distribution, comprises 37 species. Of those, 14 species are known exclusively from active postlarval forms (adult and/or deutonymph), 12 from larvae, and 11 from both2,3,4. Currently, the diagnosis and identification of Trombidium spp. are based on morphology and refer mainly to the shape and length of dorsal opisthosomal setae (pDS) in postlarval forms and to the structure of tritorostral setae (hypostomalae, bs) and traits of the posterior dorsal sclerite (scutellum) in larvae. Following the latter, two species groups could be provisionally distinguished within the genus: the ‘holosericeum’ group, in which postlarval forms possess elongate pDS, while the bs setae in larvae are developed in the form of obliquely truncated calyxes, with finger-like digitations, and the ‘brevimanum’ group, in which the pDS are shorter and distinctly widened terminally, whereas the bs setae are slenderer and possess single, short branches5. The first group, in addition to Trombidium holosericeum (L.), was likely to comprise, among others, T. geniculatum (Feider, 1955), T. latum C.L. Koch, 1837, known from all active instars, and T. heterotrichum (Berlese, 1910), whose knowledge has been limited to adults and deutonymphs. Trombidium heterotrichum, hitherto known exclusively from active postlarval forms, is one of the most rarely recorded members of the genus; its occurrence has been reported in a few localities in the Western Palaearctic2,6, in the absence of any data on the larvae and host range. Trombidium brevimanum larvae are known to exploit spiders as regular hosts and their feeding preferences are restricted to arachnids7,8, while T. holosericeum hosts cover a wide range of various hexapods8.
Here, we present the results of a survey of Trombidium aimed at verifying the species identity of specimens observed in the following seasons within the same allotment. The differences in colour of the active postlarval forms and macroscopically assessed differences in the size of the genital plate underlie the hypothesis on the occurrence of more than one species in the area. The identity of potentially co-occurring species was tested with an integrative approach, including mitochondrial COI gene analysis followed by species delimitation, as well as morphological analyses of all active instars and experimental rearing.
Materials and methods
Sampling at the study plot
The mites representing Trombidium spp. were collected in 2022 and 2023 in an allotment garden (c. 300 m2) in Wrocław, Poland [locality I, see Table 1].
The regular (at c. 7–day intervals) search for representatives of active postlarval forms was carried out from the early spring to late summer and encompassed the onset and decline, until the complete absence, of individuals in the field. Hosts together with parasitizing larvae were searched for using an entomological sweep net, considering the similar time assumptions (the onset and then the lack of larvae on hosts, sampling at the same time intervals). Larvae, at least partly engorged, were transferred to rearing vials (see Laboratory Experiments section) to trace further stages of the life cycle, whereas the unengorged larvae were directly preserved in EtOH.
Other material examined
The representatives of active postlarval forms of Trombidium spp. were collected from various localities in Poland [localities II–XI, for details – see Table 1]. The material served for morphological and molecular comparisons aimed at confirming the distinct status of the species under study. The vouchers are deposited at the Department of Invertebrate Systematic and Ecology, Wrocław University of Environmental and Life Sciences (DISE-WUELS).
Laboratory experiments
The field-collected specimens (active postlarval forms, larvae collected with hosts, and larvae that detached from hosts) were placed separately in glass rearing vials (34 × ⌀ 24 mm) with tight semi-transparent plastic lids; the vials were filled to 2/3 with a charcoaled plaster of Paris as a substratum. The material was kept in a MLR-352 H climate chamber [L12(22 °C): D12(15 °C) h photoperiod, 80% RH] until oviposition, followed by egg incubation, larval emergence, and parasitism experiments. The contents of the vials were checked at regular 3-day intervals.
The females were transferred to 96% EtOH after oviposition and identified soon after oviposition. In the subsequent part of the experiment, only the eggs laid by T. heterotrichum were considered (see the protocol for mounting and identification below).
Larvae not supplied with food were transferred to EtOH for up to a few days after emergence. Randomly selected larvae that hatched from different clutches of preidentified females were used in experiments aimed at evaluating host preferences and the success of parasitism. Three types of transparent, tight plastic containers (⌀ 30 × 15 mm; ⌀ 30 × 50 mm; 40 × 40 × 40 mm) filled to 1/3 with charcoaled plaster of Paris served as arenas. Larvae (10–20 specimens) obtained from one female were introduced to the arena and exposed to potential hosts (1–5 host specimens of each taxon at a time). The following arthropods were offered as potential hosts to mite larvae: Araneae: Micrommata virescens (Sparassidae), Enoplognatha ovata (Theridiidae), Misumena vatia, Xysticus sp. (Thomisidae); Diptera: Calliphoridae sp., Dolichopodidae sp., Muscidae spp., Syrphidae sp.; Coleoptera: Cetonia aurata (Scarabaeidae), Coccinella septempunctata (Coccinellidae), Rhagonyha fulva (Cantharidae); Hemiptera: Aelia acuminata (Pentatomidae), Eurygaster maura (Scutelleridae), Pyrrhocoris apterus (Pyrrhocoridae), Aphididae spp., Miridae spp., Cicadellidae sp.; Lepidoptera: Pieridae sp. The containers were checked daily or every two days to record the possible onset of parasitism as well as its termination and the transformation of mite larvae into subsequent developmental instars. After the termination of the experiment, all specimens were preserved in EtOH.
For larvae collected with hosts, and larvae that detached from hosts the parasitism success measured by the transformation into subsequent developmental stages, was checked.
Morphological identification
The mite material intended for morphological studies was preserved on microscopic slides; PVA (KOLCHEM) was used as a mounting medium for larvae, and Hoyer’s medium was used for deutonymphs and adults. The morphological identification of Trombidium species followed Mąkol5.
Twelve females, 10 males, five deutonymphs, and 30 larvae of T. heterotrichum were selected for detailed morphological studies. The measurements (given in micrometres) were taken with a Nikon Eclipse E600 microscope equipped with differential interference contrast (DIC) and coupled with NIS Elements BR software version 3.22.14 (www.nis-elements.com), whereas drawings were taken under a Nikon Eclipse 80i microscope equipped with DIC and Y-IDT drawing attachment. The drawings were converted into digital illustrations using Procreate® v. 5.3.7. For morphological terminology, see Vercammen-Grandjean and Popp9, Southcott10, Gabryś11 and Mąkol and Wohltmann12. Other abbreviations used in the text: AD = adult(s), DN = deutonymph(s), LV = larva(e), OL = length of ocular sclerite in larva, LS = length of scutum, WS = width of scutum.
Statistical analyses
To test the morphological differences between the metric traits of larvae representing three congeneric species, as the normal distribution was not confirmed, the non-parametric ANOVA (Kruskal-Wallis) was used. The Kruskal-Wallis tests were applied separately for all dependent variables and the false discovery rate was controlled using the Benjamini-Hochberg stepwise adjustment, and a two-tailed p < 0.001 was considered statistically significant. For each statistically significant variable in the Kruskal-Wallis test, Dunn’s multiple comparison was used (p < 0.05 was considered statistically significant). To discover clusters based on the differences between larvae of all three species, the Principal Component Analysis (PCA) was applied. The metric traits of scutum in larvae (a sclerite whose dimensions are not influenced by the degree of larval engorgement) were considered in the analysis. Only larvae with complete set of measurements were included in the PCA. All analyses were performed using R statistical software version 4.4.113. The principal components were visualized using ‘ggbiplot’ v.0.55 function of the biplot package implemented in R.
Molecular identification
For molecular identification, representatives of putatively distinct species of Trombidium spp. occurring at the same locality (see also ‘Sampling at the study plot’ section, above) and of the genus members collected at other localities in Poland (see ‘Other material examined’, above) were used. For the DNA extraction, isolation protocol, and amplification of the DNA barcode region (cytochrome c oxidase 1 subunit), see Mąkol and Felska14 (excluding the BalaCOIF and BalaCOIR primers, which were not used in the present study). The amplification product was purified using a QIAquick PCR purification kit (Qiagen) and sequenced on both strands (Genomed S.A., Poland). Multiple sequence alignment and calculation of distances between sequences, initialized with default parameters, were performed in Geneious Prime15. The sequences were deposited in GenBank (for accession numbers, see the Table 1).
Delimitation of species. For species delimitation, the distance matrix methods ABGD (automatic barcode gap discovery16) and ASAP (assembly of species by automatic partitioning17) were applied using online tools (https://bioinfo.mnhn.fr/abi/public/abgd/, and https://bioinfo.mnhn.fr/abi/public/asap/). An alignment composed of identified haplotype sequences of Trombidium spp. served as a base. The ABGD analyses were carried out with the following default options: Pmin = 0.001, Pmax = 0.1, steps = 10, and Nb bins (for distance distribution) = 20, with a relative gap width (X) = 1.5. For both ABGD and ASAP, the Kimura (K80) substitution model with a transition-transversion (ts/tv) ratio = 1.5 was used.
Phylogenetic analyses
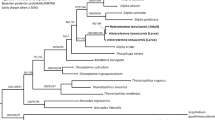
The support for species delineated using morphological and molecular approaches was tested on a dataset consisting of haplotype COI sequences of Trombidium spp. obtained during the present study (see the Table 1) and COI sequences retrieved from GenBank: KR071845 (Trombiculidae: Hirsutiella zachvatkini), OP945736 (Trombiculidae: Blankaartia acuscutellaris), KY888693 (Trombiculidae: Neotrombicula vulgaris), OL619431 (Trombiculidae: Leptotrombidium russicum), KR633049 (Microtrombidiidae: Milandanielia intermedia), MK995459 (Microtrombidiidae: Platytrombidium fasciatum), MK995458 (Podothrombiidae: Podothrombium sp.), and KM100983 (Bdellidae). The analyses were carried out using Bayesian inference implemented in MrBayes ver. 3.2.518. The GTR + I + G model of evolution was chosen using Mega X19. The multiple sequence alignment format was converted from FASTA to NEXUS using ALTER20. The consensus maximum likelihood tree was visualized with FigTree ver. 1.4.421.
Results
Field data
The representatives of active postlarval forms of Trombidium spp. were observed at the study plot (locality I) from April to July. The adults and deutonymphs were collected directly from the soil surface or uncultivated grassy areas. The macroscopic differences between individuals, pertaining most of all to body colour, allowed for the preliminary distinction between crimson red representatives of Trombidium brevimanum (Berlese, 1910) and bright red representatives of other Trombidium species. No microhabitat segregation was observed for specimens preliminarily assigned to different groups.
Parasitizing larvae were collected in the second half of July. Larvae attached to spiders were preliminarily assigned to T. brevimanum based on earlier assumptions (e.g7,8), related to host-parasite associations, whereas the larvae collected from insects were tentatively regarded as representing other Trombidium spp.
The morphology-based examination of active postlarval forms and larvae confirmed the presence of T. brevimanum and allowed the detection of two other species, T. holosericeum and T. heterotrichum, in the material. Among the other representatives of terrestrial Parasitengona, only Platytrombidium fasciatum (Microtrombidiidae), with active postlarval forms having white transverse stripes against the bright red coloration of the idiosoma dorsum and larvae that parasitize Diptera, was detected in the study plot (locality I).
Larvae of T. brevimanum were collected from Tetragnatha sp. (Tetragnathidae), Enoplognatha ovata (Theridiidae), and Xysticus ulmi (Thomisidae), larvae of T. holosericeum parasitized the true bugs (Hemiptera), including indet. Miridae and Palomena prasina (Pentatomidae), but also scorpionflies (Mecoptera) - Panorpa communis (Panorpidae), and beetles (Coleoptera) - Rhagonyha fulva (Cantharidae), whereas the larvae of T. heterotrichum were found only on hemipteran Javesella sp. (Delphacidae).
In total, 91 representatives of active postlarval forms of T. heterotrichum and 14 larvae attached to nine hosts were collected from the study plot (locality I). The number of larvae parasitizing one host varied from one to three (one larva was identified on five host specimens, two larvae on three hosts, and three larvae on one host). Of the 14 larvae recorded, seven were attached to the dorso-lateral side of the thorax, two to the ventral side of the thorax, and two to the thorax/abdomen border. For the remaining three larvae that dropped off the host, the attachment site was not recorded.
Four species of Trombidium, T. holosericeum, T. latum, T. brevimanum, and T. conisetum Mąkol, 2005, were detected in the material originating from localities II–XI (Table 1) based on morphology.
Laboratory data
The laboratory rearing of field-collected ovigerous females of T. heterotrichum resulted in the first obtaining of larvae of the species hitherto known exclusively from active postlarval forms. Trombidium heterotrichum larvae were obtained from 42 field-collected females. Oviposition was observed from early May to mid-July. On average, 135 eggs were deposited by field-collected females (48–259, for n = 7 females) in one or two clutches. The eggs, which were yellowish and 165 μm in diameter (148–177, for n = 210 eggs deposited by 21 females), developed into prelarvae within 15 days (7–25, for n = 14 clutches deposited by different females) and into larvae within the next 13 days (7–23, for n = 13 clutches deposited by different females). The total development time between oviposition and larval emergence was 26 days (22–37, for n = 23 clutches deposited by different females). The emergence of larvae was observed from mid-June to mid-August.
A selection of field-collected arthropods (see taxa listed in the Laboratory experiments section of Materials and methods) was offered to larvae that emerged in the laboratory, however, in no case did the larvae enter the parasitic phase, and in no case did the potential hosts represent the planthoppers of the Delphacidae family. Parasitism success, expressed as the emergence of deutonymphs from larvae collected in the field together with hosts, was recorded in the second half of August, i.e., 27 days on average (24–29, n = 6) after the larvae detached from the host. The time between detachment and entry into the calyptostatic protonymph was 14 days on average (7–21, n = 5), whereas the time between the onset of the protonymph and emergence of the deutonymph was 13 days on average (3–31, n = 5).
Statistical inference. Fifty out of 57 analyzed metric characters of larvae differed significantly between three species (p < 0.001) (Table 2). The Dunn’s tests showed differences between each three species with respect to 22 traits. Forty-five characters differed between T. heterotrichum and T. holosericeum, and also between T. holosericeum and T. brevimanum, whereas only 28 - between T. heterotrichum and T. brevimanum (Table 3).
Exploring the metric data sets for larvae with PCA clearly confirmed the presence of three distinct species-specific clusters in the studied material. The first two principal components explained together 85.7% of the total variation, with the first component (PCA 1) explaining 67.7% and the second (PCA 2) 18% of the variation (Fig. 1).
Molecular studies and phylogenetic inference
The amplification of the barcode COI region, followed by sequencing, resulted in 18 COI sequences of Trombidium spp. (Table 1). Of those, 11 sequences represented unique haplotypes, including four from the study plot (locality I) and seven from other localities (II–IV, VI, VII, IX, and XI) in Poland. As a result of multiple alignments of single haplotype sequences of Trombidium spp., we obtained a 606 bp data block with 81.7 to 99.8% identity (translating into one up to 111 nonidentical bases) between sequences.
The molecular species delimitation based on a dataset consisting of 11 sequences resulted in five groups, both with ASAP and with ABGD, with a barcode gap between intra- and interspecific pairwise distances of congeneric sequences of 2–14%. The haplotype sequences of all three species observed in the study plot (locality I), i.e., T. brevimanum, T. holosericeum, and T. heterotrichum, were assigned to different groups along with the selected sequences from other localities (III, IV, VI, IX, and XI). Two unique groups were formed by sequences of T. latum and T. conisetum, the species not detected within the study plot, which originated from localities II and VII and VI, respectively (Table 1). In the maximum likelihood (ML) tree, based on the COI dataset (550 bp data block) consisting of haplotype sequences of Trombidium spp. and sequences of Podothrombium sp., Trombiculidae, Microtrombidiidae, and Bdellidae, all five species of Trombidium formed a well-supported clade, and high support was also received independently by each of the species belonging to this clade (Fig. 2).Trombidium holosericeum was recovered as a sister taxon to the four remaining representatives of the genus considered in the analysis.
The first formal description of T. heterotrichum larvae, supplemented with data on active postlarval forms, is provided below.
Taxonomy
Laboratory-reared larvae of T. heterotrichum (n = 30) were subjected to detailed morphological studies. Morphometric data on field-collected females (n = 12) and males (n = 10) and laboratory-reared deutonymphs (n = 5) add to the previous characteristics of the species.
-
Trombidiidae Leach, 1815.
-
Trombidium Fabricius, 1775.
-
Type species: Acarus holosericeus Linnaeus, 1758.
-
Trombidium heterotrichum (Berlese, 1910).
-
Sericothrombium heterotrichum Berlese, 1910.
Morphology
Diagnosis. Adult and deutonymph. See Mąkol6,22.
Larva. Colour in life yellowish-orange to reddish. Hypostomalae (tritorostral setae, bs) in the shape of a slender, oblique calyx, with sharp-ended branches arising around the edges. The scutellum delicately concave anteriorly. Setae c1 arising slightly before half the length of the sclerite. ƒD formula: 2(2)2-6-4-4-4. Setae h2 shifted to the ventral side of the idiosoma. Solenidion on tarsus I in the proximal part of the segment; the famulus located distal to the solenidion. All tarsi terminated with paired claws and empodium. The inner claw on tarsus III spur-like and much shorter than the outer claw. Sword-like seta, only slightly shorter than the normally developed anterior claw, present at tarsus III termination; ogive-shaped seta not detected.
Description. Adult and deutonymph - the complementary metric data to the characteristics provided by Mąkol6 are provided in Table 4.
Larva. The standard measurements are shown in Table 2.
Gnathosoma (Fig. 3). Hypostomalae (tritorostral setae, bs) in the shape of a slender, oblique calyx, with several sharply terminated branchlets of different lengths arising around the calyx edge. The cheliceral blade curved, with one small subterminal tooth-like process at the inner edge of the blade. Setae or (= cs) short, acicular. fPp = 0-N-0-BNB-BBBζζω. Seta placed dorsally on the palp femur short, thorn-like; the most proximal and the most distal setae on the palp tibia long, with indistinct setules or barbs, seta located between them the shortest, thorn-like; six setae on the palp tarsus – the longest seta setulated, two shorter setae with barbs, two eupathidia and one solenidion. Odontus divided on most of its length, except for the most basal part.
Idiosoma, dorsum (Fig. 4a). Dorsal sclerites porous on the whole surface, except for the linear folds of the cuticle in the antero-median part of the scutum. The scutum quasirectangular in outline, protruded and rounded antero-medially, with three pairs of nonsensillary setae (AM smooth or with minute barbs, AL and PL setulose along the whole shaft) and a pair of sensilla (S). The sensilla with sparsely distributed setules along the distal part of the stem. The scutellum oval in outline, with a delicate concavity at the anterior edge; a pair of c1 setae shifted to the anterior half of the sclerite. Paired eyes on each side of the prodorsum at the level of the posterior part of the scutum. The anterior lens larger than the posterior lens, and both slightly protruded above the idiosoma surface and situated on the common oval sclerite. Dorsal idiosomal setae arranged in rows C–H (c1–c3, d1–d3, e1–e2, f1–f2, h1–h2), all setulose. The setae in row H the longest; h2 shifted to the ventral side of the idiosoma.
Idiosoma, venter (Fig. 4b). Coxal plates well delimited. fCx = NBN-BB-B. Supracoxala I (elc 1) short, acicular. Claparède’s organ inserted between coxae I and II. Setae 3a inserted between coxal plates III. fV = 4u-2 (excluding h2); all setae covered with setulae, and the shafts of the ventral setae slightly thinner than those of the dorsal setae. Anus surrounded by membranous valves.
Legs (Fig. 5a–c). Leg I: Tr (1n) – Fe (5n) – Ge (4n, 2σ, 1κ) – Ti (5n, 2φ, 1κ) – Ta (15n, 1ω, 1ε, 2ζ); leg II: Tr (1n) – Fe (4n) – Ge (3n, 1σ, 1κ) – Ti (5n, 2φ) – Ta (13(12)n, 1ω, 1ε, 1ζ); leg III: Tr (1n) – Fe (4n) – Ge (3n, 1σ) – Ti (5n) – Ta (12n). Solenidion on tarsus I at c. 0.375 segment length; famulus shifted to the more distal position; solenidion on tarsus II at approximately half the length of the segment; famulus almost levelled with solenidion. Tarsi I-III terminated with paired claws and unpaired, claw-like empodia. Claws of tarsi I-II of comparable length. The inner claw on tarsus III is spur-like and much shorter than the outer claw. A sword-like seta similar in length to the outer claw placed subterminally at tarsus III.
Distribution. Western Palaearctic (Austria, Czech Republic, Finland, France, Norway, Poland, The Netherlands, [?] Switzerland).
Remarks. Trombidium heterotrichum remains the only member of the genus in which active postlarval forms have some of the posterodorsal setae pDS (i.e., pDS II) that narrow apically (for details see6); for all other members of the genus, the pDS are either parallel-sided or enlarged at termination. A feature that manifests sexual dimorphism and can be observed only in adults is the varied size of the genital plates, specifically the epivalves - in males of T. heterotrichum, the epivalves are larger than those in females (Fig. 6). A similar difference has thus far been observed in representatives of Trombidium geniculatum5,23, while in all other Trombidium spp., the plates of both sexes are of similar size and do not show a tendency toward hypertrophy.
For differences between the active postlarval forms of T. heterotrichum and other European members of the genus that have been described or redescribed in recent decades, see Mąkol5.
The qualitative traits of T. heterotrichum larvae are similar to those of T. holosericeum, T. geniculatum, and T. latum, and the species can be distinguished by the shape of the hypostomal setae (more slender digitations in T. heterotrichum, more stout in T. holosericeum, T. geniculatum, and T. latum) and the termination of tarsus III (ogive-shaped seta not detected at segment termination in T. heterotrichum, present in the remaining three species; sword-like seta more stout and covered with bristle-like setules in T. holosericeum, more slender, smooth in T. heterotrichum). Less pronounced differences also apply to the general shapes of the scutum and scutellum.
Discussion
The overall difficulties in determining the species limits between Trombidium spp. (e.g7), which are commonly used for morphology-based identification, necessitate the use of other sources of information. This problem is equally important in the case of various systematic groups. Oh et al.24, in their studies of leptonetid spiders, reported that for closely related species that are microhabitat specialists and morphologically difficult to identify, the use of molecular analyses is essential for confirming cases of congeneric sympatry. Molecular data provide significant support for this supposition; however, given the relatively low barcoding success rate and continuing difficulties in obtaining representative strains of many mite species for comparative purposes, the results of these studies are very slowly translated into effects. One should also keep in mind that delimiting the closely related species with the application of one marker only may be of limited credibility25, and instead the integrative approach considering various evidence should be implemented. The data on life cycle and ecology may thus constitute another useful source of complementary information. The latter, however, being relatively well recognized for most common species, remains in a state of flux for an array of other taxa, making a comprehensive comparison and species resolution at the intrageneric level difficult.
A comparison of morphological and molecular studies confirmed the preliminary assignment of the examined specimens and sequences to five named species (Table 1). The conspecificity of morphologically distinguished representatives of Trombidium spp. was supported by earlier examination of the type material of all species in question22.
The molecular delimitation confirmed the presence of five species of Trombidium in the examined material, including three species occurring synchronously and syntopically in the study plot. Of the three, T. holosericeum and T. brevimanum have been adequately described, both concerning the morphology, ecology, and biology of all active instars, while the knowledge of T. heterotrichum has been limited to active postlarval forms. The scarcity of records on the occurrence of this species may be at least partly attributed to difficulties in discriminating between the larvae of T. holosericeum and T. heterotrichum. Despite the more pronounced differences between active postlarval forms, deutonymphs and adults are of less interest to researchers due to their lack of potential economic importance. During present study, the differences between T. heterotrichum, T. holosericeum and T. latum were confirmed by statistical inference in relation to larvae.
Phylogenetic analyses have shown that the shape of posterodorsal setae in active postlarval forms of Trombidium but also the shape of hypostomalae in larvae, contrary to earlier suppositions (e.g5), are unlikely to differentiate the species groups, as various shapes of pDS could be observed in the group constituting a sister taxon of T. holosericeum. The strong support received by all species of Trombidium considered in the analysis, combined with the knowledge of all active instars of three species recorded from the study plot, constitutes yet another proof of the separate status of all three species. However, the relatively indistinct qualitative differences between the larvae of T. holosericeum and T. heterotrichum necessitate careful identification of Trombidium members, especially because the evidence is limited to morphology or based on only single life instars.
In addition to the distinct structure of dorsal opisthosomal setae in postlarval forms, which is not known for other Trombidium species, the presence of enlarged external genital sclerites (epivalves) in males of T. heterotrichum is noteworthy. Enlarged sclerites have been confirmed only for T. geniculatum; however, their adaptive role remains unknown.
The first description of the larvae of T. heterotrichum contributes to the knowledge of the life cycle of Trombidium spp. and terrestrial Parasitengona as a whole. Like T. holosericeum and T. brevimanum7,12, T. heterotrichum also seems to represent a semi-univoltine species with an iteroparous reproduction mode. The duration of development, from oviposition to larval stage, was comparable to that recorded for other representatives of the genus. Despite the relatively wide range of time observed for several clutches of T. heterotrichum as far as the egg-prelarva and prelarva-larva stages are concerned, the overall time recorded for the egg-larva phase is compensated for by particular clutches and characterized by a smaller variability range.
Trombidium heterotrichum is the twelfth of 37 nominal species in the genus and is known from larvae and active postlarval forms. Some of the abovementioned species require redescription due to laconic descriptions that do not provide grounds for correct species discrimination.
In the present work, we also document the first, to the best of our knowledge, case of sympatric, syntopic, and synchronous occurrence of three representatives of the same genus of trombidiid mites at all stages of their life cycle. Mąkol and Wohltmann12 suggested that differences between some Trombidium spp. may predominantly apply to the range of parasitized hosts. Trombidium brevimanum was reported to parasitize arachnids7,8, and adequate preferences were also confirmed in the present study. Trombidium holosericeum larvae are known for their associations with various hexapods8, and despite the wide range of hosts available for reappraisal, this species tends to prefer hemipteran Miridae. Notably, during our survey, both T. holosericeum and T. heterotrichum were collected from Hemiptera; however, members of distinct families, Miridae and Delphacidae, were parasitized by larvae of each species. This may lead to the assumption that the actual host spectrum of members of Trombidium spp., with special reference to the most common species, can be narrower than previously reported; however, more studies should be carried out to confirm this hypothesis. Nevertheless, the lack of Delphacidae in the array of potential hosts offered to larvae of T. heterotrichum in the laboratory may further explain the failure of parasitism experiments, and at the same time, preferences for Miridae and Delphacidae observed in the natural environment may constitute preliminary confirmation of niche segregation between two representatives of Trombidium.
Data availability
All data analyzed in this study are included in this article or are available from the corresponding author on request.
Change history
28 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-98295-3
References
Wohltmann, A. The evolution of life histories in Parasitengona (Acari: Prostigmata). Acarologia 41, 145–204 (2000).
Mąkol, J. & Wohltmann, A. An annotated checklist of terrestrial Parasitengona (actinotrichida: Prostigmata) of the World, excluding Trombiculidae and Walchiidae. Ann. Zool. 62, 359–562 (2012).
Saboori, A., Šundic, M. & Pešic, V. A new species of the genus Trombidium Fabricius (Acari: Trombidiidae), with a checklist of terrestrial parasitengone mites of Montenegro. Syst. Appl. Acarol. 22, 584–601 (2017).
Sevsay, S., Buğa, E. & Elveri̇ci̇, M. A new species of the genus Trombidium (Acari: Trombidioidea) parasitic on spider from Turkey. Acarol Stud. 2, 34–40 (2020).
Mąkol, J. Trombidiidae (Acari: Actinotrichida: Trombidioidea) of Poland. Fauna Poloniae. Mus. Inst. Zool. PAS, Natura Optima Dux Foundation, Warsaw, 1 [NS], 1–259 (2005).
Mąkol, J. A redescription of Trombidium heterotrichum (Berlese, 1910) (Acari: Actinotrichida, Trombidioidea) from Berlese Acaroteca. Redia LXXXVI, 71–76 (2003).
Wohltmann, A. On the biology of Trombidium brevimanum (Berlese, 1910) (Acari: Prostigmata: Parasitengonae: Trombidiidae) with a redescription of all active instars. Mitt Hamburg Zool. Mus. Inst. 96, 159–170 (1999).
Felska, M., Wohltmann, A. & Mąkol, J. A synopsis of host-parasite associations between Trombidioidea (Trombidiformes: Prostigmata, Parasitengona) and arthropod hosts. Syst. Appl. Acarol. 23, 1375–1479 (2018).
Vercammen-Grandjean, P. H. & Popp, E. Atomus rhopalicus n. sp., a parasite of RhopaTutelatutela Walker (Hymenoptera) from Germany (Trombidiidae: Acarina). Opusc Zool. 95, 1–8 (1967).
Southcott, R. V. Studies on the taxonomy and biology of the subfamily Trombidiinae (Acarina: Trombidiidae) with a critical revision of the genera. Aust J. Zool. Suppl. Ser. 123, 1–116 (1986).
Gabryś, G. The World genera of Microtrombidiidae (Acari, Actinedida, Trombidioidea). Monogr. Up. Sil Mus. 2, 361 (1999).
Mąkol, J. & Wohltmann, A. A redescription of Trombidium holosericeum (Linnaeus, 1758) (Acari: Actinotrichida: Trombidioidea) with characteristics of all active instars and notes on taxonomy and biology. Ann. Zool. 50, 67–91 (2000).
R Core Team R. a language and environment for statistical computing. R foundation for statistical computing, Vienna; (2024). https://www.R-project.org/
Mąkol, J. & Felska, M. Intraspecific variation of morphological traits backed up with molecular evidence votes for reappraisal of hitherto distinguished Balaustium species—a case study of Balaustium murorum (Acariformes: Parasitengona, Erythraeidae). Exp. Appl. Acarol. 91, 585–601. https://doi.org/10.1007/s10493-023-00859-3 (2023).
Geneious, P. ® 2023.0.3 Biomatters Ltd. (2023). https://www.geneious.com).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x (2012).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: assemble species by automatic partitioning. Mol. Ecol. Resour. 21, 609–620. https://doi.org/10.1111/1755-0998.13281 (2021).
Ronquist, F. et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F. & Posada, D. ALTER: program-oriented format conversion of DNA and protein alignments. Nucl. Acids Res. Web Serv. Issue. ISSN, 0305–1048 (2010). http://www.sing-group.org/ALTER/
Rambaut, A. FigTree v1.4.4.; (2018). http://tree.bio.ed.ac.uk/software/figtree/
Mąkol, J. Generic level review and phylogeny of Trombidiidae and Podothrombiidae (Acari: Actinotrichida: Trombidioidea) of the World. Ann. Zool. 57, 1–194 (2007).
Robaux, P. Contribution a l’étude des Acariens Trombidiidae d’Europe. I. Etude Des Thrombidions adultes de la Péninsule Ibérique. II. Liste critique des thrombidions d’Europe. Mém Mus. Natl. Hist. Nat. Sér Zool. 46 (1), 1–124 (1967).
Oh, J. H., Kim, S. & Lee, S. DNA barcodes reveal population-dependent cryptic diversity and various cases of sympatry of Korean leptonetid spiders (Araneae: Leptonetidae). Sci. Rep. 12, 15528. https://doi.org/10.1038/s41598-022-18666-y (2022).
Dupuis, J. R., Roe, A. D. & Sperling, F. A. H. Multi-locus species delimitation in closely related animals and fungi: one marker is not enough. Mol. Ecol. 21, 4422–4436. https://doi.org/10.1111/j.1365-294X.2012.05642.x (2012).
Acknowledgements
Our thanks go to Anna and Grzegorz Zaleśny for their kind approval for taking samples in their allotment garden. We are grateful to the anonymous reviewers for their valuable comments which improved the quality of the manuscript. The APC is financed by Wroclaw University of Environmental and Life Sciences.
Author information
Authors and Affiliations
Contributions
J.M.: conceptualization, study design, molecular analyses, preparation of figures, writing the original draft, review, editing. M.F.: conceptualization, study design, sampling, data curation, material preparation, morphometric analyses, laboratory experiments, statistical analyses, review, editing. Both authors read, commented on, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Tables 2, 3 and 4 were incorrectly given as Tables 3, 4 and 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mąkol, J., Felska, M. The syntopic occurrence of velvet mites Trombidium spp. (Acariformes: Trombidiidae) with the first characteristics of the larva of Trombidium heterotrichum. Sci Rep 14, 32155 (2024). https://doi.org/10.1038/s41598-024-81750-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81750-y